Adsorption Characteristics of Phenolic Compounds on Graphene Oxide and Reduced Graphene Oxide: A Batch Experiment Combined Theory Calculation
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Preparation of RGO
2.3. Characterization
2.4. Batch Adsorption Experiments
3. Results and Discussion
3.1. Characterization of GO and RGO
3.2. Effect of Contact Time
3.3. Effect of pH on Adsorption
3.4. Adsorption Isotherms and Thermodynamics
3.5. Effect of GO Reduction on Adsorption
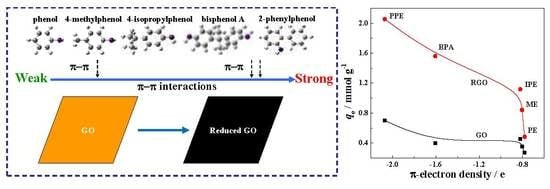
3.6. Effect of the Chemical Structure of Phenols on Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Liu, Q.S.; Zheng, T.; Wang, P.; Jiang, J.P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Acikgoz, E.; Ozcan, B. Phenol biodegradation by halophilic archaea. Int. Biodeter. Biodegr. 2016, 107, 140–146. [Google Scholar] [CrossRef]
- Ma, W.W.; Han, Y.X.; Xu, C.Y.; Han, H.J.; Ma, W.C.; Zhu, H.; Li, K.; Wang, D.X. Enhanced degradation of phenolic compounds in coal gasification wastewater by a novel integration of micro-electrolysis with biological reactor (MEBR) under the micro-oxygen condition. Bioresour. Technol. 2018, 251, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Renuka, M.K.; Gayathri, V. UV/solar light induced photocatalytic degradation of phenols and dyes by Fe(PS-BBP)Cl3. J. Photochem. Photobiol. A Chem. 2018, 353, 477–487. [Google Scholar] [CrossRef]
- Xiao, J.D.; Xie, Y.B.; Han, Q.Z.; Cao, H.B.; Wang, Y.J.; Nawaz, F.; Duan, F. Superoxide radical-mediated photocatalytic oxidation of phenolic compounds over Ag+/TiO2: Influence of electron donating and withdrawing substituents. J. Hazard. Mater. 2016, 304, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Li, H.C.; Shan, C.; Pan, B.C. Fe(III)-doped g-C3N4 mediated peroxymonosulfate activation for selective degradation of phenolic compounds via high-valent iron-oxo species. Environ. Sci. Technol. 2018, 52, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.H.; Gao, Z.T.; Zhang, L.Z.; He, W.W.; Yin, J.J. Core–shell Structure dependent reactivity of Fe@Fe2O3 nanowires on aerobic degradation of 4-chlorophenol. Environ. Sci. Technol. 2013, 47, 5344–5352. [Google Scholar] [CrossRef] [PubMed]
- Capocelli, M.; Prisciandaro, M.; Lancia, A.; Musmarra, D. Hydrodynamic cavitation of p-nitrophenol: A theoretical and experimental insight. Chem. Eng. J. 2014, 254, 1–8. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Degradation of 2,4-dinitrophenol using a combination of hydrodynamic cavitation, chemical and advanced oxidation processes. Ultrason. Sonochem. 2013, 20, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Bernal, A.M.; Gómez-Granados, F.; Giraldo, L.; Moreno-Piraján, J.C. A sudy of the interactions of activated carbon-phenol in aqueous solution using the determination of immersion enthalpy. Appl. Sci. 2018, 8, 843. [Google Scholar] [CrossRef]
- Sanchez-Montero, M.J.; Pelaz, J.; Martin-Sanchez, N.; Izquierdo, C.; Salvador, F. Supercritical regeneration of an activated carbon fiber exhausted with phenol. Appl. Sci. 2018, 8, 81. [Google Scholar] [CrossRef]
- Yangui, A.; Abderrabba, M.; Sayari, A. Amine-modified mesoporous silica for quantitative adsorption and release of hydroxytyrosol and other phenolic compounds from olive mill wastewater. J. Taiwan Inst. Chem. Eng. 2017, 70, 111–118. [Google Scholar] [CrossRef]
- Xu, J.; Wang, L.; Zhu, Y.F. Decontamination of bisphenol A from aqueous solution by graphene adsorption. Langmuir 2012, 28, 8418–8425. [Google Scholar] [CrossRef] [PubMed]
