High-Performance Thin-Film-Nanocomposite Cation Exchange Membranes Containing Hydrophobic Zeolitic Imidazolate Framework for Monovalent Selectivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Characterization and Performance Assessment
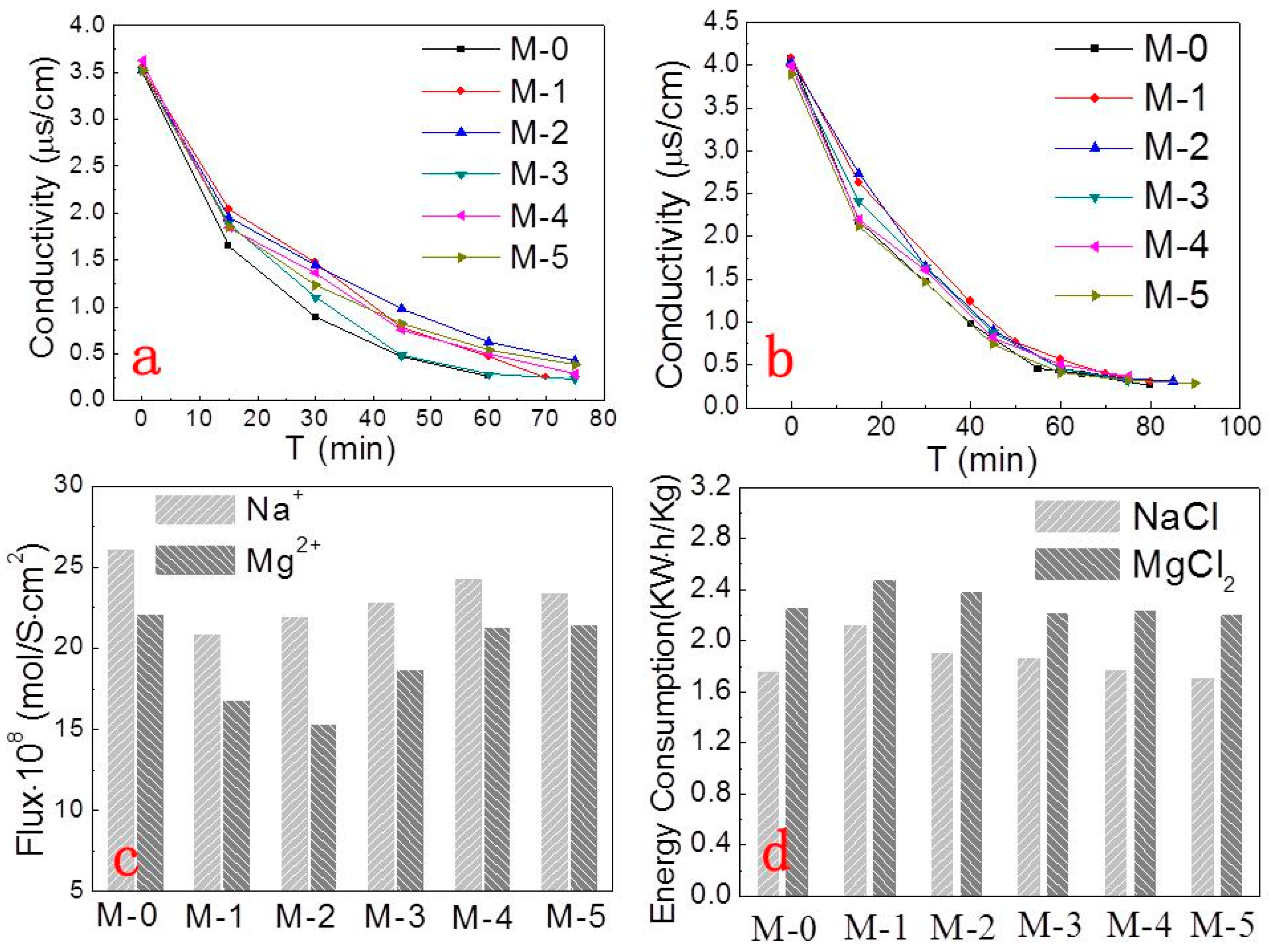
3. Results and Discussion
3.1. SEM and Zeta Potential
3.2. IEC and Water Uptake
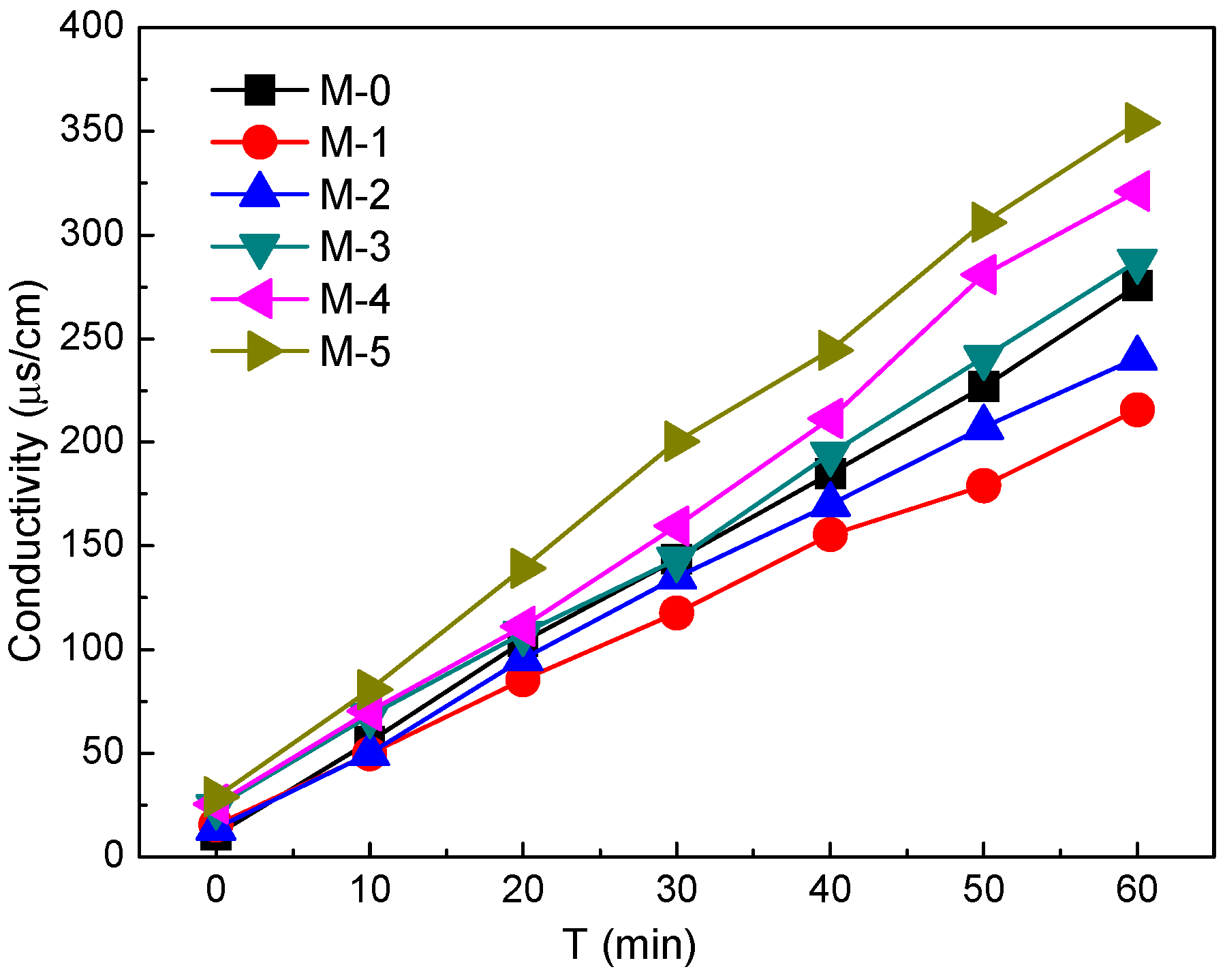
3.3. Diffusion Dialysis
3.4. Membrane Resistance
3.5. Electrodialysis Experiments
3.6. Monovalent Selectivity
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Johnson, R.J.; Stenvinkel, P.; Jensen, T.; Lanaspa, M.A.; Roncal, C.; Song, Z.; Bankir, L.; Sánchez-Lozada, L.G. Metabolic and kidney diseases in the setting of climate change, water shortage, and survival factors. J. Am. Soc. Nephrol. 2016, 27, 2247–2256. [Google Scholar] [CrossRef] [PubMed]
- Pendergast, M.M.; Hoek, E.M. A review of water treatment membrane nanotechnologies. Energy Environ. Sci. 2011, 4, 1946–1971. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.; Gosling, S.N.; Kummu, M.; Flörke, M.; Pfister, S.; Hanasaki, N.; Wada, Y.; Zhang, X.; Zheng, C. Water scarcity assessments in the past, present and future. Earths Future 2017, 5, 545–559. [Google Scholar] [CrossRef]
- Khawaji, A.D.; Kutubkhanah, I.K.; Wie, J.-M. Advances in seawater desalination technologies. Desalination 2008, 221, 47–69. [Google Scholar] [CrossRef]
