Synthesis and Biophysical Insights into the Binding of a Potent Anti-Proliferative Non-symmetric Bis-isatin Derivative with Bovine Serum Albumin: Spectroscopic and Molecular Docking Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemistry
2.2.1. Synthesis of 5-Bromo-3-hydrazonoindolin-2-one
2.2.2. Synthesis of 1-benzylindoline-2,3-dione (2)
2.2.3. Synthesis of 1-Benzyl-3-((5-bromo-2-oxoindolin-3-ylidene)hydrazono)indolin-2-one (3)
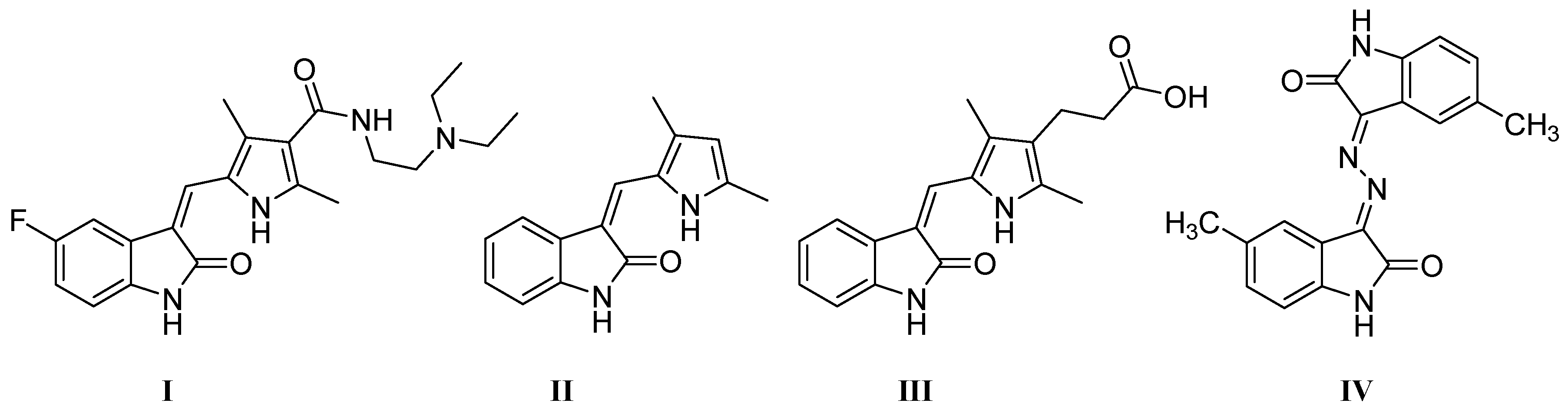
2.3. Anti-Proliferative Activity
2.4. Sample Preparation for BSA Binding Studies
2.5. Instruments and Conditions
2.6. Protein Concentration Determination
2.7. Fluorescence Quenching Studies
2.8. Synchronous and Three Dimensional Fluorescence Measurements
2.9. Competitive Binding Using Site Markers
2.10. UV-Vis Spectrophotometric Determinations
2.11. Molecular Docking Studies
3. Results and Discussion
3.1. Synthesis of the Title Compound 3
3.2. Anti-Proliferative Activity
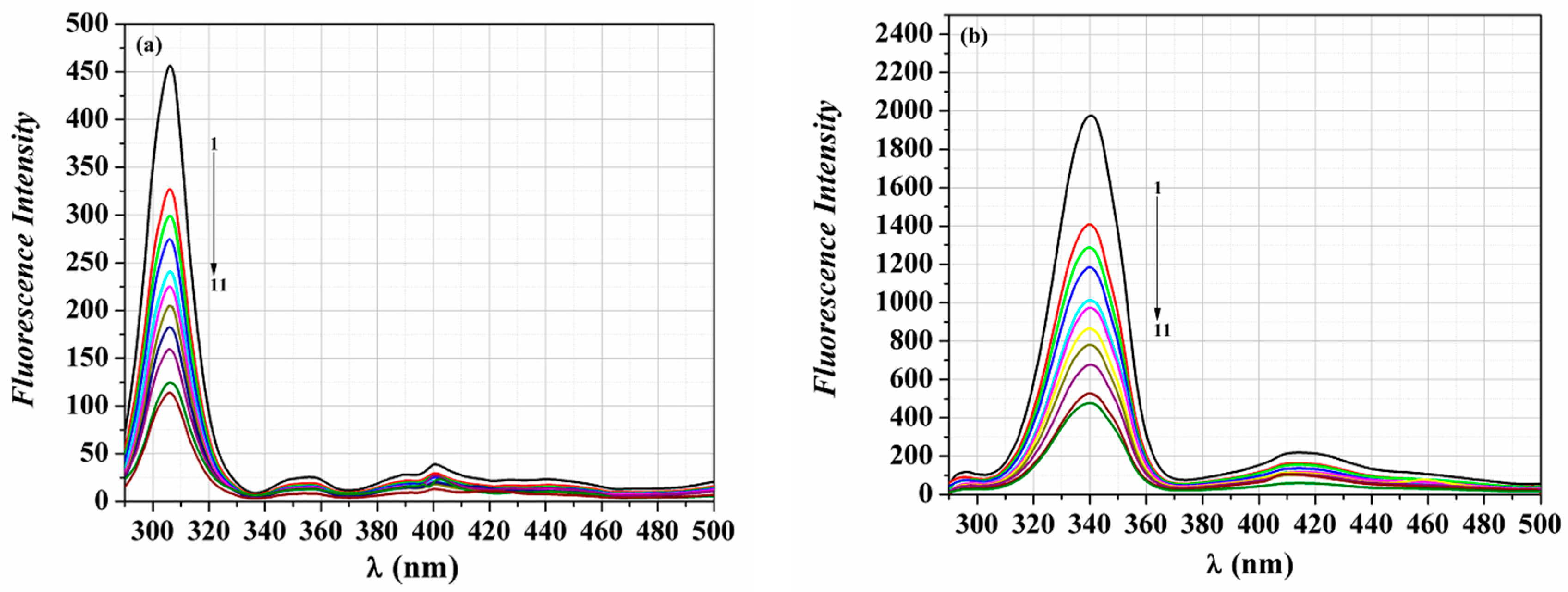
3.3. Fluorescence Spectroscopic Investigation of the Binding Mechanism
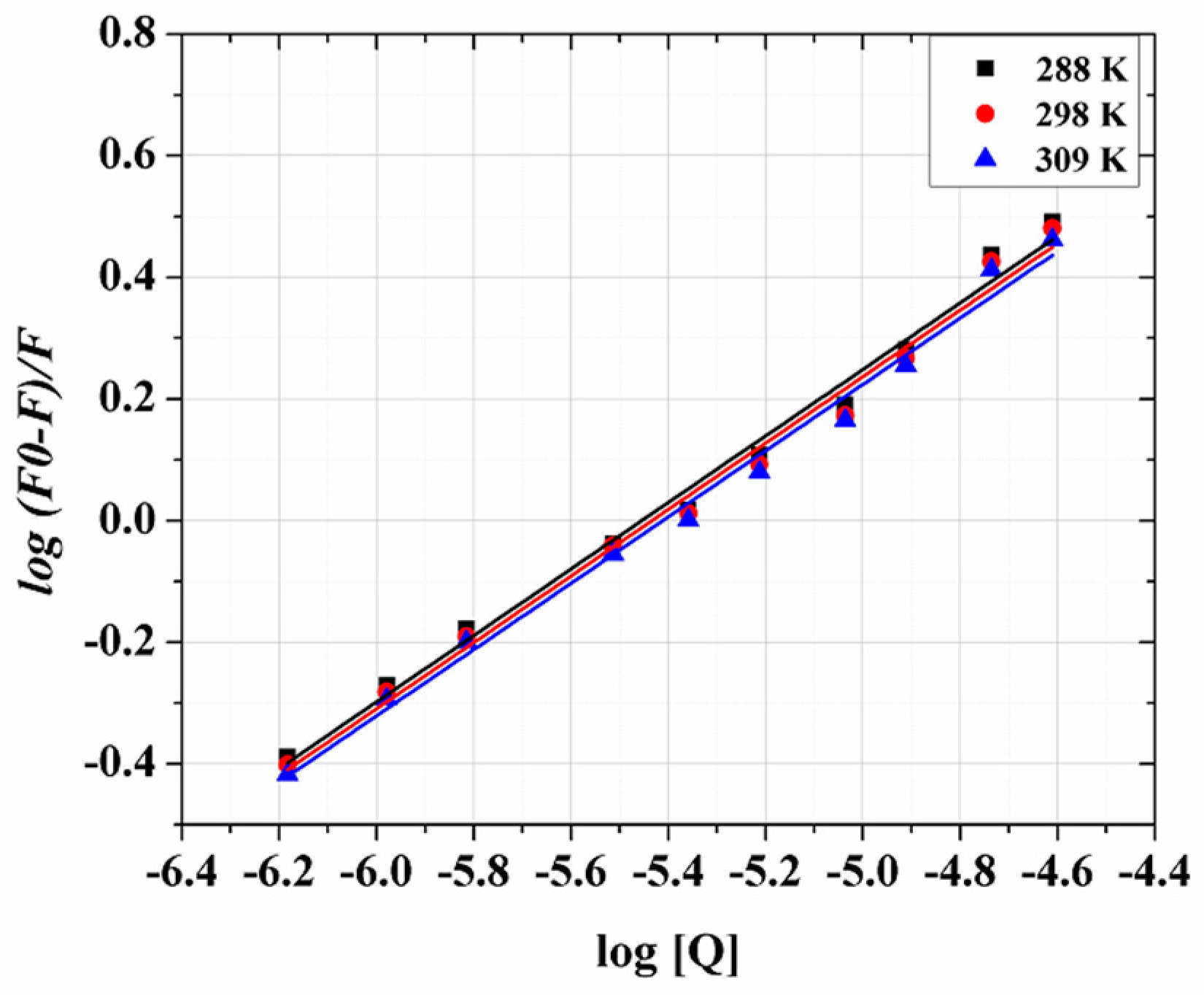
3.4. Binding Constant and Number of Binding Sites
3.5. Thermodynamics Parameters and Nature of the Binding Forces
3.6. UV-Vis Absorption Spectra
3.7. Effect of Compound 3 on BSA Conformation
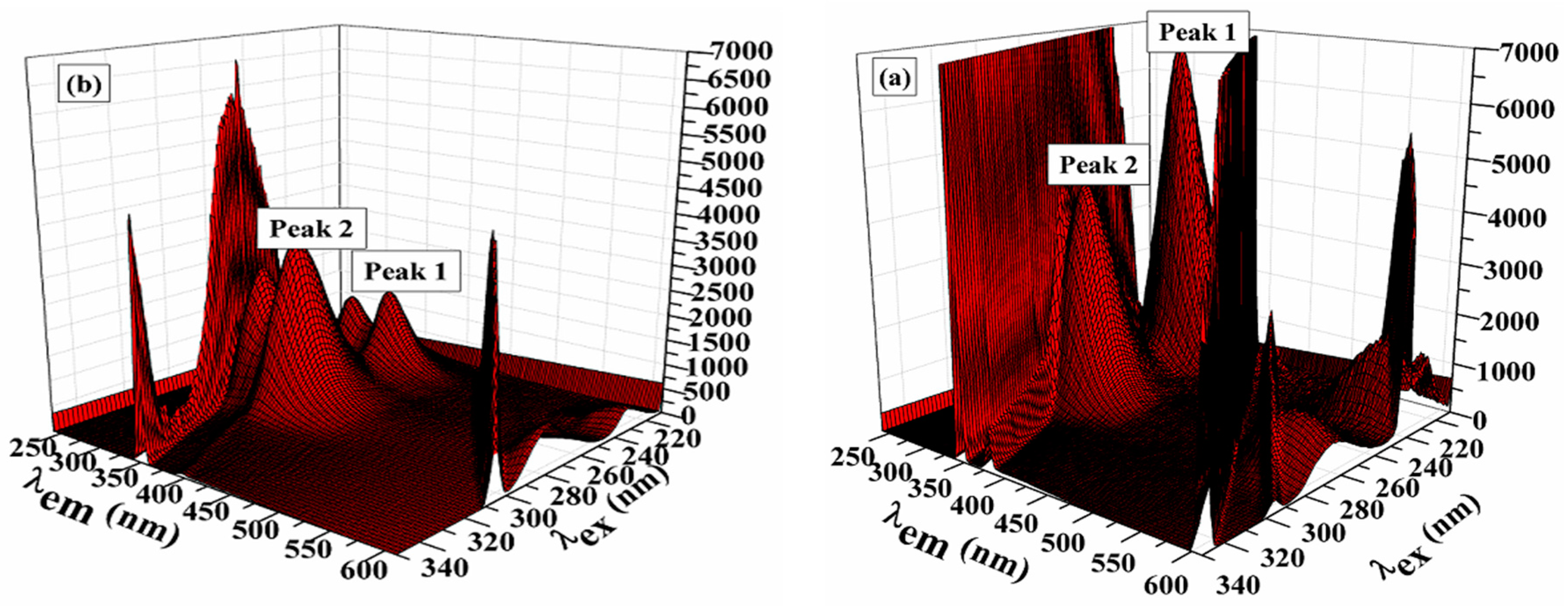