- Morozov, S.V.; Novoselov, K.S.; Schedin, F.; Jiang, D.; Firsov, A.A.; Geim, A.K. Two-dimensional electron and hole gases at the surface of graphite. Phys. Rev. B 2005, 72, 201401. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Apul, O.G.; Karanfil, T. Adsorption of halogenated aliphatic contaminants by graphene nanomaterials. Water Res. 2015, 79, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majidi, R.; Karami, A.R. Caffeine and nicotine adsorption on perfect, defective and porous graphene sheets. Diam. Relat. Mater. 2016, 66, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Cao, Z.; Zhang, Y.L.; Yuan, Z.L.; Lou, Z.M.; Xu, X.H.; Wang, X.K. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Z.; Shi, Z.; Yuan, M.Y.; Liu, L.S. Adsorption of trivalent antimony from aqueous solution using graphene oxide: Kinetic and thermodynamic studies. J. Chem. Eng. Data 2015, 60, 806–813. [Google Scholar] [CrossRef]
- Ramesha, G.K.; Kumara, A.V.; Muralidhara, H.B.; Sampath, S. Graphene and graphene oxide as effective adsorbents toward anionic and cationic dyes. J. Colloid Interface Sci. 2011, 361, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Mu’azu, N.D.; Jarrah, N.; Zubair, M.; Alagha, O. Removal of phenolic compounds from water using sewage sludge-based activated carbon adsorption: A review. Int. J. Environ. Res. Public Health 2017, 14, 1094. [Google Scholar] [CrossRef] [PubMed]
- Erto, A.; Chianese, S.; Lancia, A.; Musmarra, D. On the mechanism of benzene and toluene adsorption in single-compound and binary systems: Energetic interactions and competitive effects. Desalin. Water Treat. 2017, 86, 259–265. [Google Scholar] [CrossRef]
- Björk, J.; Hanke, F.; Palma, C.A.; Samori, P.; Cecchini, M.; Persson, M. Adsorption of aromatic and anti-aromatic systems on graphene through π-π Stacking. J. Phys. Chem. Lett. 2010, 1, 3407–3412. [Google Scholar] [CrossRef]
- Zhao, G.X.; Jiang, L.; He, Y.D.; Li, J.X.; Dong, H.L.; Wang, X.K.; Hu, W.P. Sulfonated Graphene for Persistent Aromatic Pollutant Management. Adv. Mater. 2011, 23, 3959–3963. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Min, K.A.; Hong, S.; Kim, G. Ab initio study of adsorption properties of hazardous organicmolecules on graphene: Phenol, phenyl azide, and phenylnitrene. Chem. Phys. Lett. 2015, 618, 57–62. [Google Scholar] [CrossRef]
- He, L.; Liu, F.F.; Zhao, M.Y.; Qi, Z.; Sun, X.F.; Afzal, M.Z.; Sun, X.M.; Li, Y.H.; Hao, J.C.; Wang, S.G. Electronic-property dependent interactions between tetracycline and graphene nanomaterials in aqueous solution. J. Environ. Sci. 2018, 66, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Chen, B.L. Macroscopic and spectroscopic investigations of the adsorption of nitroaromatic compounds on graphene oxide, reduced graphene oxide, and graphene nanosheets. Environ. Sci. Technol. 2015, 49, 6181–6189. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.G.; Yan, D.X.; Ji, X.; Chen, T.; Li, Z.M. Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology 2011, 22, 055705. [Google Scholar] [CrossRef] [PubMed]
- Jiao, T.F.; Guo, H.Y.; Zhang, Q.R.; Peng, Q.M.; Tang, Y.F.; Yan, X.H.; Li, B.B. Reduced graphene oxide-based silver nanoparticle-containing composite hydrogel as highly efficient dye catalysts for wastewater treatment. Sci. Rep. 2015, 5, 11873. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Tang, H.Q.; Huang, S.S.; Zhu, L.H. Fast and facile microwave-assisted synthesis of graphene oxide nanosheets. RSC Adv. 2014, 4, 60102–60105. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, D.; Chen, X.; Lin, Y. Adsorption of bisphenol A from water by surfactant-modified zeolite. J. Colloid Interface Sci. 2010, 348, 585–590. [Google Scholar] [CrossRef] [PubMed]
- De Souza, Í.F.T.; Petri, D.F.S. β-Cyclodextrin hydroxypropyl methylcellulose hydrogels for bisphenol A adsorption. J. Mol. Liq. 2018, 266, 640–648. [Google Scholar] [CrossRef]
- Wang, L.C.; Ni, X.J.; Cao, Y.H.; Cao, G.Q. Adsorption behavior of bisphenol A on CTAB-modified graphite. Appl. Surf. Sci. 2018, 428, 165–170. [Google Scholar] [CrossRef]
- Zhang, D.F.; Huo, P.L.; Liu, W. Behavior of phenol adsorption on thermal modified activated carbon. Chin. J. Chem. Eng. 2016, 24, 446–452. [Google Scholar] [CrossRef]
