Toward Defect Engineering Strategies to Optimize Energy and Electronic Materials
Abstract
:1. Introduction
2. Advanced Materials for Nuclear Applications
2.1. Background
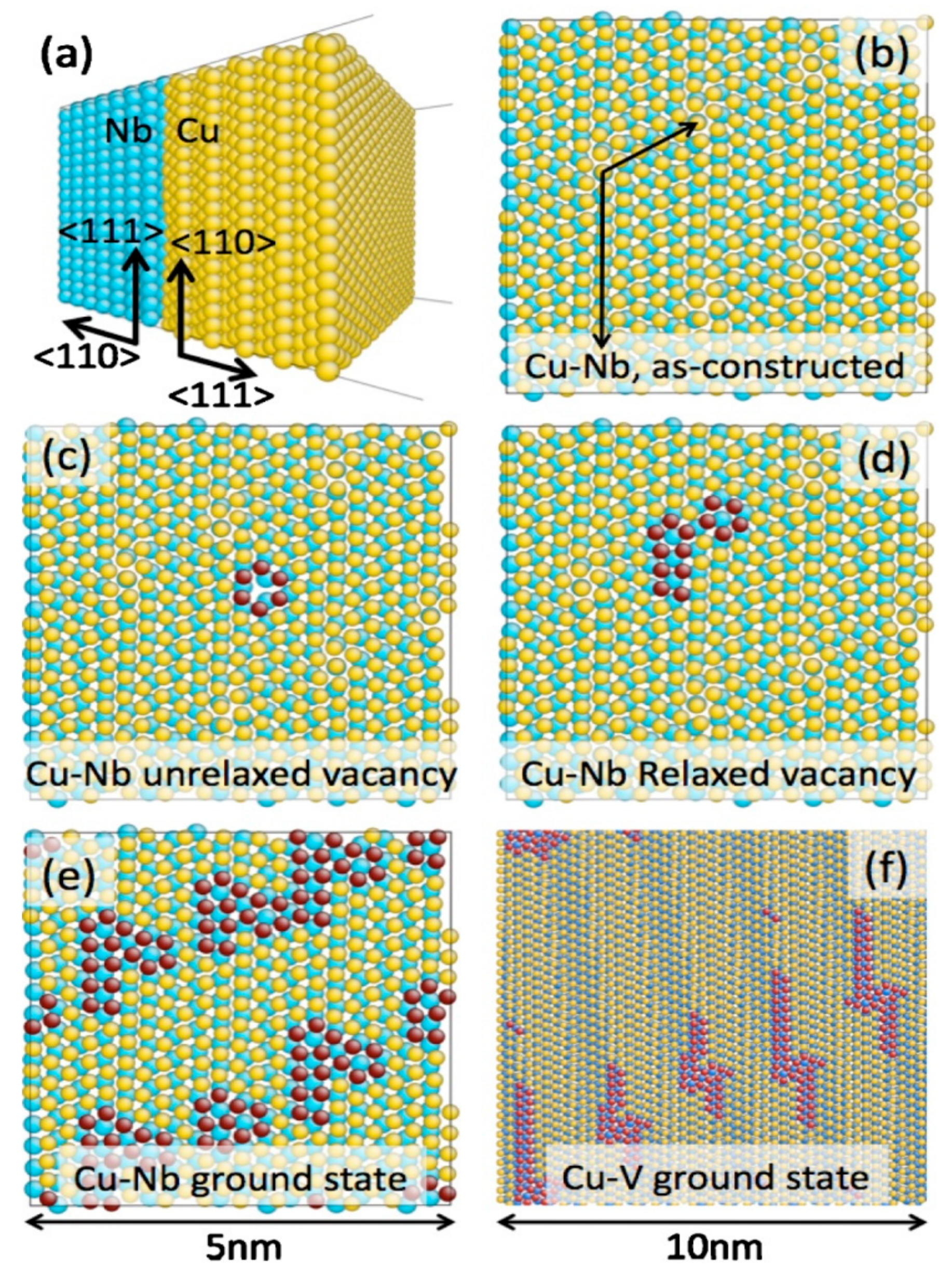
2.2. Radiation Tolerant Materials
3. Defect Engineering Oxygen Diffusion in Ceria
3.1. Motivation
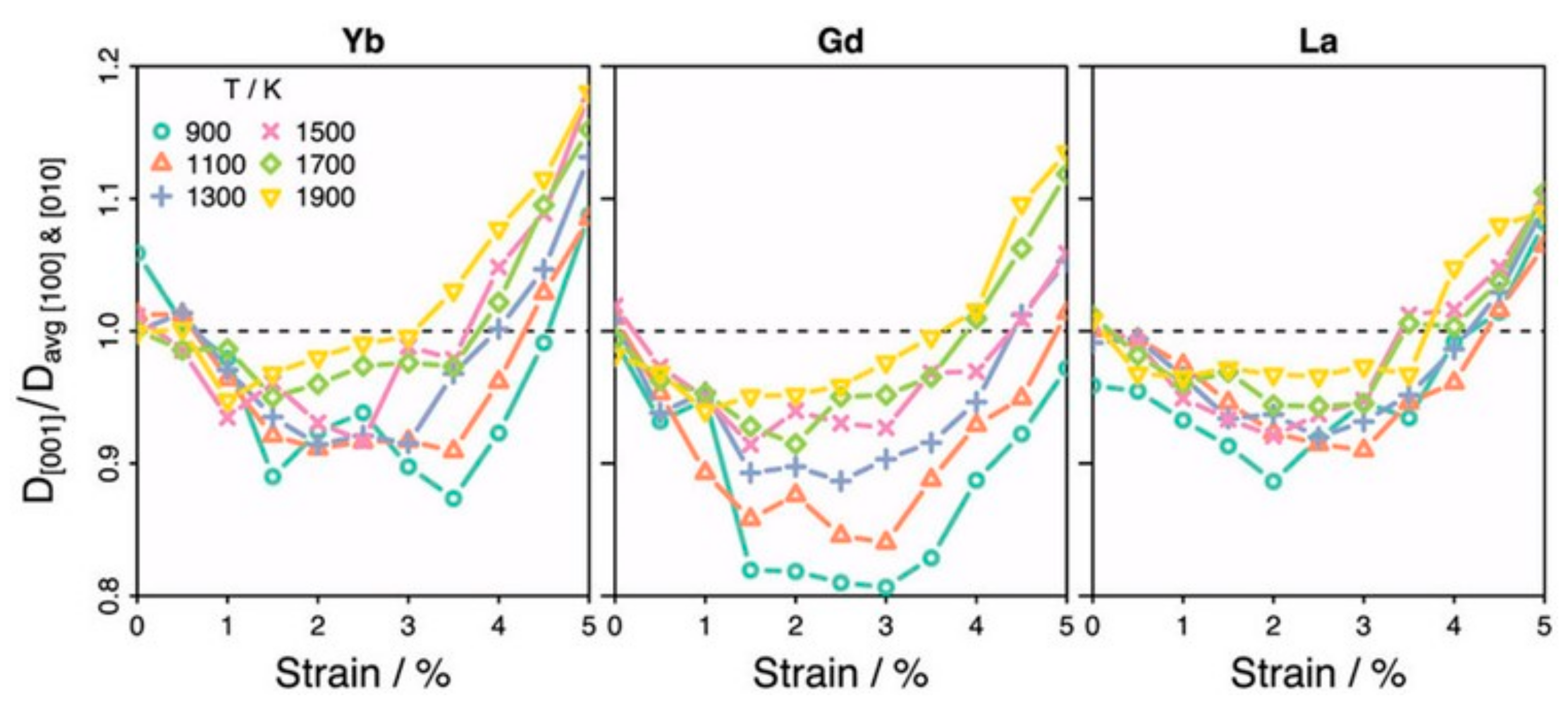
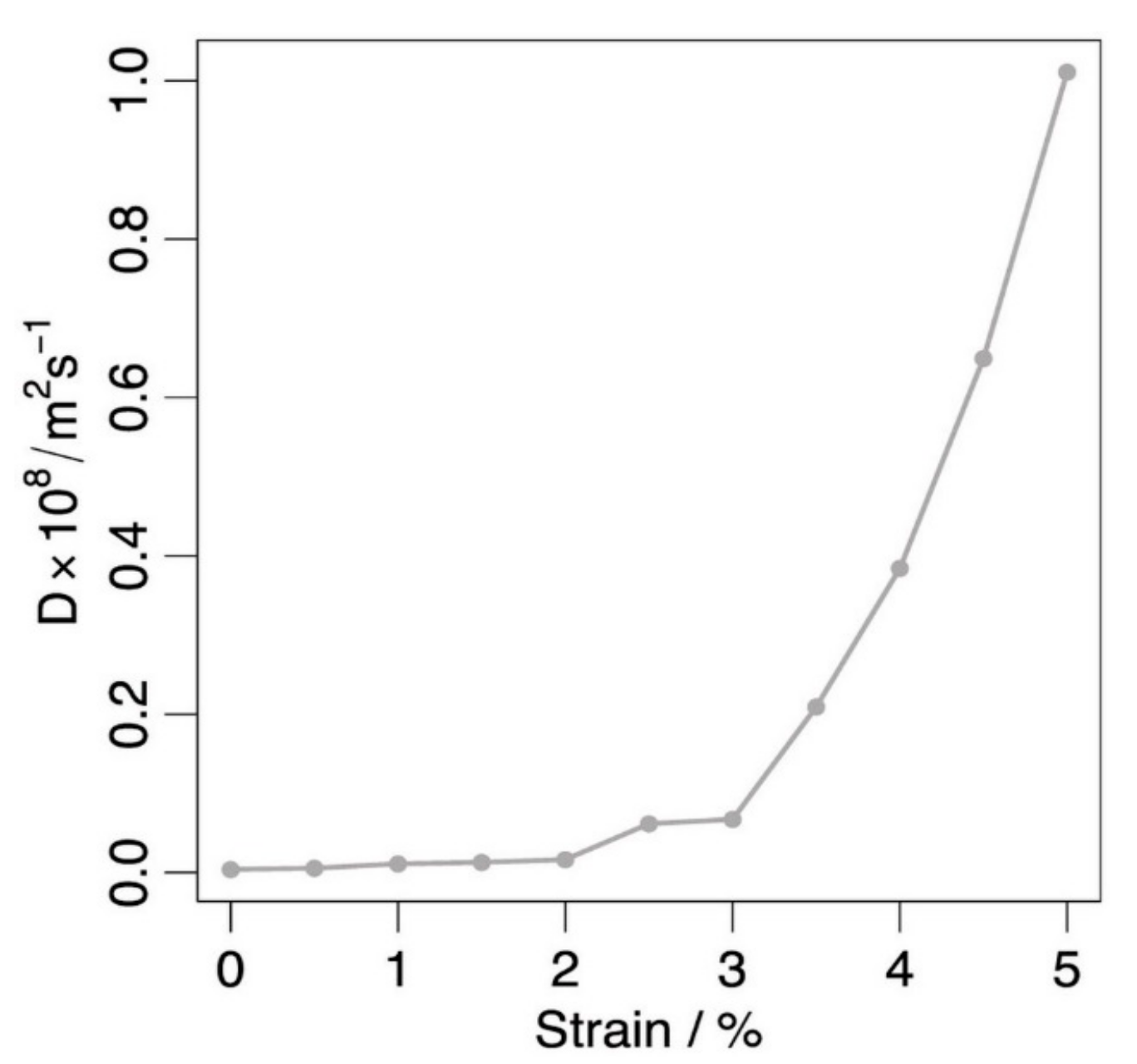
3.2. Doping and Strain Strategies
4. Doping Strategies in Silicon and Germanium
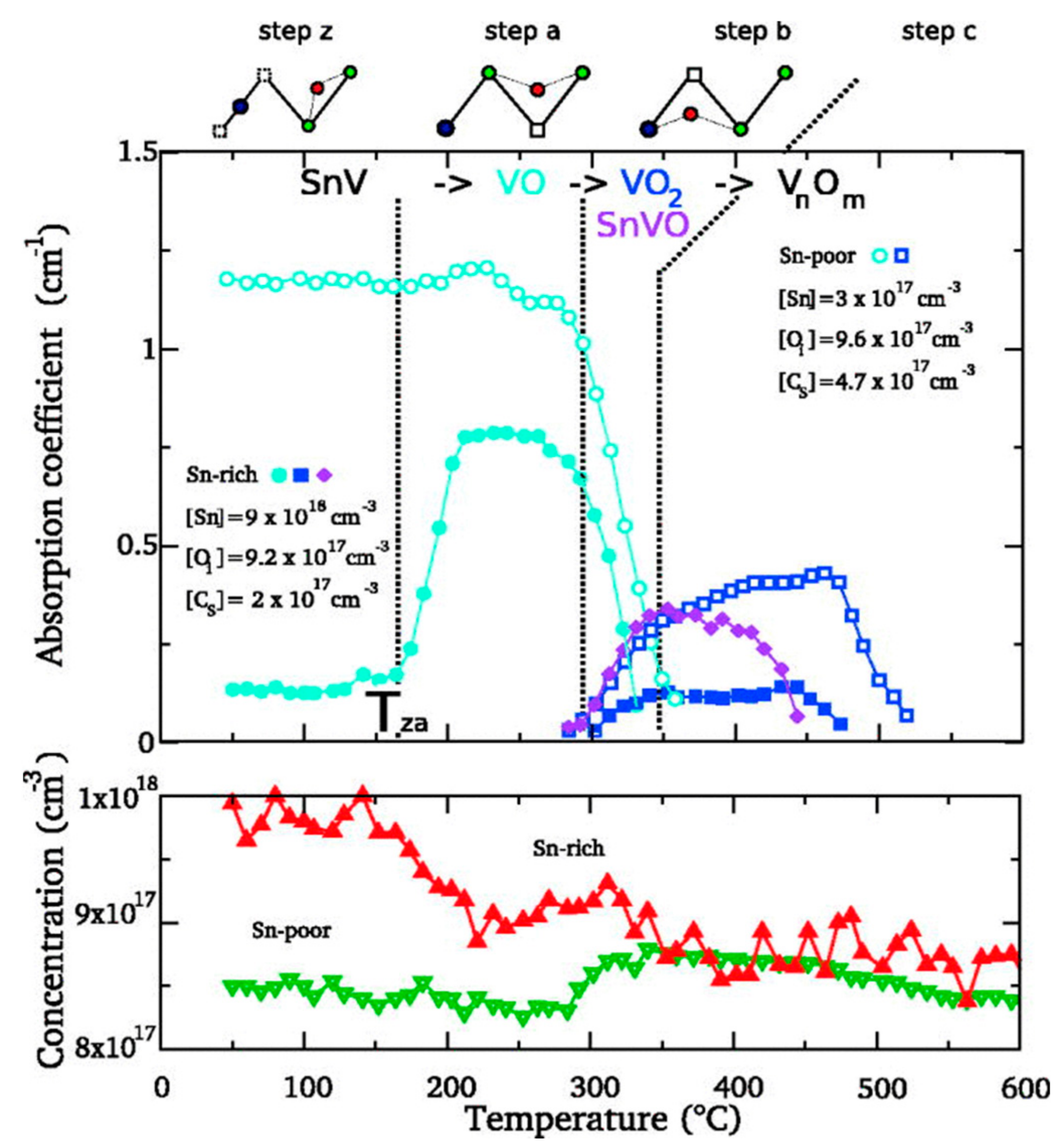
4.1. Isovalent Doping in Silicon
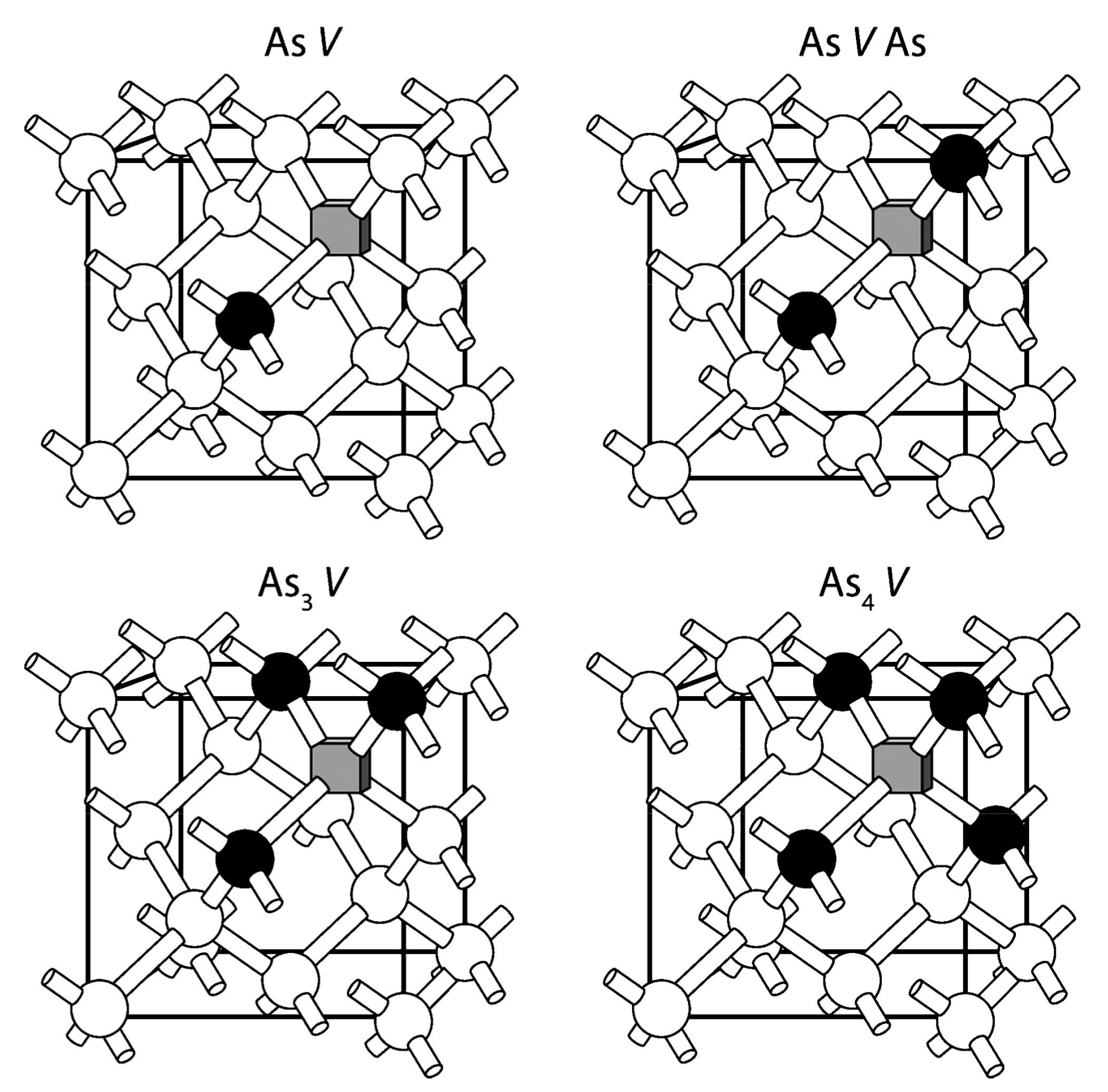
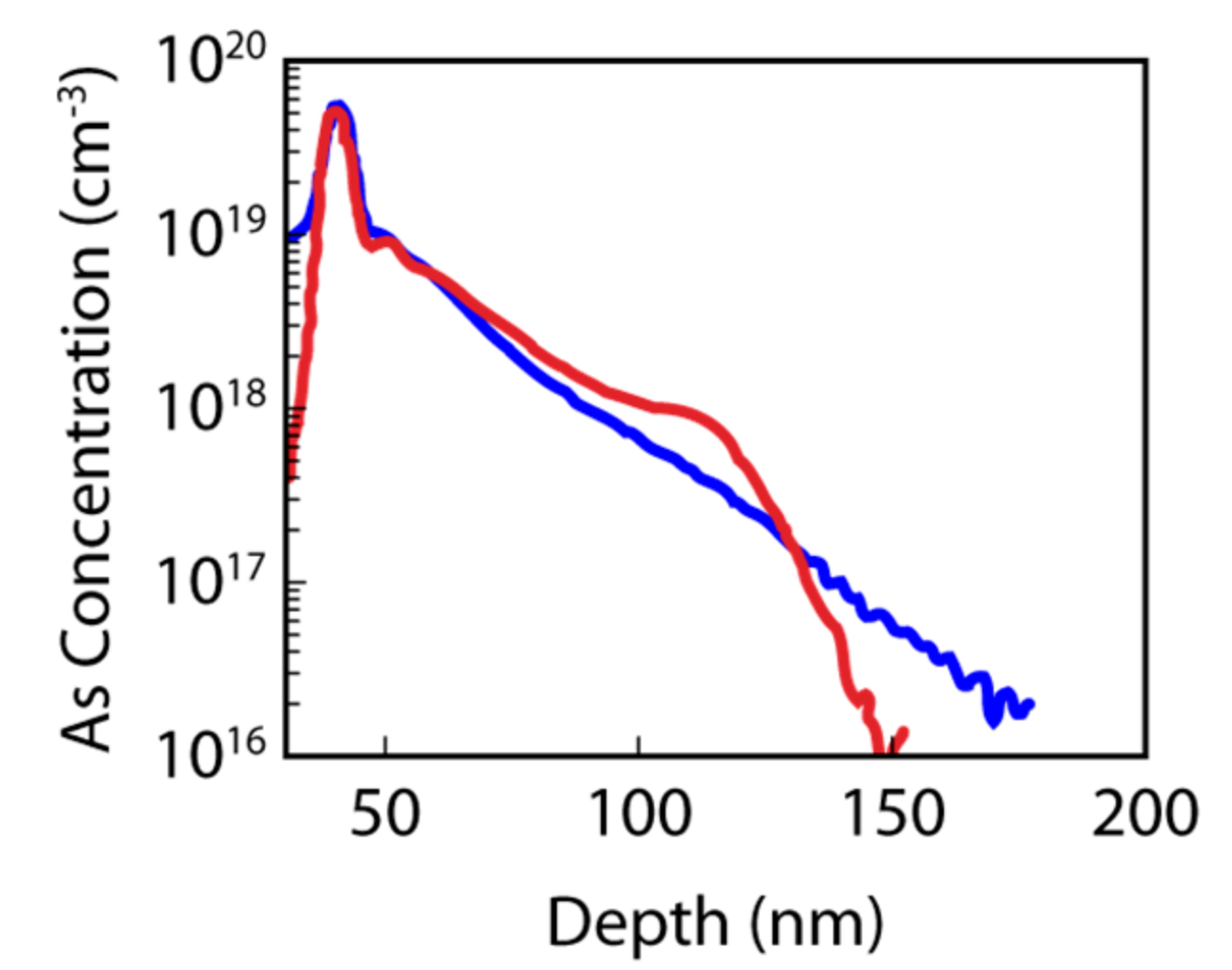
4.2. Fluorine Doping in Germanium
5. Tuning Metal Oxides for Catalysis
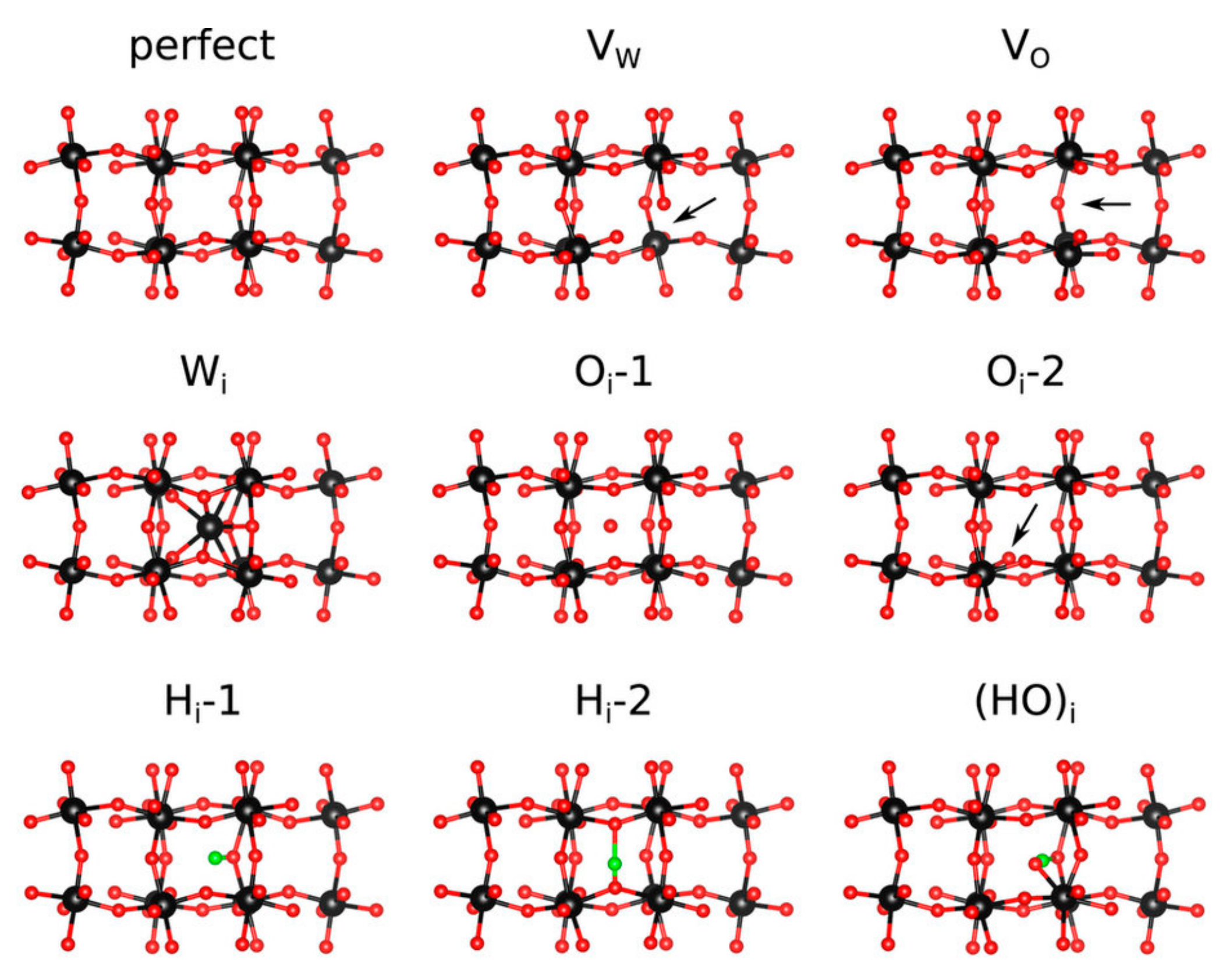
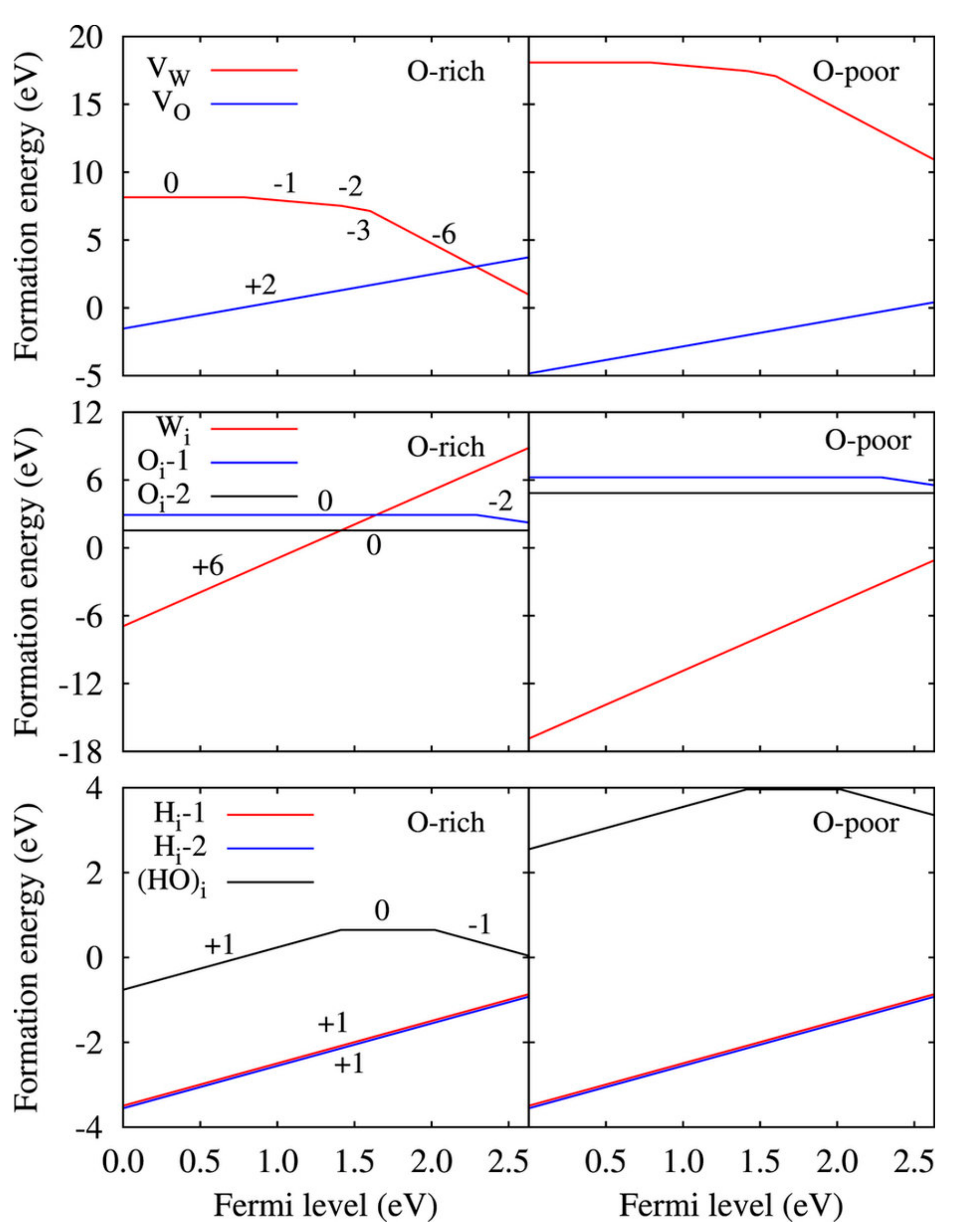
5.1. Hydrogen Doping in WO3
5.2. Codoping in TiO2
6. Summary and Future Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seymour, I.D.; Chroneos, A.; Kilner, J.A.; Grimes, R.W. Defect processes in orthorhombic LnBaCo2O5.5 double perovskites. Phys. Chem. Chem. Phys. 2011, 13, 15305–15310. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.T.; Chroneos, A.; Jiang, C.; Schwingenschlögl, U.; Grimes, R.W. Deviations from Vegard’s law in ternary III-V alloys. Phys. Rev. B 2010, 82, 073201. [Google Scholar] [CrossRef]
- Chroneos, A.; Bracht, H. Diffusion of n-type dopants in germanium. Appl. Phys. Rev. 2014, 1, 011301. [Google Scholar] [CrossRef]
- Horlait, D.; Middleburgh, S.C.; Chroneos, A.; Lee, W.E. Synthesis and DFT investigation of new bismuth-containing MAX phases. Sci. Rep. 2016, 6, 18829. [Google Scholar] [CrossRef] [PubMed]
- Solovjov, A.L.; Tkachenko, M.A.; Vovk, R.V.; Chroneos, A. Fluctuation conductivity and pseudogap in HoBa2Cu3O7−δ single crystals under pressure with transport current flowing under an angle 45° to the twin boundaries. Phys. C Supercond. 2014, 501, 24–31. [Google Scholar] [CrossRef]
- Horlait, D.; Grasso, S.; Chroneos, A.; Lee, W.E. Attempts to synthesise quaternary MAX phases (Zr,M)2AlC and Zr2(Al,A)C as a way to approach Zr2AlC. Mater. Res. Lett. 2016, 4, 137–144. [Google Scholar] [CrossRef]
- Zapata-Solvas, E.; Christopoulos, S.R.G.; Ni, N.; Parfitt, D.C.; Horlait, D.; Fitzpatrick, M.E.; Chroneos, A.; Lee, W.E. Experimental synthesis and density functional theory investigation of radiation tolerance of Zr3(Al1−xSix)C2 MAX phases. J. Am. Ceram. Soc. 2017, 100, 1377–1387. [Google Scholar] [CrossRef]
- Chroneos, A.; Grimes, R.W.; Uberuaga, B.P.; Brotzmann, S.; Bracht, H. Vacancy-arsenic clusters in germanium. Appl. Phys. Lett. 2007, 91, 192106. [Google Scholar] [CrossRef]
- Vovk, R.V.; Obolenskii, M.A.; Zavgorodniy, A.A.; Goulatis, I.L.; Beleskii, V.I.; Chroneos, A. Structural relaxation, metal to insulator transition and pseudo-gap in oxygen deficient HoBa2Cu3O7−δ single crystals. Phys. C Supercond. 2009, 469, 203–206. [Google Scholar] [CrossRef]
- Vovk, R.V.; Vovk, N.R.; Shekhovtsov, O.V.; Goulatis, I.L.; Chroneos, A. c-axis hopping conductivity in heavily Pr-doped YBCO single crystals. Supercond. Sci. Technol. 2013, 26, 085017. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Review of solutions to global warming, air pollution, and energy security. Energy Environ. Sci. 2009, 2, 148–173. [Google Scholar] [CrossRef]