3.7.1. Synchronous Fluorescence
3.7.2. Three Dimensional Fluorescence Measurements
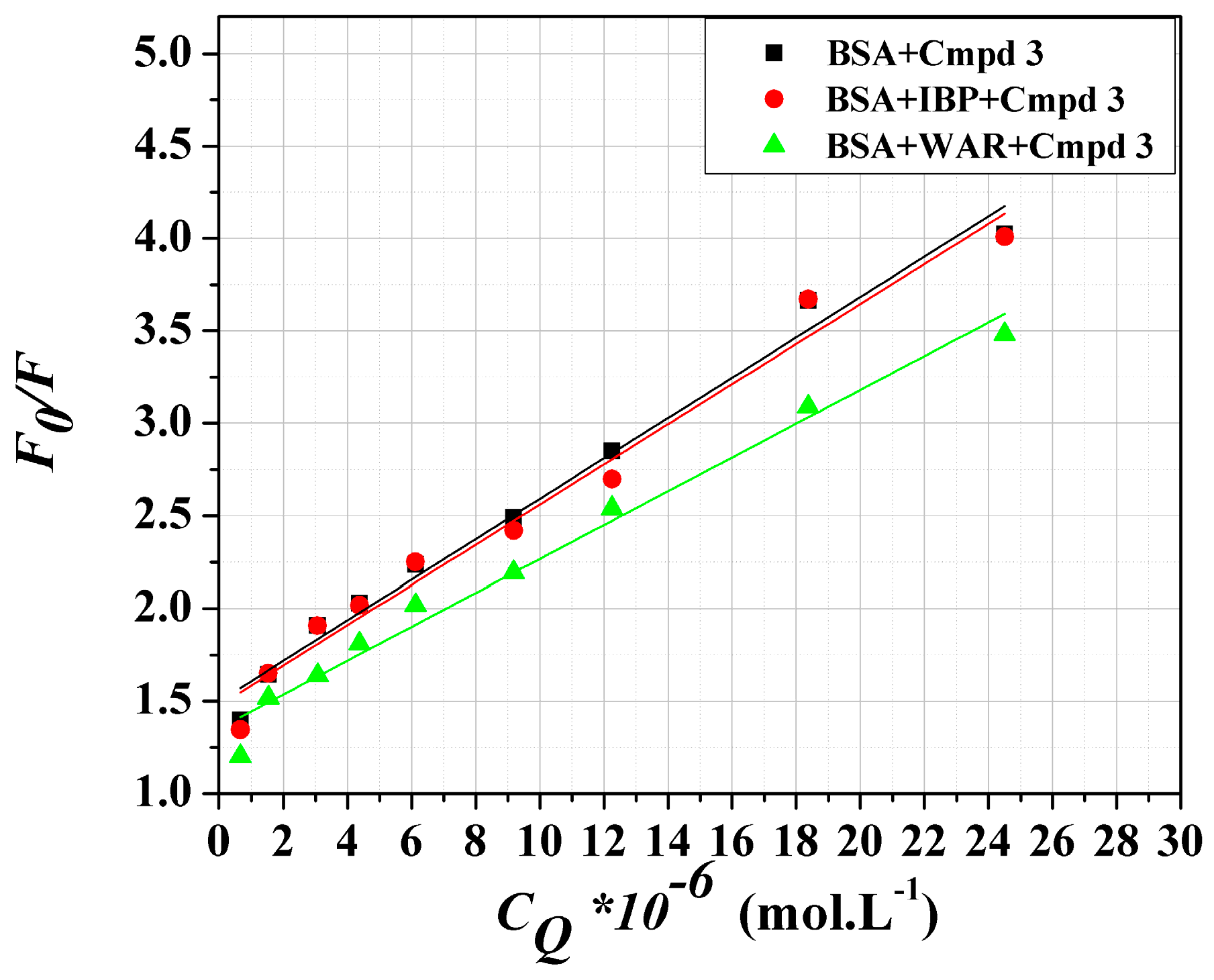
3.8. Site Markers Competitive Binding
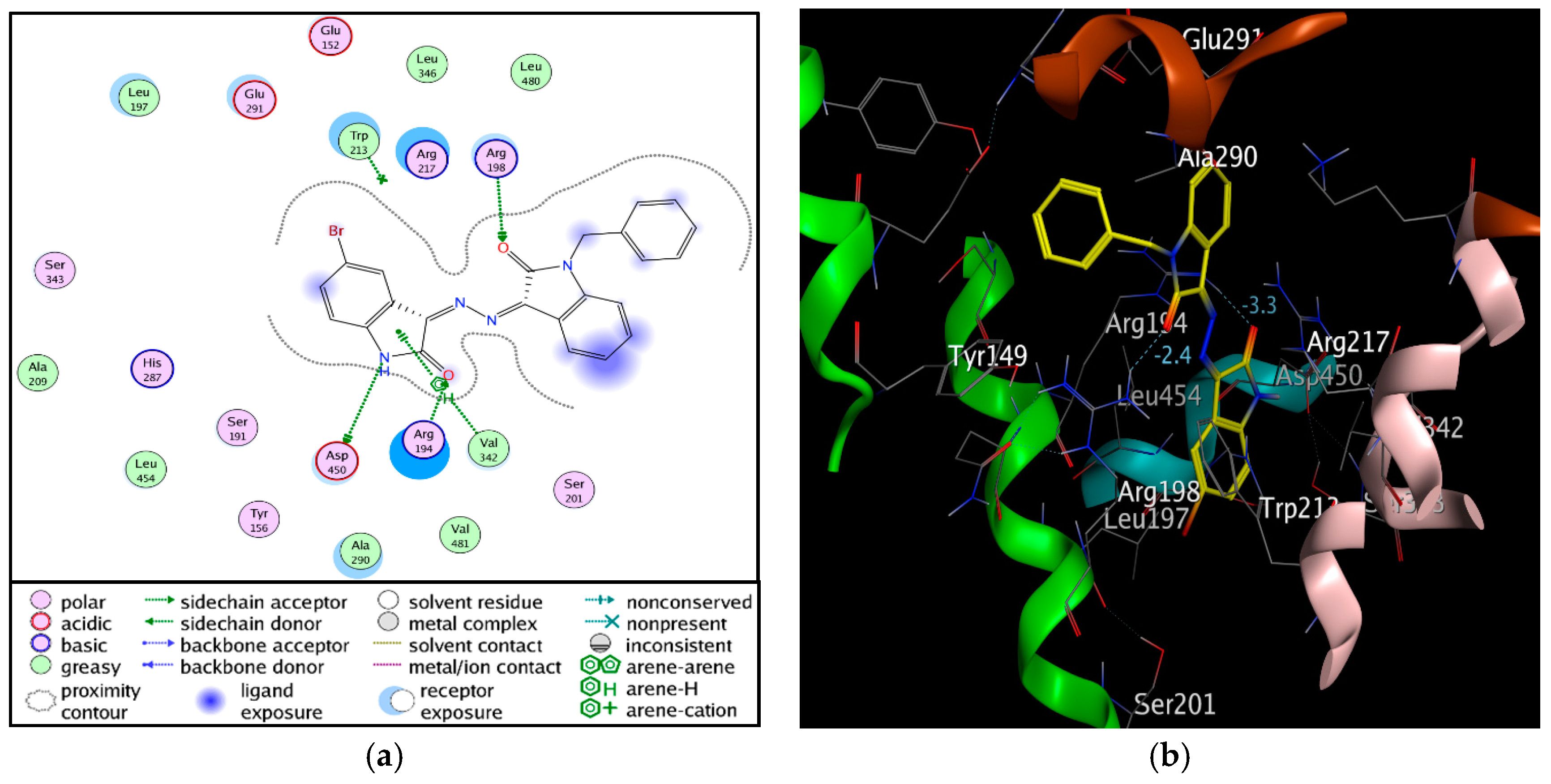
3.9. Molecular Docking
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Avendaño, C.; Menendez, J.C. Medicinal Chemistry of Anticancer Drugs; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Glover, V.; Halket, J.M.; Watkins, P.J.; Clow, A.; Goodwin, B.L.; Sandler, M. Isatin: Identity with the purified endogenous monoamine oxidase inhibitor tribulin. J. Neurochem. 1988, 51, 656–659. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.M.; Prasanna, P.; Seshu, K.V.; Renuka, B.; Rao, C.V.; Kumar, G.S.; Narasimhulu, C.P.; Babu, P.A.; Puranik, R.C.; Subramanyam, D.; et al. Novel indolo[2,1-b]quinazoline analogues as cytostatic agents: Synthesis, biological evaluation and structure-activity relationship. Bioorg. Med. Chem. lett. 2002, 12, 2303–2307. [Google Scholar] [CrossRef]
- Gursoy, A.; Karali, N. Synthesis and primary cytotoxicity evaluation of 3-[[(3-phenyl-4(3H)-quinazolinone-2-yl)mercaptoacetyl]hydrazono]-1H-2-indolinones. Eur. J. Med. Chem. 2003, 38, 633–643. [Google Scholar] [CrossRef]
- Pandeya, S.N.; Smitha, S.; Jyoti, M.; Sridhar, S.K. Biological activities of isatin and its derivatives. Acta Pharm. 2005, 55, 27–46. [Google Scholar] [PubMed]