- Cheng, W.P.; Gao, W.; Cui, X.Y.; Ma, J.H.; Li, R.F. Phenol adsorption equilibrium and kinetics on zeolite X/activated carbon composite. J. Taiwan Inst. Chem. Eng. 2016, 62, 192–198. [Google Scholar] [CrossRef]
- Mohammed, N.A.S.; Abu-Zurayk, R.A.; Hamadneh, I.; Al-Dujaili, A.H. Phenol adsorption on biochar prepared from the pine fruit shells: Equilibrium, kinetic and thermodynamics studies. J. Environ. Manag. 2018, 226, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.; Biggs, M.J.; Skinner, W.; Bandosz, T.J. The effects of activated carbon surface features on the reactive adsorption of carbamazepine and sulfamethoxazole. Carbon 2014, 80, 419. [Google Scholar] [CrossRef]
- Pei, Z.G.; Li, L.Y.; Sun, L.X.; Zhang, S.Z.; Shan, X.Q.; Yang, S.; Wen, B. Adsorption characteristics of 1,2,4-trichlorobenzene, 2,4,6-trichlorophenol, 2-naphthol and naphthalene on graphene and graphene oxide. Carbon 2013, 51, 156–163. [Google Scholar] [CrossRef] [Green Version]









| Adsorbent | Phenolic Initial Concentration (mg L−1) | Adsorption Capacity (mg g−1) | Reference |
|---|---|---|---|
| GO/RGO | 40 (PE) | 25/45 | Our work |
| GO/RGO | 40 (BPA) | 91/355 | Our work |
| surfactant-modified zeolite | 100 (BPA) | 60 | [31] |
| β-Cyclodextrin hydroxypropyl methylcellulose hydrogels | 99.54 (BPA) | 8 | [32] |
| CTAB-modified graphite | 300 (BPA) | 125 | [33] |
| Thermal modified activated carbon | 1000 (PE) | 90 | [34] |
| zeolite X/activated carbon | 103 (PE) | 14 | [35] |
| biochar | 50 (PE) | 28 | [36] |
| Phenols | Adsorbents | QF (mmol1−1/n L1/n g−1) | 1/n | Km (mmoln−1 L g−n) | R2 |
|---|---|---|---|---|---|
| Phenol (PE) | GO | 0.645 | 0.745 | 0.555 | 0.997 |
| RGO | 0.882 | 0.758 | 0.847 | 0.992 | |
| 4-methylphenol (ME) | GO | 0.939 | 0.751 | 0.92 | 0.986 |
| RGO | 1.695 | 0.703 | 2.118 | 0.993 | |
| 4-isopropylphenol (IPE) | GO | 1.264 | 0.723 | 1.382 | 0.995 |
| RGO | 2.393 | 0.599 | 4.292 | 0.981 | |
| Bisphenol A (BPA) | GO | 1.124 | 0.566 | 1.229 | 0.992 |
| RGO | 3.675 | 0.321 | 57.667 | 0.973 | |
| 2-phenylphenol (PPE) | GO | 2.194 | 0.667 | 3.248 | 0.99 |
| RGO | 3.819 | 0.272 | 137.89 | 0.968 |
| Adsorbents | Phenolics | ΔG0 (kJ/mol) | ΔS0 (J/mol) | ΔH0 (kJ/mol) | ||
|---|---|---|---|---|---|---|
| 298 K | 313 K | 333 K | ||||
| RGO | PE | −1.37 | −0.88 | −0.39 | −27.33 | −9.48 |
| ME | −3.42 | −3.28 | −2.55 | −24.32 | −10.74 | |
| IPE | −4.99 | −4.73 | −4.20 | −21.77 | −11.50 | |
| BPA | −9.33 | −9.00 | −8.87 | −12.21 | −12.92 | |
| PPE | −10.57 | −10.04 | −10.31 | −6.76 | −12.44 | |
| GO | PE | −0.51 | −0.34 | −0.12 | −10.92 | −3.76 |
| ME | −1.63 | −1.47 | −1.11 | −14.38 | −5.93 | |
| IPE | −2.43 | −1.91 | −1.51 | −25.68 | −10.03 | |
| BPA | −3.39 | −3.01 | −2.82 | −15.91 | −8.09 | |
| PPE | −4.37 | −3.876 | −3.583 | −21.786 | −10.8 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Hu, Y.; Min, J.; Li, S.; Deng, X.; Yuan, S.; Zuo, X. Adsorption Characteristics of Phenolic Compounds on Graphene Oxide and Reduced Graphene Oxide: A Batch Experiment Combined Theory Calculation. Appl. Sci. 2018, 8, 1950. https://doi.org/10.3390/app8101950
Wang X, Hu Y, Min J, Li S, Deng X, Yuan S, Zuo X. Adsorption Characteristics of Phenolic Compounds on Graphene Oxide and Reduced Graphene Oxide: A Batch Experiment Combined Theory Calculation. Applied Sciences. 2018; 8(10):1950. https://doi.org/10.3390/app8101950
Chicago/Turabian StyleWang, Xiaobo, Yanhui Hu, Jianhua Min, Sijie Li, Xiangyi Deng, Songdong Yuan, and Xiaohua Zuo. 2018. "Adsorption Characteristics of Phenolic Compounds on Graphene Oxide and Reduced Graphene Oxide: A Batch Experiment Combined Theory Calculation" Applied Sciences 8, no. 10: 1950. https://doi.org/10.3390/app8101950
APA StyleWang, X., Hu, Y., Min, J., Li, S., Deng, X., Yuan, S., & Zuo, X. (2018). Adsorption Characteristics of Phenolic Compounds on Graphene Oxide and Reduced Graphene Oxide: A Batch Experiment Combined Theory Calculation. Applied Sciences, 8(10), 1950. https://doi.org/10.3390/app8101950