Development and Characterization of a Poly (Vinyl Alcohol)/Graphene Oxide Composite Hydrogel as An Artificial Cartilage Material
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of GO
2.2. X-ray Photoelectron Spectroscopy (XPS) Spectrum Measurement of GO
2.3. Preparation of PVA-H/PVA-GO-H
2.4. SEM Measurements of PVA-H/PVA-GO-H
2.5. Contact Angle Measurements
2.6. Tensile Test
2.7. Cell Culture
3. Results
3.1. XPS Spectrum of GOs
3.2. SEM Images of PVA-H/PVA-GO-H
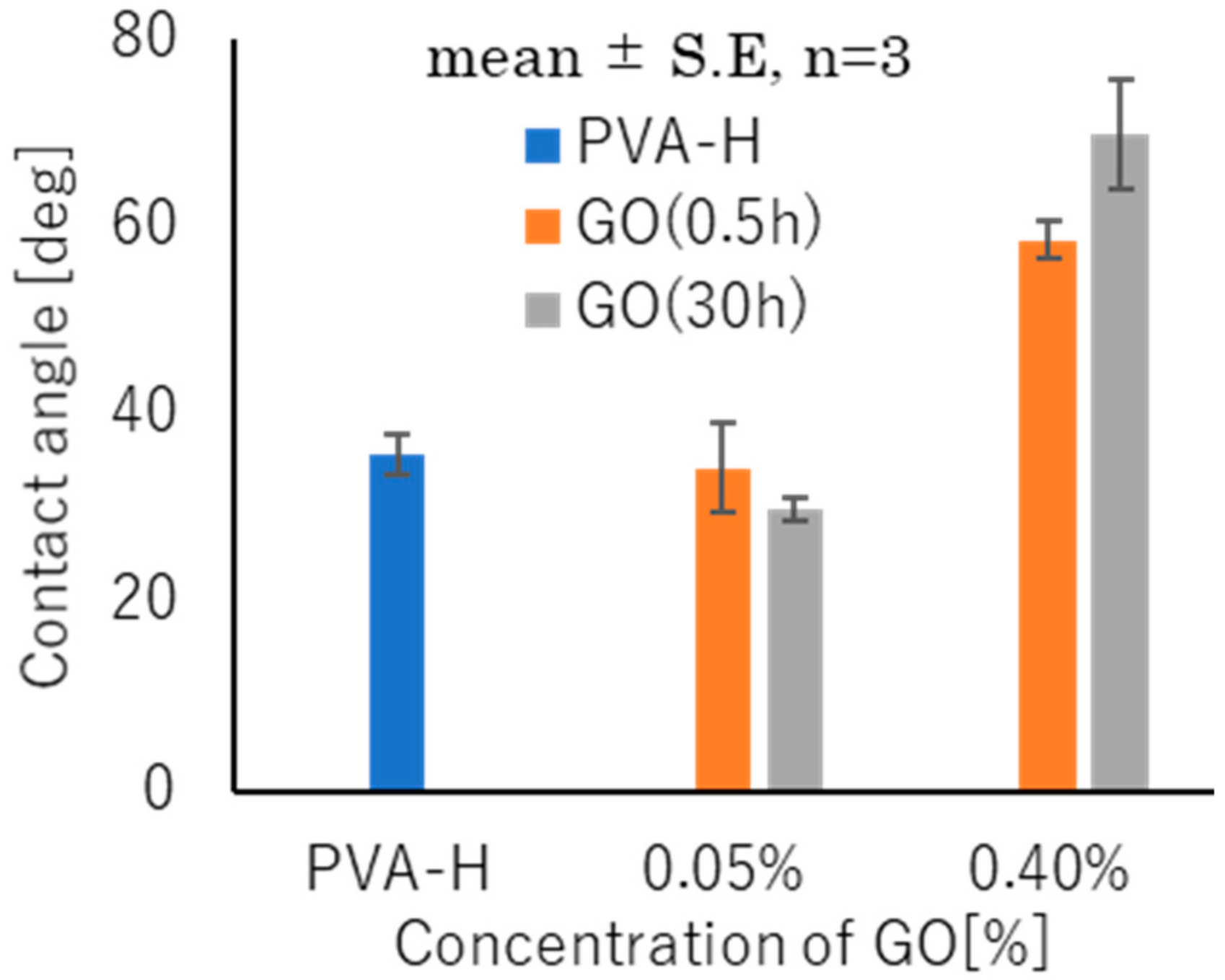
3.3. Hydrophilicity Evaluation
3.4. Control of Water Content
3.5. Mechanical Properties
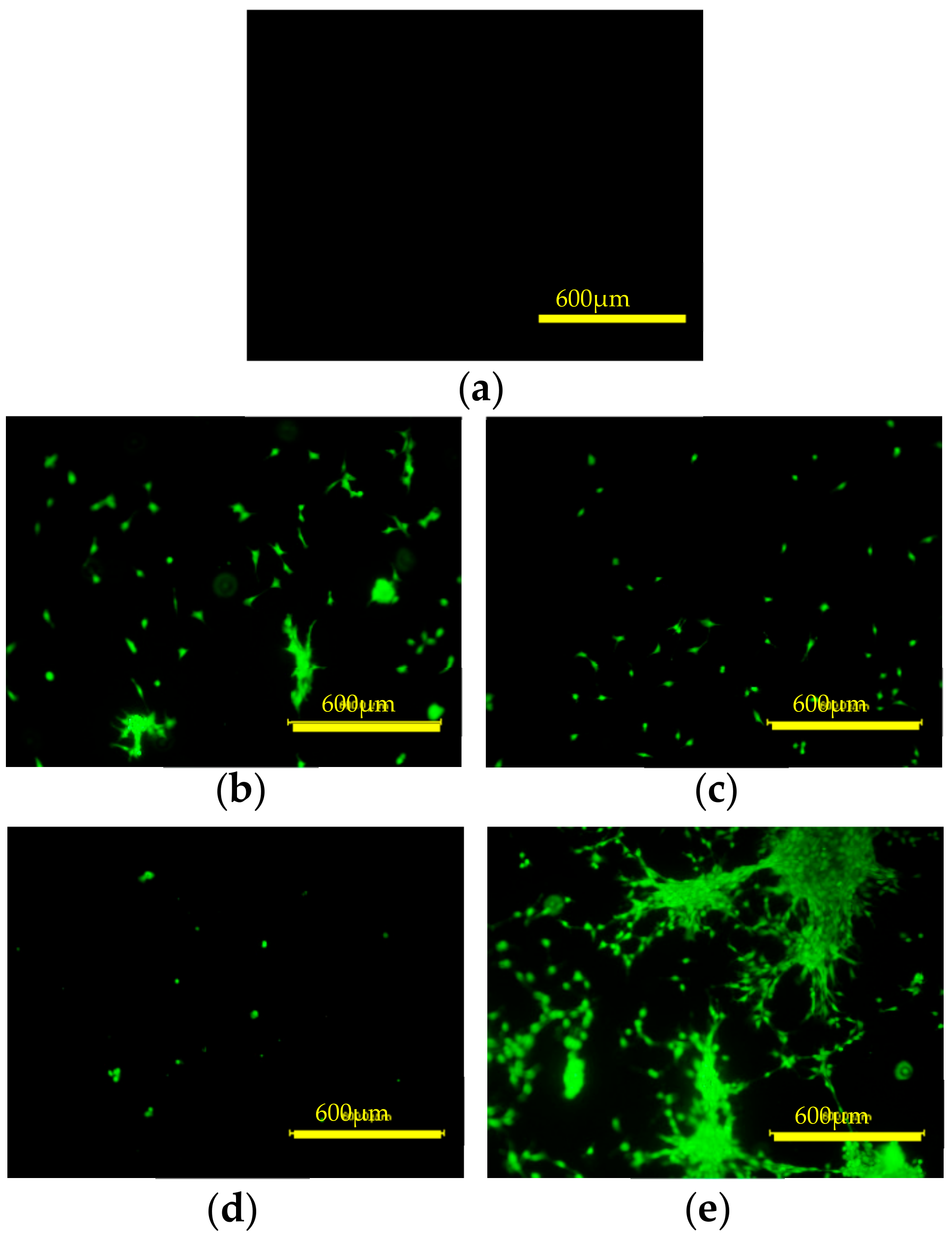
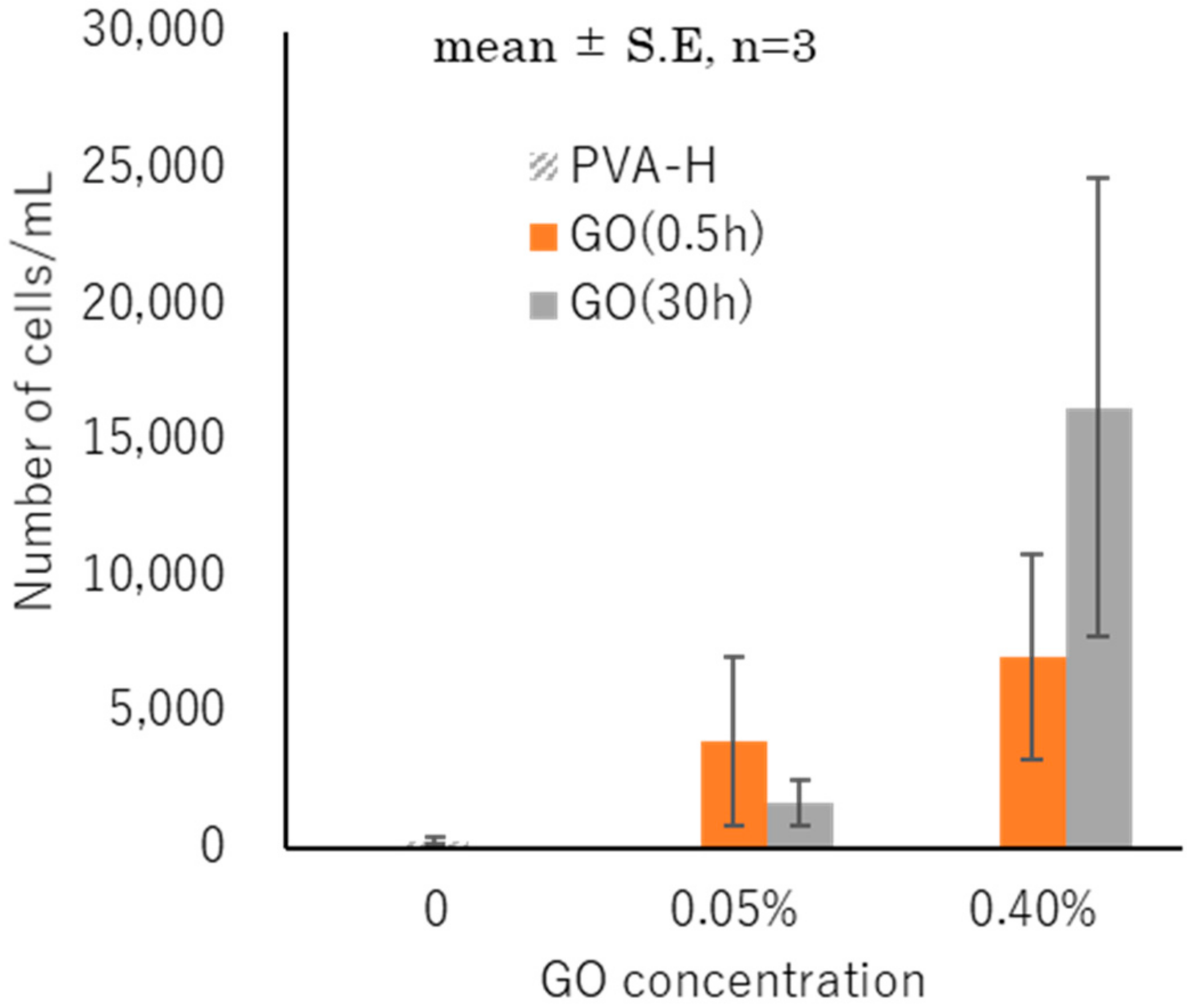
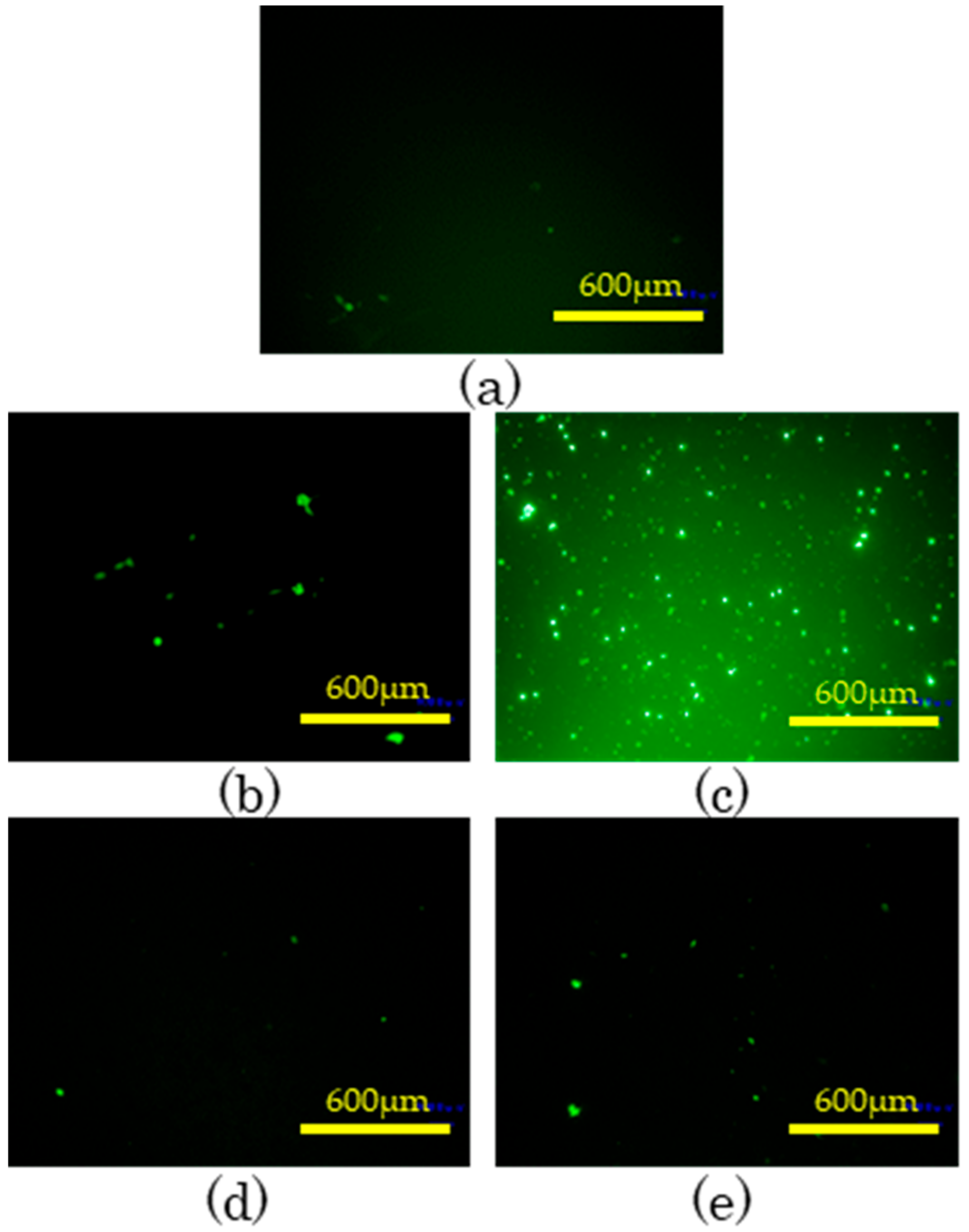
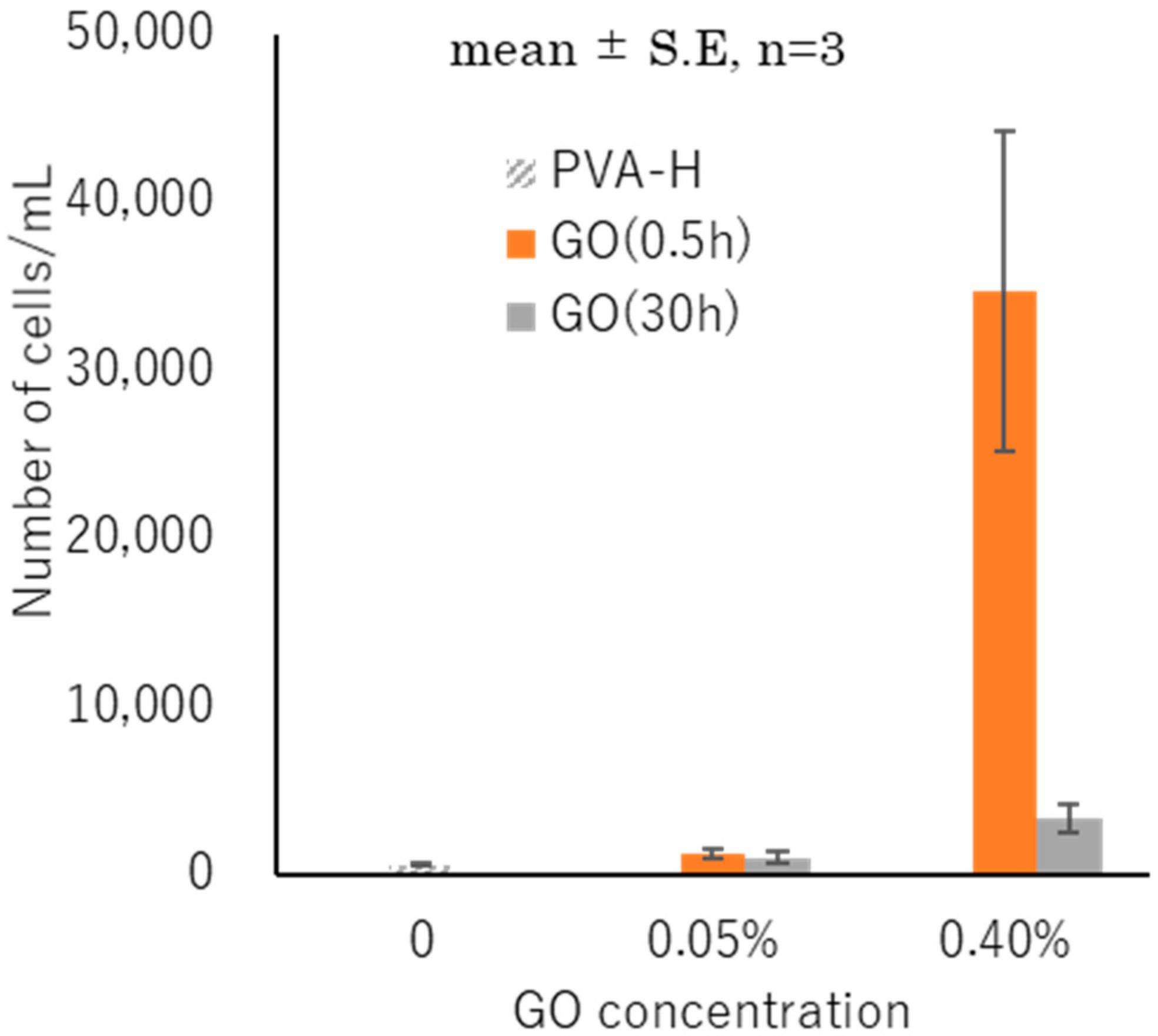
3.6. Cell Attachment and Proliferation
4. Discussions
Author Contributions
Funding
Conflicts of Interest
References
- Mayer, F.; Dickhuth, H.H. Physical activity after total joint replacement. Int. SportMed J. 2008, 9, 39–43. [Google Scholar]