- Moon, M.J.; Lee, S.K.; Lee, J.W.; Song, W.K.; Kim, S.W.; Kim, J.I.; Cho, C.; Choi, S.J.; Kim, Y.C. Synthesis and structure-activity relationships of novel indirubin derivatives as potent anti-proliferative agents with CDK2 inhibitory activities. Bioorg. Med. Chem. 2006, 14, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Cerchiaro, G.; Ferreira, A.M.D.C. Oxindoles and copper complexes with oxindole-derivatives as potential pharmacological agents. J. Braz. Chem. Soc. 2006, 17, 1473–1485. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Almahli, H.; Al-Ansary, G.H.; Ghabbour, H.A.; Aly, M.H.; Ismael, O.E.; Al-Dhfyan, A.; Abdel-Aziz, H.A. Synthesis and in vitro anti-proliferative activity of some novel isatins conjugated with quinazoline/phthalazine hydrazines against triple-negative breast cancer MDA-MB-231 cells as apoptosis-inducing agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 600–613. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Liang, C.; Shirazian, S.; Zhou, Y.; Miller, T.; Cui, J.; Fukuda, J.Y.; Chu, J.Y.; Nematalla, A.; Wang, X.; et al. Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl) amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase. J. Med. Chem. 2003, 46, 1116–1119. [Google Scholar] [PubMed]