- Devanathan, R.; Weber, W.J.; Gale, G.D. Radiation tolerance of ceramics- insights from atomistic simulation of damage accumulation in pyrochlores. Energy Environ. Sci. 2010, 3, 1551–1559. [Google Scholar] [CrossRef]
- Grimes, R.W.; Nuttal, W.J. Generating the option of a two-stage nuclear renaissance. Science 2010, 329, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Degueldre, C.; Bertsch, J.; Kuri, G.; Martin, M. Nuclear fuel in generation II and III reactors: Research issues related to high burn-up. Energy Environ. Sci. 2011, 4, 1651–1661. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. Towarss an electricity-powered world. Energy Environ. Sci. 2011, 4, 3193–3222. [Google Scholar]
- Grimes, R.W.; Konings, R.J.M.; Edwards, L. Greater tolerance for nuclear materials. Nat. Mater. 2008, 7, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Ewing, R.C. Nuclear waste forms for actinides. Proc. Natl. Acad. Sci. USA 1999, 96, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Winograd, I.J.; Roseboom, E.H., Jr. Yucca mountain revisited. Science 2008, 320, 1426–1427. [Google Scholar] [CrossRef] [PubMed]
- Sickafus, K.E.; Minervini, L.; Grimes, R.W.; Valdez, J.A.; Ishimaru, M.; Li, F.; McClellan, K.J.; Hartmann, T. Radiation tolerance of complex oxides. Science 2000, 289, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Demkowicz, M.J.; Bhattacharyya, D.; Usov, I.; Wang, Y.Q.; Nastasi, M.; Misra, A. The effect of excess atomic volume on He bubble formation at fcc-bcc interfaces. Appl. Phys. Lett. 2010, 97, 161903. [Google Scholar] [CrossRef]
- Bai, X.M.; Voter, A.F.; Hoagland, R.G.; Nastasi, M.; Uberuaga, B.P. Efficient annealing of radiation damage near grain boundaries via interstitial emission. Science 2010, 327, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.C. Advances in solid oxide fuel cell technology. Solid State Ionics 2000, 135, 305–313. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Minh, N.Q.; Takahashi, T. Science and Technology of Ceramic Fuel Cells; Elsevier: Amsterdam, The Netherlands, 1995. [Google Scholar]
- Jacobson, A.J. Materials for solid oxide fuel cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Rushton, M.J.D.; Chroneos, A. Impact of uniaxial strain and doping on oxygen diffusion in CeO2. Sci. Rep. 2014, 4, 6068. [Google Scholar] [CrossRef] [PubMed]
- Steele, B.C.H. Appraisal of Ce1−yGdyO2−y/2 electrolytes for IT-SOFC operation at 500 °C. Solid State Ionics 2000, 129, 95–110. [Google Scholar] [CrossRef]
- Sata, N.; Eberman, K.; Eberl, K.; Maier, J. Mesoscopic fast ion conduction in nanometre scale planar heterostructures. Nature 2000, 408, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Kushima, A.; Yildiz, B. Oxygen ion diffusivity in strained yttria stabilized zirconia: Where is the fastest strain? J. Mater. Chem. 2010, 20, 4809–4819. [Google Scholar] [CrossRef]
- Guo, X.X.; Matei, I.; Lee, J.-S.; Maier, J. Ion conduction across nanosized CaF2/BaF2 multilayer heterostructures. Appl. Phys. Lett. 2007, 91, 103102. [Google Scholar] [CrossRef]