- Lattemann, S.; Höpner, T. Environmental impact and impact assessment of seawater desalination. Desalination 2008, 220, 1–15. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wu, B.; Yu, D.; Mondal, A.N.; Hou, L.; Afsar, N.U.; Li, Q.; Xu, T.; Miao, J.; Xu, T. Monovalent cation perm-selective membranes (MCPMs): New developments and perspectives. Chin. J. Chem. Eng. 2017, 25, 1606–1615. [Google Scholar] [CrossRef]
- Ge, L.; Wu, B.; Li, Q.; Wang, Y.; Yu, D.; Wu, L.; Pan, J.; Miao, J.; Xu, T. Electrodialysis with nanofiltration membrane (EDNF) for high-efficiency cations fractionation. J. Membr. Sci. 2016, 498, 192–200. [Google Scholar] [CrossRef]
- Lau, W.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Chen, J.P.; Ismail, A. A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.; Jahanshahi, M.; Rahimpour, A. Synthesis of novel thin film nanocomposite (TFN) forward osmosis membranes using functionalized multi-walled carbon nanotubes. J. Membr. Sci. 2013, 435, 233–241. [Google Scholar] [CrossRef]
- Safarpour, M.; Khataee, A.; Vatanpour, V. Thin film nanocomposite reverse osmosis membrane modified by reduced graphene oxide/TiO2 with improved desalination performance. J. Membr. Sci. 2015, 489, 43–54. [Google Scholar] [CrossRef]