- Moti, L.T.; Hatem, E.S. Cartilage regeneration for treatment of osteoarthritis: A paradigm for nonsurgical intervention. Ther. Adv. Musculoskelet. Dis. 2015, 7, 76–87. [Google Scholar]
- Wagner, H. Surface replacement arthroplasty of the hip. Clin. Orthop. Relat. Res. 1978, 134, 102–130. [Google Scholar] [CrossRef]
- Allan, S.H. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar]
- Gong, J.P.; Kurokawa, T.; Narita, T.; Kagata, G.; Osada, Y.; Nishimura, G.; Kinjo, M. Synthesis of hydrogels with extremely low surface friction. J. Am. Chem. Soc. 2001, 123, 5582–5583. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Tabata, Y.; Ikada, Y. Comparison of body distribution of poly(vinyl alcohol) with other water-soluble polymers after intra-venous administration. J. Pharm. Pharmacol. 1995, 47, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zheng, Y.; Wang, Y.; Wu, G. Review of poly(vinyl alcohol) hydrogel and its compounds in the application of artificial cartilage materials. J. Biomed. Eng. 2003, 20, 742–745. [Google Scholar]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-network hydrogels with extremely high mechanical strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Liu, Y.; Vrana, N.E.; Cahill, P.A.; McGuinness, G.B. Physically crosslinked composite hydrogels of PVA with natural macromolecules: Structure, mechanical properties, and endothelial cell compatibility. J. Biomed. Mater. Res. Part B 2009, 90B, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Koyano, T.; Minoura, N.; Nagura, M.; Kobayashi, K. Attachment and growth of cultured fibroblast cells on PVA/chitosan-blended hydrogels. J. Biomed. Mater. Res. 1998, 39, 486–490. [Google Scholar] [CrossRef]
- Ushio, K.; Oka, M.; Hyon, S.-H.; Hayami, T.; Yura, S.; Matsumura, K.; Toguchida, J.; Nakamura, T. Attachment of artificial cartilage to underlying bone. J. Biomed. Mater. Res. B. 2004, 68B, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.Y.; Tai, Z.X.; Sun, D.F.; Chen, J.T.; Ma, H.B.; Yan, X.B.; Liu, B.; Xue, Q.J. Fabrication and characterization of poly(vinyl alcohol)/graphene oxide nanofibrous biocomposite scaffolds. J. Appl. Polym. Sci. 2013, 127, 1885–1894. [Google Scholar] [CrossRef]