- Kilner, J.A. Ionic conductors feel the strain. Nat. Mater. 2008, 7, 838–839. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Jiang, J.; Hertz, J.L. Reduced ionic conductivity in biaxially compressed ceria. RSC Adv. 2014, 4, 21625–21630. [Google Scholar] [CrossRef]
- Garcia-Barriocanal, J.; Rivera-Calzada, A.; Varela, M.; Sefrioui, Z.; Iborra, E.; Leon, C.; Pennycook, S.J.; Santamaria, J. Colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures. Science 2008, 321, 676–680. [Google Scholar] [CrossRef] [PubMed]
- De Souza, R.A.; Ramadan, A.; Hörner, S. Modifying the barriers for oxygen vacancy migration in fluorite structured CeO2 electrolytes through strain: A computer simulation study. Energy Environ. Sci. 2011, 5, 5445–5453. [Google Scholar] [CrossRef]
- Kilner, J.A.; Waters, C.D. The effects of dopant cation oxygen vacancy complexes on the anion transport properties of nonstoichiometric fluorite oxides. Solid State Ionics 1982, 6, 253–259. [Google Scholar] [CrossRef]
- Minervini, L.; Zacate, M.O.; Grimes, R.W. Defect cluster formation in M2O3 doped CeO2. Solid State Ionics 1999, 116, 339–349. [Google Scholar] [CrossRef]
- Rushton, M.J.D.; Chroneos, A.; Skinner, S.J.; Kilner, J.A.; Grimes, R.W. Effect of strain on the oxygen diffusion in yttria and gadolinia co-doped ceria. Solid State Ionics 2013, 230, 37–42. [Google Scholar] [CrossRef]
- Chroneos, A.; Sgourou, E.N.; Londos, C.A.; Schwingenschlögl, U. Oxygen defect processes in silicon and silicon germanium. Appl. Phys. Rev. 2015, 2, 021306. [Google Scholar] [CrossRef]
- Chroneos, A.; Londos, C.A.; Sgourou, E.N.; Pochet, P. Point defect engineering strategies to supress A-center formation in silicon. Appl. Phys. Lett. 2011, 99, 241901. [Google Scholar] [CrossRef]
- Chroneos, A.; Londos, C.A.; Sgourou, E.N. Effect of tin doping on oxygen and carbon related defects in Czochralski silicon. J. Appl. Phys. 2011, 110, 093507. [Google Scholar] [CrossRef]
- Sgourou, E.N.; Timerkaeva, D.; Londos, C.A.; Aliprantis, D.; Chroneos, A.; Caliste, D.; Pochet, P. Impact of isovalent doping on the trapping of vacancy and interstitial related defects in Si. J. Appl. Phys. 2013, 113, 113506. [Google Scholar] [CrossRef]
- Simoen, E.; Clays, C.; Neimash, V.; Kraitchinskii, A.; Kras’ko, N.; Puzenko, O.; Blondeel, A.; Clauws, P. Deep levels in high energy proton irradiated tin-doped n-type Czochralski silicon. Appl. Phys. Lett. 2000, 76, 2838. [Google Scholar] [CrossRef]
- Khirunenko, L.I.; Kobzar, O.A.; Pomozov, Y.V.; Shakhovstov, V.I.; Sosnin, M.G.; Tripachko, N.A.; Markevich, V.P.; Murin, L.I.; Peaker, A.R. Defect-impurity interactions in irradiated tin-doped Cz-Si crystals. Phys. Status Solidi C 2003, 0, 694–697. [Google Scholar] [CrossRef]
- Larsen, A.N.; Goubet, J.J.; Mejlholm, P.; Christensen, J.S.; Fanciulli, M.; Gunnlaugsson, H.P.; Weyer, P.; Petersen, J.W.; Resende, A.; Kaukonen, N.; et al. Tin-vacancy acceptor levels in electron irradiated n-type silicon. Phys. Rev. B 2000, 62, 4535–4544. [Google Scholar] [CrossRef]