- Peyravi, M.; Jahanshahi, M.; Rahimpour, A.; Javadi, A.; Hajavi, S. Novel thin film nanocomposite membranes incorporated with functionalized TiO2 nanoparticles for organic solvent nanofiltration. Chem. Eng. J. 2014, 241, 155–166. [Google Scholar] [CrossRef]
- Kitagawa, S. Metal–organic frameworks (MOFs). Chem. Soc. Rev. 2014, 43, 5415–5418. [Google Scholar]
- Sorribas, S.; Gorgojo, P.; Téllez, C.; Coronas, J.; Livingston, A.G. High flux thin film nanocomposite membranes based on metal–organic frameworks for organic solvent nanofiltration. J. Am. Chem. Soc. 2013, 135, 15201–15208. [Google Scholar] [CrossRef] [PubMed]
- Van Goethem, C.; Verbeke, R.; Hermans, S.; Bernstein, R.; Vankelecom, I. Controlled positioning of MOFs in interfacially polymerized thin-film nanocomposites. J. Mater. Chem. A 2016, 4, 16368–16376. [Google Scholar] [CrossRef]
- Wee, L.H.; Lescouet, T.; Ethiraj, J.; Bonino, F.; Vidruk, R.; Garrier, E.; Packet, D.; Bordiga, S.; Farrusseng, D.; Herskowitz, M.; et al. Hierarchical Zeolitic Imidazolate Framework-8 Catalyst for Monoglyceride Synthesis. ChemCatChem 2013, 5, 3562–3566. [Google Scholar] [CrossRef]
- Duan, J.; Pan, Y.; Pacheco, F.; Litwiller, E.; Lai, Z.; Pinnau, I. High-performance polyamide thin-film-nanocomposite reverse osmosis membranes containing hydrophobic zeolitic imidazolate framework-8. J. Membr. Sci. 2015, 476, 303–310. [Google Scholar] [CrossRef]