- Liang, J.J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.F.; Guo, T.Y.; Chen, Y.S. Molecular-level dispersion of graphene into poly(vinyl alcohol) and effective reinforcement of their nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Sharma, S.K.; Prakash, J.; Rujari, P.K. Effects of the molecular level dispersion of graphene oxide on the free volume characteristics of poly(vinyl alcohol) and its impact on the thermal and mechanical properties of their nanocomposites. Phys. Chem. Chem. Phys. 2015, 17, 29201–29209. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.P.; Xu, C.; Li, Y.; Gao, J.P.; Wang, W.; Liu, Y. High strength graphene oxide/polyvinyl alcohol composite hydrogels. J. Mater. Chem. 2011, 21, 10399–10406. [Google Scholar] [CrossRef]
- Shi, X.T.; Chang, H.X.; Chen, S.; Lai, C.; Khademhosseini, A.; Wu, H.K. Regulating cellular behavior on few-layer reduced graphene oxide films with well-controlled reduction states. Adv. Funct. Mater. 2012, 22, 751–759. [Google Scholar] [CrossRef]
- Matsumura, K.; Hayami, T.; Hyon, S.H.; Tsutsumi, S. Control of proliferation and differentiation of osteoblasts on apatite-coated poly(vinyl alcohol) hydrogel as an artificial articular cartilage material. J. Biomed. Mater. Res. Part A 2010, 92A, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, M.; Inoue, T.; Miyasaka, K. Light-scattering studies on the sol–gel transition in aqueous solutions of poly(vinyl alcohol). Polym. Prepr. Jpn. 1986, 24, 303–311. [Google Scholar] [CrossRef]
- Peppas, N.A. Turbidimetric studies of aqueous poly(vinyl alcohol) solutions. Makromol. Chem. 1975, 176, 3433–3440. [Google Scholar] [CrossRef]
- Hyon, S.-H.; Cha, W.-I.; Ikada, Y. Preparation of transparent poly(vinyl alcohol) hydrogel. Polym. Bull. 1989, 22, 119–122. [Google Scholar] [CrossRef]
- William, S.H.; Richard, E.O. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339–1339. [Google Scholar]