- Lavrov, E.V.; Fancialli, M.; Kaukonen, M.; Jones, R.; Briddon, P.R. Carbon-tin defects in silicon. Phys. Rev. B 2001, 64, 125212. [Google Scholar] [CrossRef]
- Londos, C.A.; Aliprantis, D.; Sgourou, E.N.; Chroneos, A.; Pochet, P. Formation and evolution of oxygen-vacancy clusters in lead and tin doped silicon. J. Appl. Phys. 2012, 111, 123508. [Google Scholar] [CrossRef]
- Wang, H.; Chroneos, A.; Londos, C.A.; Sgourou, E.N.; Schwingenschlögl, U. Doping strategies to control A-centres in silicon: Insights from hybrid density functional theory. Phys. Chem. Chem. Phys. 2014, 16, 8487–8492. [Google Scholar] [CrossRef] [PubMed]
- David, M.L.; Simoen, E.; Clays, C.; Neimash, V.; Kras’ko, N.; Kraitchinskii, A.; Voytovych, V.; Kabaldin, A.; Barbot, J.F. Electrically active defects in irradiated n-type Czochralski silicon doped with group IV impurities. J. Phys. Condens. Matter 2005, 17, S2255. [Google Scholar] [CrossRef]
- Fanciulli, M.; Byberg, J.R. Tin-vacancy complexes in e irradiated n-type silicon. Phys. Rev. B 1999, 273–274, 524–527. [Google Scholar] [CrossRef]
- Simoen, E.; Clays, C.; Privitera, V.; Coffa, S.; Larsen, A.N.; Clauws, P. High energy proton radiation induced defects in tin doped n-type silicon. Phys. Rev. B 2001, 308–310, 477–480. [Google Scholar] [CrossRef]
- Ghao, C.; Ma, X.; Zhao, J.; Yang, D. Effect of tin on point defects and oxygen precipitation on Czochralski silicon: Experimental and theoretical studies. J. Appl. Phys. 2013, 113, 093511. [Google Scholar] [CrossRef]
- Heyns, M.; Tsai, W. Ultimate scaling of CMOS logic devices with Ge and III-V materials. MRS Bull. 2009, 34, 485–492. [Google Scholar]
- Tsoutsou, D.; Panayiotatos, Y.; Sotiropoulos, A.; Mavrou, G.; Golias, E.; Galata, S.F.; Dimoulas, A. Chemical stability of lanthanum germanate passivating layer on Ge upon high-k deposition: A photoemission study on the role of La in the interface chemistry. J. Appl. Phys. 2010, 108, 064115. [Google Scholar] [CrossRef]
- Chroneos, A.; Schwingenschlögl, U.; Dimoulas, A. Impurity diffusion, point defect engineering and surface/interface passivation in germanium. Ann. Phys. 2012, 524, 123–132. [Google Scholar] [CrossRef]
- Galata, S.F.; Evangelou, E.K.; Panayiotatos, Y.; Sotiropoulos, A.; Dimoulas, A. Post deposition annealing studies of lanthanum aluminate and ceria high-k dielectrics on germanium. Microelectron. Reliab. 2007, 47, 532–535. [Google Scholar] [CrossRef]
- Chen, D.K.; Schrimpf, R.D.; Fleetwood, D.M.; Galloway, K.F.; Pantelides, S.T.; Dimoulas, A.; Mavrou, G.; Sotiropoulos, A.; Panayiotatos, Y. Total dose response of Ge MOS capasitors with HfO2/Dy2O3 gate stacks. IEEE Trans. Nucl. Sci. 2007, 54, 971–974. [Google Scholar] [CrossRef]
- Chroneos, A.; Skarlatos, D.; Tsamis, C.; Christofi, A.; McPhail, D.S.; Hung, R. Implantation an diffusion of phosphorous in germanium. Mater. Sci. Semicond. Process 2006, 9, 640–643. [Google Scholar] [CrossRef]
- Chroneos, A. Dopant-vacancy cluster formation in germanium. J. Appl. Phys. 2010, 107, 076102. [Google Scholar] [CrossRef]