- Wang, L.; Fang, M.; Liu, J.; He, J.; Li, J.; Lei, J. Layer-by-layer fabrication of high-performance polyamide/ZIF-8 nanocomposite membrane for nanofiltration applications. ACS Appl. Mater. Interfaces 2015, 7, 24082–24093. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.M.; Yang, T.; Chung, T.S. Polybenzimidazole (PBI)/zeolitic imidazolate frameworks (ZIF-8) mixed matrix membranes for pervaporation dehydration of alcohols. J. Membr. Sci. 2012, 415, 577–586. [Google Scholar] [CrossRef]
- Bazinet, L.; Moalic, M. Coupling of porous filtration and ion-exchange membranes in an electrodialysis stack and impact on cation selectivity: A novel approach for sea water demineralization and the production of physiological water. Desalination 2011, 277, 356–363. [Google Scholar] [CrossRef]
- Kang, K.C.; Linga, P.; Park, K.-N.; Choi, S.-J.; Lee, J.D. Seawater desalination by gas hydrate process and removal characteristics of dissolved ions (Na+, K+, Mg2+, Ca2+, B3+, Cl−, SO42−). Desalination 2014, 353, 84–90. [Google Scholar] [CrossRef]
- Kotov, V.; Peregonchaya, O.; Selemenev, V. Electrodialysis of binary electrolyte mixtures with membranes modified by organic species. Russ. J. Electrochem. 2002, 38, 927–929. [Google Scholar] [CrossRef]
- Tansel, B.; Sager, J.; Rector, T.; Garland, J.; Strayer, R.F.; Levine, L.; Roberts, M.; Hummerick, M.; Bauer, J. Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes. Sep. Purif. Technol. 2006, 51, 40–47. [Google Scholar] [CrossRef]