- Matsuura, K.; Umahara, Y.; Gotoh, K.; Hoshijima, Y.; Ishida, H. Surface modification effects on the tensile properties of functionalised graphene oxide epoxy films. RSC Adv. 2018, 8, 9677–9684. [Google Scholar] [CrossRef] [Green Version]
- Cha, W.I.; Hyon, S.H.; Ikada, Y. Microstructure of poly(vinyl alcohol) hydrogels investigated with differential scanning calorimetry. Die Makromol. Chem. 1993, 194, 2433–2441. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Nagano, S.; Hara, M.; Hyon, S.H.; Patel, M.; Matsumura, K. Facile preparation of transparent poly (vinyl alcohol) hydrogels with uniform microcrystalline structure by hot-pressing without using organic solvents. Polym. J. 2017, 49, 535–542. [Google Scholar] [CrossRef]
- Otsuka, E.; Komiya, S.; Sasaki, S.; Xing, J.W.; Bando, T.; Hirashima, Y.; Sugiyama, M.; Suzuki, A. Effects of preparation temperature on swelling and mechanical properties of PVA cast gels. Soft Matter 2012, 8, 812–8136. [Google Scholar] [CrossRef]









| Oxidation Time (h) | 0.5 | 30 |
|---|---|---|
| C:O | 71:28 | 69:29 |
| Functional Group | GO (0.5 h) | GO (30 h) |