- Bracht, H.; Schneider, S.; Klug, J.N.; Liao, C.J.; Lundsgaard Hansen, J.; Haller, E.E.; Nylandsted Larsen, A.; Bougeard, D.; Posselt, M.; Wündisch, C. Interstitial-mediated diffusion in germanium under proton irradiation. Phys. Rev. Lett. 2009, 103, 255501. [Google Scholar] [CrossRef] [PubMed]
- Brotzmann, S.; Bracht, H. Intrinsic and extrinsic diffusion of phosphorous, arsenic, and antimony in germanium. J. Appl. Phys. 2008, 103, 033508. [Google Scholar] [CrossRef]
- Chroneos, A.; Bracht, H.; Grimes, R.W.; Uberuaga, B.P. Vacancy-mediated dopant diffusion activation enthalpies for germanium. Appl. Phys. Lett. 2008, 92, 172103. [Google Scholar] [CrossRef]
- Tsouroutas, P.; Tsoukalas, D.; Bracht, H. Experiments and simulation on diffusion and activation of codoped with arsenic and phosphorous germanium. J. Appl. Phys. 2010, 108, 024903. [Google Scholar] [CrossRef]
- Brotzmann, S.; Bracht, H.; Lundsgaard Hansen, J.; Nylandsted Larsen, A.; Simoen, E.; Haller, E.E.; Christensen, J.S.; Werner, P. Diffusion and defect reactions between donors, C, and vacancies in Ge. I Experimental results. Phys. Rev. B 2008, 77, 235207. [Google Scholar] [CrossRef]
- Chroneos, A.; Grimes, R.W.; Uberuaga, B.P.; Bracht, H. Diffusion and defect reactions between donors, C, and vacancies in Ge. II Atomistic calculations of related complexes. Phys. Rev. B 2008, 77, 235208. [Google Scholar] [CrossRef]
- Diebel, M.; Dunham, S.T. Ab initio calculations to model anomalous fluorine behavior. Phys. Rev. Lett. 2004, 93, 245901. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, F.; dos Santos, J.H.R.; Behar, M. Lattice site investigation of F in preamorphized Si. Phys. Rev. B 2007, 76, 033201. [Google Scholar] [CrossRef]
- Boninelli, S.; Impellizzeri, G.; Mirabella, S.; Priolo, F.; Napolitani, E.; Cherkashin, N.; Cristiano, F. Formation and evolution of F nanobubbles in amorphous and crystalline Si. Appl. Phys. Lett. 2008, 93, 061906. [Google Scholar] [CrossRef]
- Chroneos, A.; Grimes, R.W.; Bracht, H. Fluorine codoping in germanium to suppress donor diffusion and deactivation. J. Appl. Phys. 2009, 106, 063707. [Google Scholar] [CrossRef]
- Impellizzeri, G.; Boninelli, S.; Priolo, F.; Napolitani, E.; Spinella, C.; Chroneos, A.; Bracht, H. Fluorine effect on As diffusion in Ge. J. Appl. Phys. 2011, 109, 113527. [Google Scholar] [CrossRef]
- Jung, W.S.; Park, J.H.; Nainani, S.; Nam, D.; Saraswat, K.C. Fluorine passivation of vacancy defects in bulk germanium for Ge metal oxide semiconductor field effect transistor application. Appl. Phys. Lett. 2012, 101, 072104. [Google Scholar] [CrossRef]
- Sanson, A.; El Mubarek, H.A.W.; Gandy, A.S.; De Salvador, D.; Napolitani, E.; Carnera, A. Formation of F6V2 complexes in F-implanted Ge determined by X-ray absorption near edge structure spectroscopy. Mater. Sci. Semicond. Process. 2017, 62, 205–208. [Google Scholar] [CrossRef]
- Boninelli, S.; Impellizzeri, G.; Priolo, F.; Napolitani, E.; Spinella, C. Fluorine in Ge: Segregation and EOR defects stabilization. Nucl. Instrum. Methods Phys. Res. Sect. B 2012, 282, 21–24. [Google Scholar] [CrossRef]