- Firdaous, L.; Malériat, J.P.; Schlumpf, J.P.; Quéméneur, F. Transfer of monovalent and divalent cations in salt solutions by electrodialysis. Sep. Sci. Technol. 2007, 42, 931–948. [Google Scholar] [CrossRef]
- Han, L.; Galier, S.; Balmann, H.R. Ion hydration number and electro-osmosis during electrodialysis of mixed salt solution. Desalination 2015, 373, 38–46. [Google Scholar] [CrossRef]
- Li, J.; Yuan, S.; Wang, J.; Zhu, J.; Shen, J.; van der Bruggen, B. Mussel-inspired modification of ion exchange membrane for monovalent separation. J. Membr. Sci. 2018, 553, 139–150. [Google Scholar] [CrossRef]
- Sun, F.; Wu, C.; Wu, Y.; Xu, T. Porous BPPO-based membranes modified by multisilicon copolymer for application in diffusion dialysis. J. Membr. Sci. 2014, 450, 103–110. [Google Scholar] [CrossRef]
- Lind, M.L.; Ghosh, A.K.; Jawor, A.; Huang, X.; Hou, W.; Yang, Y.; Hoek, E.M. Influence of zeolite crystal size on zeolite-polyamide thin film nanocomposite membranes. Langmuir 2009, 25, 10139–10145. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Fang, M.; Liu, J.; He, J.; Deng, L.; Li, J.; Lei, J. The influence of dispersed phases on polyamide/ZIF-8 nanofiltration membranes for dye removal from water. RSC Adv. 2015, 5, 50942–50954. [Google Scholar] [CrossRef]