|---|---|---|
| C–C | 1.00 | 1.00 |
| C–OH | 0.60 | 0.30 |
| C–O (epoxy) | 0.87 | 0.90 |
| C=O | 0.087 | 0.094 |
| O–C=O | 0.082 | 0.099 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Terai, W.; Hoshijima, Y.; Gotoh, K.; Matsuura, K.; Matsumura, K. Development and Characterization of a Poly (Vinyl Alcohol)/Graphene Oxide Composite Hydrogel as An Artificial Cartilage Material. Appl. Sci. 2018, 8, 2272. https://doi.org/10.3390/app8112272
Zhao Y, Terai W, Hoshijima Y, Gotoh K, Matsuura K, Matsumura K. Development and Characterization of a Poly (Vinyl Alcohol)/Graphene Oxide Composite Hydrogel as An Artificial Cartilage Material. Applied Sciences. 2018; 8(11):2272. https://doi.org/10.3390/app8112272
Chicago/Turabian StyleZhao, Yibo, Wataru Terai, Yuko Hoshijima, Kazuma Gotoh, Koji Matsuura, and Kazuaki Matsumura. 2018. "Development and Characterization of a Poly (Vinyl Alcohol)/Graphene Oxide Composite Hydrogel as An Artificial Cartilage Material" Applied Sciences 8, no. 11: 2272. https://doi.org/10.3390/app8112272