- Prenen, H.; Cools, J.; Mentens, N.; Folens, C.; Sciot, R.; Schoffski, P.; Van Oosterom, A.; Marynen, P.; Debiec-Rychter, M. Efficacy of the kinase inhibitor SU11248 against gastrointestinal stromal tumor mutants refractory to imatinib mesylate. Clin. Cancer Res. 2006, 12, 2622–2627. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Michaelson, M.D.; Redman, B.G.; Hudes, G.R.; Wilding, G.; Figlin, R.A.; Ginsberg, M.S.; Kim, S.T.; Baum, C.M.; DePrimo, S.E.; et al. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2006, 24, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Krug, M.; Hilgeroth, A. Recent advances in the development of multi-kinase inhibitors. Mini Rev. Med. Chem. 2008, 8, 1312–1327. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Xia, J.; Lei, D.; Li, X.; Yao, Q.; Gao, J. Synthesis, in vitro and in vivo antitumor activity of symmetrical bis-Schiff base derivatives of isatin. Eur. J. Med. Chem. 2014, 74, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Kragh-Hansen, U. Molecular aspects of ligand binding to serum albumin. Pharmacol. Rev. 1981, 33, 17–53. [Google Scholar] [PubMed]
- He, X.M.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.A.; Grune, T.; Curry, S. Crystallographic analysis reveals modes of binding of medium and long chain fatty acids to human serum albumin. J. Mol. Biol. 2000, 303, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Sattar, Z.; Iranfar, H.; Asoodeh, A.; Saberi, M.R.; Mazhari, M.; Chamani, J. Interaction