- Erbil, H.Y.; Demirel, A.L.; Avcı, Y.; Mert, O. Transformation of a simple plastic into a superhydrophobic surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Nabe, A.; Staude, E.; Belfort, G. Surface modification of polysulfone ultrafiltration membranes and fouling by BSA solutions. J. Membr. Sci. 1997, 133, 57–72. [Google Scholar] [CrossRef]
- Kang, G.-D.; Cao, Y.-M. Development of antifouling reverse osmosis membranes for water treatment: A review. Water Res. 2012, 46, 584–600. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Li, J.; Zhu, J.; Yuan, S.; Li, X.; Zhao, Z.; Zhao, Y.; Liu, Y.; Volodine, A.; Li, J.; Shen, J.; et al. Mussel-inspired monovalent selective cation exchange membranes containing hydrophilic mil53 (al) framework for enhanced ion flux. Ind. Eng. Chem. Res. 2018, 57, 6275–6283. [Google Scholar] [CrossRef]
- Sata, T. Studies on anion exchange membranes having permselectivity for specific anions in electrodialysis—Effect of hydrophilicity of anion exchange membranes on permselectivity of anions. J. Membr. Sci. 2000, 167, 1–31. [Google Scholar] [CrossRef]
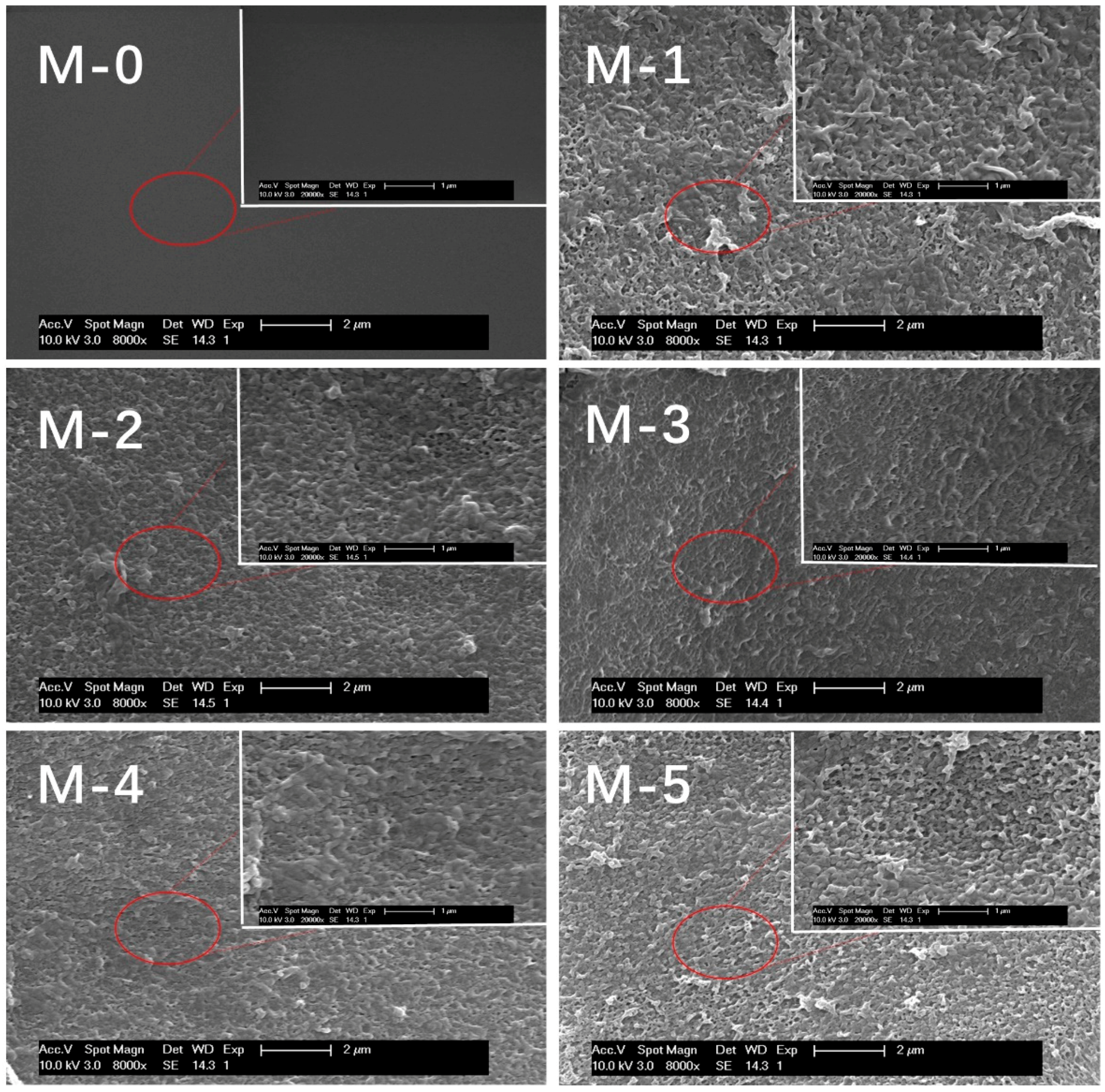

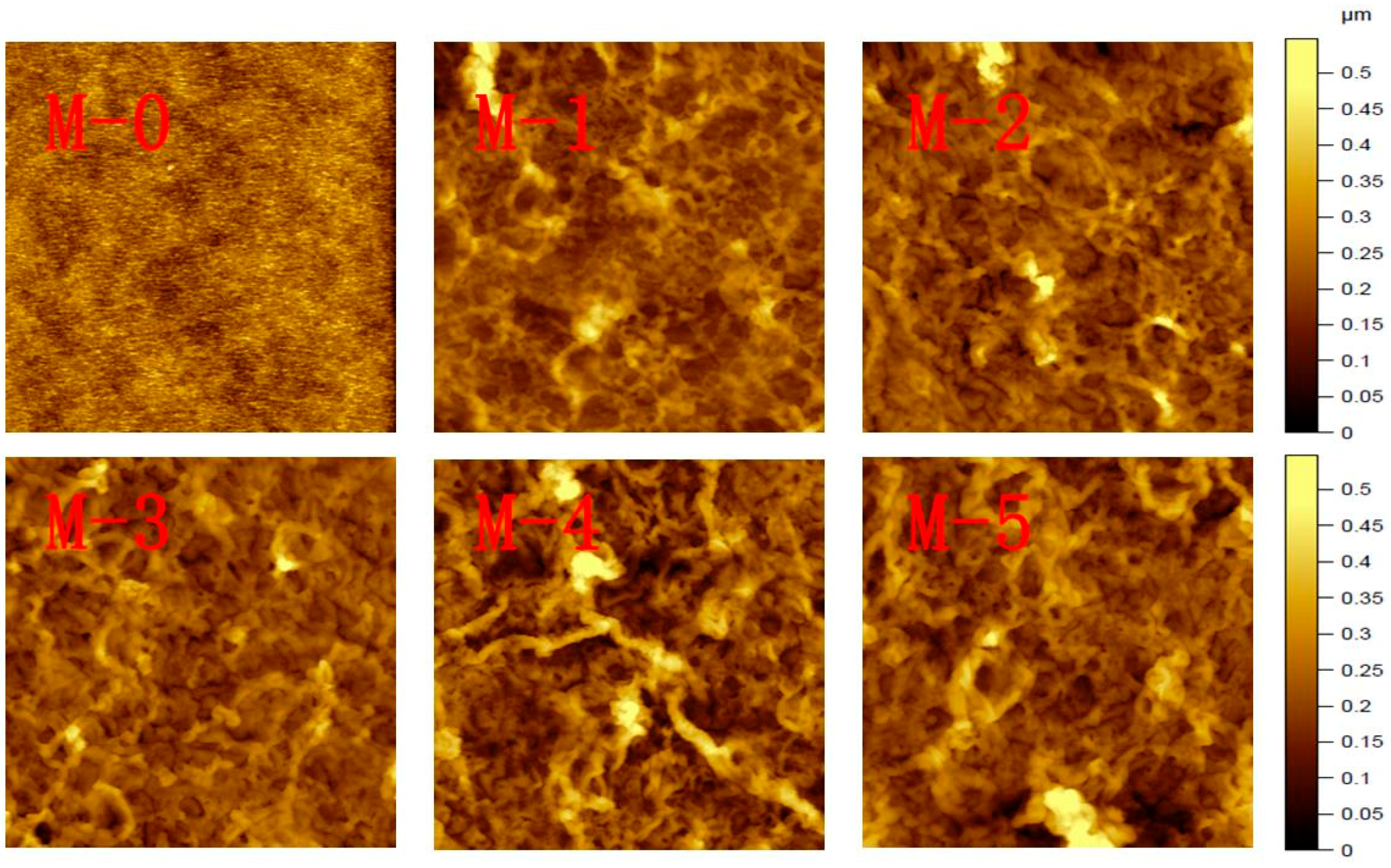



| Membrane Type | C (%) | N (%) | O (%) | Zn (%) |
|---|---|---|---|---|
| M-0 | 56.80 | 14.31 | 28.89 | - |
| M-1 | 59.35 | 14.78 | 25.87 | - |
| M-2 | 57.59 | 16.60 | 25.78 | 0.03 |
| M-3 | 57.77 | 16.14 | 26.01 | 0.08 |