- Edwardson, C.J.; Coleman, P.G.; El Mubarek, H.A.W. Positron annihilation studies of fluorine-vacancy complexes in phosphorus and fluorine implanted germanium. Semicond. Sci. Technol. 2014, 29, 035005. [Google Scholar] [CrossRef]
- Granqvist, C.G. Handbook of Inorganic Electrochromic Materials; Elsevier: New York, NY, USA, 1995. [Google Scholar]
- Vasilopoulou, M.; Palilis, L.C.; Georgiadou, D.G.; Douvas, A.M.; Argitis, P.; Kennou, S.; Sygellou, L.; Papadimitropoulos, G.; Kostis, I.; Stathopoulos, N.A.; et al. Reduction of tungsten oxide: A path towards dual functionality utilization for efficient anode and cathode interfacial layers in organic light emitting diodes. Adv. Funct. Mater. 2011, 21, 1489–1497. [Google Scholar] [CrossRef]
- Zhu, J.; Vasilopoulou, M.; Davazoglou, D.; Kennou, S.; Chroneos, A.; Schwingenschlögl, U. Intrinsic defects and H doping in WO3. Sci. Rep. 2017, 7, 40882. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.K. Optical and photoelectric properties and color centers in thin films of tungsten oxide. Philos. Mag. 1973, 27, 801–822. [Google Scholar] [CrossRef]
- Owen, J.F.; Teegarden, K.J.; Shanks, H.R. Optical properties of the sodium-tungsten bronzes and tungsten trioxide. Phys. Rev. B 1978, 18, 3827–3837. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Gerosa, M.; Valentin, C.D.; Onida, G.; Bottani, C.E.; Pacchioni, G. Anisotropic effects of oxygen vacancies on electrochromic properties and conductivity of γ-monoclinic WO3. J. Phys. Chem. C 2016, 120, 11716–11726. [Google Scholar] [CrossRef]
- Wang, W.; Janotti, A.; Van de Walle, C.G. Role of oxygen vacancies in crystalline WO3. J. Mater. Chem. C 2016, 4, 6641–6648. [Google Scholar] [CrossRef]
- Wang, F.; Di Valentin, C.; Pacchioni, G. Electronic and structural properties of WO3: A systematic hybrid DFT study. J. Phys. Chem. C 2011, 115, 8345–8353. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Gratzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef] [PubMed]
- Russo, S.P.; Grey, I.E.; Wilson, N.C. Nitrogen/hydrogen codoping of anatase: A DFT study. J. Phys. Chem. C 2008, 112, 7653–7664. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Gai, Y.; Li, J.; Li, S.-S.; Xia, J.-B.; Wei, S.-H. Design of narrow-gap TiO2: A passivated codoping approach for enhanced photoelectrochemical activity. Phys. Rev. Lett. 2009, 102, 036402. [Google Scholar] [CrossRef] [PubMed]
- Vasilopoulou, M.; Georgiadou, D.G.; Soultati, A.; Boukos, N.; Gardelis, S.; Palilis, L.C.; Fakis, M.; Skoulatakis, G.; Kennou, S.; Botzakaki, M.; et al. Atomic layer deposited aluminium and zirconium oxides for surface passivation of TiO2 in high-efficiency organic photovoltaics. Adv. Energy Mater. 2014, 4, 1400214. [Google Scholar] [CrossRef]
- Sivula, K.; van de Krol, R. Semiconducting materials for photoelectrochemical energy conversion. Nat. Mater. Rev. 2016, 1, 15010. [Google Scholar] [CrossRef]