between holo transferrin and HSA–PPIX complex in the presence of lomefloxacin: An evaluation of PPIX aggregation in protein–protein interactions. Spectrochim. Acta Part A 2012, 97, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Liu, J.; Hu, Z.; Chen, X. Interaction of wogonin with bovine serum albumin. Bioorg. Med. Chem. 2005, 13, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, A.S. Insight into the Interaction between the HIV-1 Integrase inhibitor Elvitegravir and bovine serum albumin: A Spectroscopic study. J. Spectrosc. 2015. [Google Scholar] [CrossRef]
- Sułkowska, A. Interaction of drugs with bovine and human serum albumin. J. Mol. Struct. 2002, 614, 227–232. [Google Scholar] [CrossRef]
- Zhou, N.; Liang, Y.Z.; Wang, P. 18β-Glycyrrhetinic acid interaction with bovine serum albumin. J. Photochem. Photobiol. A 2007, 185, 271–276. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhang, H.M.; Zhang, G.C.; Tao, W.H.; Fei, Z.H.; Liu, Z.T. Spectroscopic studies on the interaction between silicotungstic acid and bovine serum albumin. J. Pharm. Biomed. Anal. 2007, 43, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.P.; Wei, Y.L.; Dong, C. Study on the interaction of 3,3-bis(4-hydroxy-1-naphthyl)-phthalide with bovine serum albumin by fluorescence spectroscopy. J. Photochem. Photobiol. A 2006, 177, 6–11. [Google Scholar] [CrossRef]