| M-4 | 58.30 | 16.92 | 24.70 | 0.08 |
| M-5 | 59.07 | 15.49 | 25.32 | 0.12 |
| Membrane | M-0 | M-1 | M-2 | M-3 | M-4 | M-5 |
|---|---|---|---|---|---|---|
| Zeta potential (mV) | −16.4 | −20.7 | −22.3 | −23.7 | −23.8 | −24.5 |
| Membrane Type | M-0 | M-1 | M-2 | M-3 | M-4 | M-5 |
|---|---|---|---|---|---|---|
| Root mean square height (Rsq) | 1.0 nm | 36.3 nm | 43.4 nm | 51.5 nm | 65.2 nm | 70.8 nm |
| Maximum height (Rsz) | 4.4 nm | 210 nm | 230 nm | 274 nm | 347 nm | 335 nm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhao, Z.; Yuan, S.; Zhu, J.; Van der Bruggen, B. High-Performance Thin-Film-Nanocomposite Cation Exchange Membranes Containing Hydrophobic Zeolitic Imidazolate Framework for Monovalent Selectivity. Appl. Sci. 2018, 8, 759. https://doi.org/10.3390/app8050759
Li J, Zhao Z, Yuan S, Zhu J, Van der Bruggen B. High-Performance Thin-Film-Nanocomposite Cation Exchange Membranes Containing Hydrophobic Zeolitic Imidazolate Framework for Monovalent Selectivity. Applied Sciences. 2018; 8(5):759. https://doi.org/10.3390/app8050759
Chicago/Turabian StyleLi, Jian, Zhijuan Zhao, Shushan Yuan, Junyong Zhu, and Bart Van der Bruggen. 2018. "High-Performance Thin-Film-Nanocomposite Cation Exchange Membranes Containing Hydrophobic Zeolitic Imidazolate Framework for Monovalent Selectivity" Applied Sciences 8, no. 5: 759. https://doi.org/10.3390/app8050759