- Khaselev, O.; Turner, J.A. A monolithic photovoltaic-photoelectrochemical device for hydrogen production via water splitting. Science 1998, 280, 425–427. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.G.; Qiu, X.F.; Iancu, V.; Chen, X.Q.; Pan, H.; Wang, W.; Dimitrijevic, N.M.; Rajh, T.; Meyer, H.M.; Paranthaman, M.P.; et al. Band gap narrowing of titanium oxide semiconductors by noncompensated anion-cation codoping for enhanced visible-light photoactivity. Phys. Rev. Lett. 2009, 103, 226401. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.J.; Tang, H.W.; Wei, S.H.; Al-Jassim, M.M.; Turner, J.; Yan, Y.F. Band structure engineering of semiconductors for enhanced photochemical water splitting: The case of TiO2. Phys. Rev. B 2010, 82, 045106. [Google Scholar] [CrossRef]
- Long, R.; English, N.J. Synergistic effects on band gap narrowing in titania by codoping from first principles calculations. Chem. Mater. 2010, 22, 1616–1623. [Google Scholar] [CrossRef]
- Liao, P.L.; Carter, E.A. New concepts and modelling strategies to design and evaluate photoelectrocatalysis based on transition metal oxides. Chem. Soc. Rev. 2013, 42, 2401–2422. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.B.; Cortie, D.; Zhang, S.Y.; Frankcombe, T.J.; She, G.W.; Gao, J.; Sheppard, L.R.; Hu, W.B.; Chen, H.; Zhuo, S.J.; et al. The formation of defect-pairs for highly efficient visible light catalysts. Adv. Mater. 2017, 29, 1605123. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhang, Y.-W.; Shenoy, V.B.; Gao, H. Effects of H-, N-, and (H, N)-doping on the photocatalytic activity of TiO2. J. Phys. Chem. C 2011, 115, 12224–12231. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Hernandez, O.J.; Sakaguchi, T.; Yajima, T.; Roisnel, T.; Tsujimoto, Y.; Morita, M.; Noda, Y.; Mogami, Y.; Kitada, A.; et al. An oxyhydride of BaTiO3 exhibiting hydride exchange and electronic conductivity. Nat. Mater. 2012, 11, 507–511. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dai, Y.; Yu, L.; Huang, B. Noble-metal-free plasmonic photocatalyst: Hydrogen doped semiconductors. Sci. Rep. 2014, 4, 3986. [Google Scholar] [CrossRef] [PubMed]
- Van de Walle, C.G.; Neugebauer, J. Universal alignment of hydrogen levels in semiconductors, insulators and solutions. Nature 2003, 423, 626–628. [Google Scholar] [CrossRef] [PubMed]
- Janotti, A.; Van de Walle, C.G. Hydrogen multicentre bonds. Nat. Mater. 2007, 6, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Varotsos, P. Calculation of the migration volume of vacancies in ionic solids from macroscopic parameters. Phys. Status Solidi A 1978, 47, K133–K136. [Google Scholar] [CrossRef]
- Varotsos, P.; Alexopoulos, K. Thermodynamics of Point Defects and Their Relation with the Bulk Properties; North-Holland: Amsterdam, The Netherlands, 1986. [Google Scholar]
- Zhang, B.; Wu, X.; Xu, J.; Zhou, R. Application of the cBΩ model for the calculation of oxygen self-diffusion coefficients in minerals. J. Appl. Phys. 2010, 108, 053505. [Google Scholar] [CrossRef]
- Vallianatos, F.; Saltas, V. Application of the cBΩ model to the calculation of diffusion parameters of He in olivine. Phys. Chem. Miner. 2014, 41, 181–188. [Google Scholar] [CrossRef]
- Cooper, M.W.D.; Grimes, R.W.; Fitzpatrick, M.E.; Chroneos, A. Modeling oxygen self-diffusion in UO2 under pressure. Solid State Ionics 2015, 282, 26–30. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, S. Application of the cBΩ model to the calculation of diffusion parameters of Si in silicates. Geochem. Geophys. Geosyst. 2015, 16, 705–718. [Google Scholar] [CrossRef]