- Bolel, P.; Mahapatra, N.; Halder, M. Optical spectroscopic exploration of binding of cochineal red A with two homologous serum albumins. J. Agric. Food Chem. 2012, 60, 3727–3734. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar] [PubMed]
- Gong, A.; Zhu, X.; Hu, Y.; Yu, S. A fluorescence spectroscopic study of the interaction between epristeride and bovin serum albumine and its analytical application. Talanta 2007, 73, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Qi, Z.D.; Xiao, Q.; Dong, J.X.; Zhang, Y.Z.; Liu, Y. Interaction of loratadine with serum albumins studied by fluorescence quenching method. J. Biochem. Biophys. Methods 2007, 70, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Eldehna, W.M.; Altoukhy, A.; Mahrous, H.; Abdel-Aziz, H.A. Design, synthesis and QSAR study of certain isatin-pyridine hybrids as potential anti-proliferative agents. Eur. J. Med. Chem. 2015, 90, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Shmidt, M.S.; Reverdito, A.M.; Kremenchuzky, L.; Perillo, I.A.; Blanco, M.M. Simple and efficient microwave assisted N-alkylation of isatin. Molecules 2008, 13, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Keeton, A.B.; Piazza, G.A.; University of South Alabama, Alabama, AL, USA. Unpublished work. 2017.
- Dufour, C.; Dangles, O. Flavonoid–serum albumin complexation: Determination of binding constants and binding sites by fluorescence spectroscopy. Biochim. Biophys. Acta Gen. Subj. 2005, 1721, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy; Springer Science & Business Media, LLC: New York, NY, USA, 2007. [Google Scholar]
- Bujacz, A.; Zielinski, K.; Sekula, B. Structural studies of bovine, equine, and leporine serum albumin complexes with naproxen. Proteins Struct. Funct. Bioinf. 2014, 82, 2199–2208. [Google Scholar] [CrossRef] [PubMed]
- Pasban Ziyarat, F.; Asoodeh, A.; Sharif Barfeh, Z.; Pirouzi, M.; Chamani, J. Probing the interaction of lysozyme with ciprofloxacin in the presence of different-sized Ag nano-particles by multispectroscopic techniques and isothermal titration calorimetry. J. Biomol. Struct. Dyn. 2014, 32, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Bourassa, P.; Bariyanga, J.; Tajmir-Riahi, H.A. Binding Sites of Resveratrol, Genistein, and Curcumin with Milk α- and β-Caseins. J. Phys. Chem. B 2013, 117, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Da, L. Insights into the selective binding and toxic mechanism of microcystin to catalase. Spectrochim. Acta Part A 2014, 121, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principle of Fluorescence Spectroscopy, 2nd ed.; Plemum Press: New York, NY, USA, 1999. [Google Scholar]
- Zhang, W.; Wang, F.; Xiong, X.; Ge, Y.; Liu, Y. Spectroscopic and molecular docking studies on the interaction of dimetridazole with human serum albumin. J. Chil. Chem. Soc. 2013, 58, 1717–1721. [Google Scholar] [CrossRef]
- Shcharbin, D.; Klajnert, B.; Mazhul, V.; Bryszewska, M. Dendrimer Interactions with Hydrophobic Fluorescent Probes and Human Serum Albumin. J. Fluoresc. 2005, 15, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Stern, O.; Volmer, M. The extinction period of fluorescence. Phys. Z. 1919, 20, 183–188. [Google Scholar]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G. Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry 1973, 12, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, Z.; Zhang, D.; Lv, J. Flow-injection analysis chemiluminescence detection combined with microdialysis sampling for studying protein binding of drug. Talanta 2001, 53, 835–841. [Google Scholar] [CrossRef]
- Ware, W.R. Oxygen quenching of fluorescence in solution: An experimental study of the diffusion process. J. Phys. Chem. 1962, 66, 455–458. [Google Scholar] [CrossRef]
- Chen, G.Z.; Huang, X.Z.; Xu, J.G. Spectrofluorimetric Analytical Method, 2nd ed.; Science Press: Beijing, China, 1990. [Google Scholar]
- Liu, J.; Tian, J.N.; Zhang, J.; Hu, Z.; Chen, X. Interaction of magnolol with bovine serum albumin: A fluorescence-quenching study. Anal. Bioanal. Chem. 2003, 376, 864–867. [Google Scholar] [PubMed]
- Forster, T.; Sinanoglu, O. Modern Quantum Chemistry; Academic Press: New York, NY, USA, 1996; p. 93. [Google Scholar]
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef] [PubMed]
- Abdelhameed, A.S.; Alanazi, A.M.; Bakheit, A.H.; Darwish, H.W.; Ghabbour, H.A.; Darwish, I.A. Fluorescence spectroscopic and molecular docking studies of the binding interaction between the new anaplastic lymphoma kinase inhibitor crizotinib and bovine serum albumin. Spectrochim. Acta Part A 2017, 171, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhao, S.; Yang, J.; Zhu, X.; Dai, G.; Liang, H.; Pan, F.; Weng, L. Interaction between Levamisole hydrochloride andbovine serum albumin and the influence ofalcohol: Spectra. J. Solut. Chem. 2009, 38, 1183–1192. [Google Scholar] [CrossRef]
- Zhang, H.X.; Huang, X.; Mei, P.; Gao, S. Interaction between Glyoxal-bis-(2-hydroxyanil) andbovine serum albumin insolution. J. Solut. Chem. 2008, 37, 631–640. [Google Scholar] [CrossRef]