- Chroneos, A.; Vovk, R.V. Modeling self-diffusion in UO2 and ThO2 by connecting point defect parameters with bulk properties. Solid State Ionics 2015, 274, 1–3. [Google Scholar] [CrossRef]
- Chroneos, A.; Vovk, R.V. Connecting bulk properties of germanium with the behavior of self- and dopant diffusion. Mater. Sci. Semicond. Proc. 2015, 36, 179–183. [Google Scholar] [CrossRef]
- Chroneos, A.; Fitzpatrick, M.E.; Tsoukalas, L.H. Describing oxygen self-diffusion in PuO2 by connecting point defect parameters with bulk properties. J. Mater. Sci. Mater. Electron. 2015, 26, 3287–3290. [Google Scholar] [CrossRef]
- Ganniari-Papageorgiou, E.; Fitzpatrick, M.E.; Chroneos, A. Germanium diffusion in aluminium: connection between point defect parameters with bulk properties. J. Mater. Sci. Mater. Electron. 2015, 26, 8421–8424. [Google Scholar] [CrossRef]
- Chroneos, A.; Vovk, R.V. Modeling indium diffusion in germanium by connecting point defect parameters with bulk properties. J. Mater. Sci. Mater. Electron. 2015, 26, 2113–2116. [Google Scholar] [CrossRef]
- Parfitt, D.C.; Cooper, M.W.D.; Rushton, M.J.D.; Christopoulos, S.-R.G.; Fitzpatrick, M.E.; Chroneos, A. Thermodynamic calculations of oxygen self-diffusion in mixed-oxide nuclear fuels. RSC Adv. 2016, 6, 74018–74028. [Google Scholar] [CrossRef]
- Saltas, V.; Chroneos, A.; Vallianatos, F.A. A thermodynamic approach of self- and hetero-diffusion in GaAs: Connecting point defect parameters with bulk properties. RSC Adv. 2016, 6, 53324–53330. [Google Scholar] [CrossRef]
- Chroneos, A. Connecting point defect parameters with bulk properties to describe diffusion in solids. Appl. Phys. Rev. 2016, 3, 041304. [Google Scholar] [CrossRef]
- Saltas, V.; Chroneos, A.; Vallianatos, F.A. A thermodynamic approach to self-diffusion in silicon: Evidence of a single diffusion mechanism? Mater. Chem. Phys. 2016, 181, 204–208. [Google Scholar] [CrossRef]
- Sarlis, N.V.; Skordas, E.S. Estimating the compressibility of osmium from recent measurements of Ir-Os alloys under high pressure. J. Phys. Chem. A 2016, 120, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Saltas, V.; Chroneos, A.; Vallianatos, F.A. Composition and temperature dependence of self-diffusion in Si1−xGex alloys. Sci. Rep. 2017, 7, 1374. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgourou, E.N.; Panayiotatos, Y.; Vovk, R.V.; Chroneos, A. Toward Defect Engineering Strategies to Optimize Energy and Electronic Materials. Appl. Sci. 2017, 7, 674. https://doi.org/10.3390/app7070674
Sgourou EN, Panayiotatos Y, Vovk RV, Chroneos A. Toward Defect Engineering Strategies to Optimize Energy and Electronic Materials. Applied Sciences. 2017; 7(7):674. https://doi.org/10.3390/app7070674
Chicago/Turabian StyleSgourou, Efstratia N., Yerasimos Panayiotatos, Ruslan V. Vovk, and Alexander Chroneos. 2017. "Toward Defect Engineering Strategies to Optimize Energy and Electronic Materials" Applied Sciences 7, no. 7: 674. https://doi.org/10.3390/app7070674