- Samanta, A.; Paul, B.K.; Guchhait, N. Spectroscopic probe analysis for exploring probe–protein interaction: A mapping of native, unfolding and refolding of protein bovine serum albumin by extrinsic fluorescence probe. Biophys. Chem. 2011, 156, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, A.; Wong, Y.H.; Korsholm, S.S.; Bahring, N.H.B.; Bobone, S.; Tayyab, S.; Van De Weert, M.; Stella, L. On the purported “backbone fluorescence” in protein three-dimensional fluorescence spectra. RSC Adv. 2016, 6, 112870–112876. [Google Scholar] [CrossRef]
- Ghuman, J.; Zunszain, P.A.; Petitpas, I.; Bhattacharya, A.A.; Otagiri, M.; Curry, S. Structural basis of the drug-binding specificity of human serum albumin. J. Mol. Biol. 2005, 353, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.K.; Ahmad, E.; Khan, J.M.; Alam, P.; Ishtikhar, M.; Khan, R.H. Elucidating the interaction of limonene with bovine serum albumin: A multi-technique approach. Mol. Biosyst. 2015, 11, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, G.; Birkett, D.; Wade, D. Further characterization of specific drug binding sites on human serum albumin. Mol. Pharmacol. 1976, 12, 1052–1061. [Google Scholar] [PubMed]







| Temperature (T) (K) | Stern–Volmer Parameters | Lineweaver–Burk Parameters | |||
|---|---|---|---|---|---|
| KSV × 105 (L·mol−1) | Kq × 1013 (L·mol−1·s−1) | r2 | KLB × 105 (L·mol−1) | r2 | |
| 288 | 1.13 ± 0.047 | 4.19 | 0.9863 | 1.06 ± 0.16 | 0.9786 |
| 298 | 1.10 ± 0.045 | 4.08 | 0.9876 | 1.04 ± 0.12 | 0.9798 |
| 309 | 1.06 ± 0.048 | 3.92 | 0.9841 | 1.01 ± 0.12 | 0.9813 |
| Temperature (T) (K) | ΔG° (kJ·mol−1) | ΔH° (kJ·mol−1) | ΔS° (J·mol−1·K−1) | K × 105 (L·mol−1) | n * | r2 |
|---|---|---|---|---|---|---|
| 288 | −32.98 ± 2.5 | −0.72 ± 0.71 | 105.09 ± 5.32 | 9.65 ± 0.95 | 0.947 ± 0.018 | 0.9955 |
| 298 | −34.04 ± 2.2 | 9.32 ± 1.20 | 0.946 ± 0.019 | 0.9952 | ||
| 309 | −35.19 ± 2.3 | 8.92 ± 0.97 | 0.945 ± 0.018 | 0.9956 |
| BSA | Compound 3-BSA | |||
|---|---|---|---|---|
| 1st Peak | 2nd Peak | 1st Peak | 2nd Peak | |
| Position of the peak (λex/λem, nm/nm) | 224/336 | 278/334 | 224/336 | 278/334 |
| Relative intensity (IF) | 9993.22 | 4258.31 | 2845.65 | 1290.87 |
| Stokes shift λ/nm | 112 | 56 | 112 | 52 |
| Systems | KSV × 105 (L·mol−1) | r2 |
|---|---|---|
| BSA + compound 3 | 1.10 ± 0.05 | 0.9876 |
| BSA + compound 3 + IBP | 1.08 ± 0.06 | 0.9782 |
| BSA + compound 3 + WAR | 0.91 ± 0.05 | 0.9823 |
| Compound 3 | BSA Residues | Interaction Type | Distance (Å) |
|---|---|---|---|
| N 27 | ASP450 | H-donor | 3.10 |
| O 1 | ARG198 | H-acceptor | 2.70 |
| O 30 | ARG194 | H-acceptor | 2.96 |
| 5-ring | VAL342 | pi-H | 4.50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhameed, A.S.; Bakheit, A.H.; Mohamed, M.S.; Eldehna, W.M.; Abdel-Aziz, H.A.; Attia, M.I. Synthesis and Biophysical Insights into the Binding of a Potent Anti-Proliferative Non-symmetric Bis-isatin Derivative with Bovine Serum Albumin: Spectroscopic and Molecular Docking Approaches. Appl. Sci. 2017, 7, 617. https://doi.org/10.3390/app7060617
Abdelhameed AS, Bakheit AH, Mohamed MS, Eldehna WM, Abdel-Aziz HA, Attia MI. Synthesis and Biophysical Insights into the Binding of a Potent Anti-Proliferative Non-symmetric Bis-isatin Derivative with Bovine Serum Albumin: Spectroscopic and Molecular Docking Approaches. Applied Sciences. 2017; 7(6):617. https://doi.org/10.3390/app7060617
Chicago/Turabian StyleAbdelhameed, Ali Saber, Ahmed H. Bakheit, Mostafa S. Mohamed, Wagdy M. Eldehna, Hatem A. Abdel-Aziz, and Mohamed I. Attia. 2017. "Synthesis and Biophysical Insights into the Binding of a Potent Anti-Proliferative Non-symmetric Bis-isatin Derivative with Bovine Serum Albumin: Spectroscopic and Molecular Docking Approaches" Applied Sciences 7, no. 6: 617. https://doi.org/10.3390/app7060617







