Control of the Polymorphism of Calcium Carbonate Produced by Self-Healing in the Cracked Part of Cementitious Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. Experimental Procedure
3. Results and Discussion
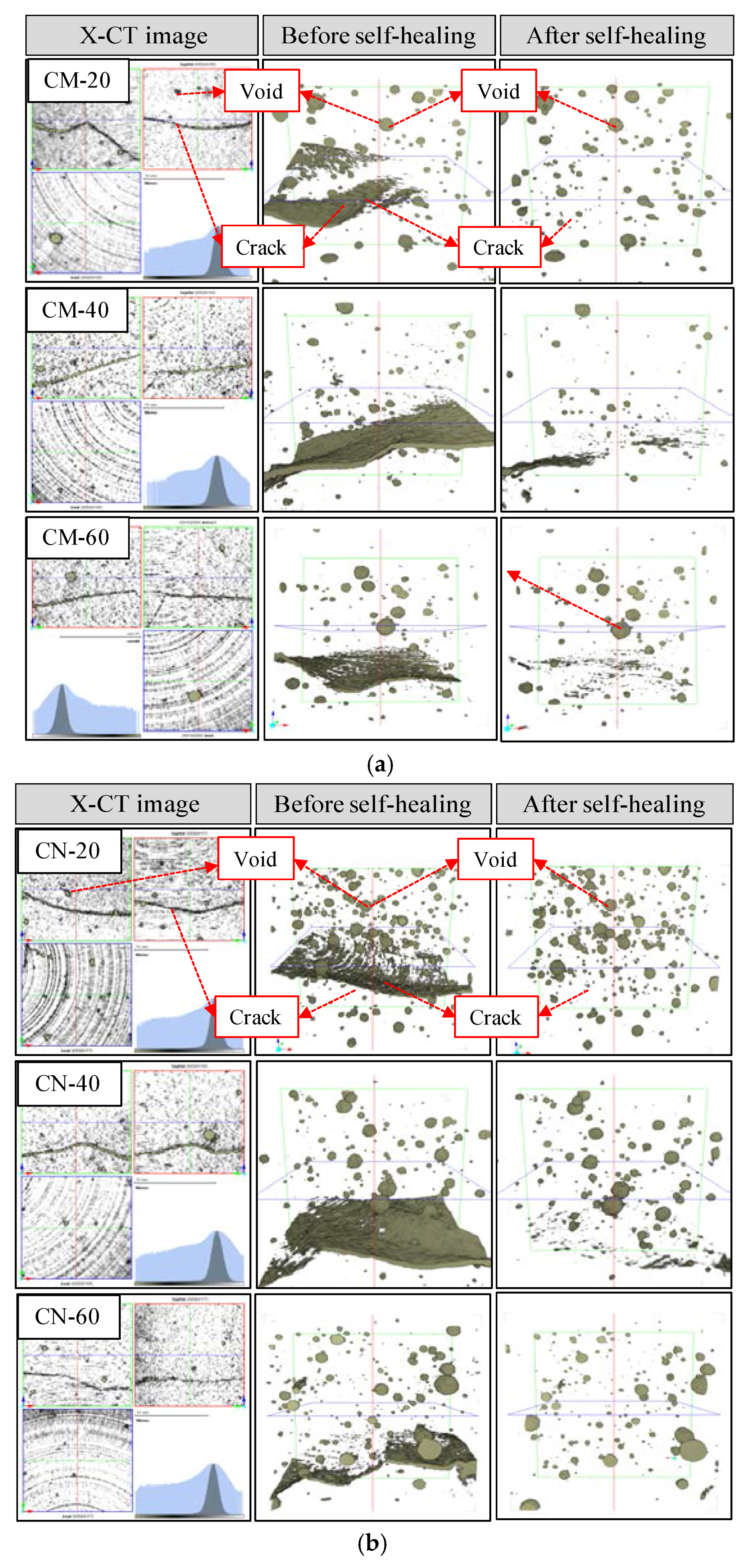
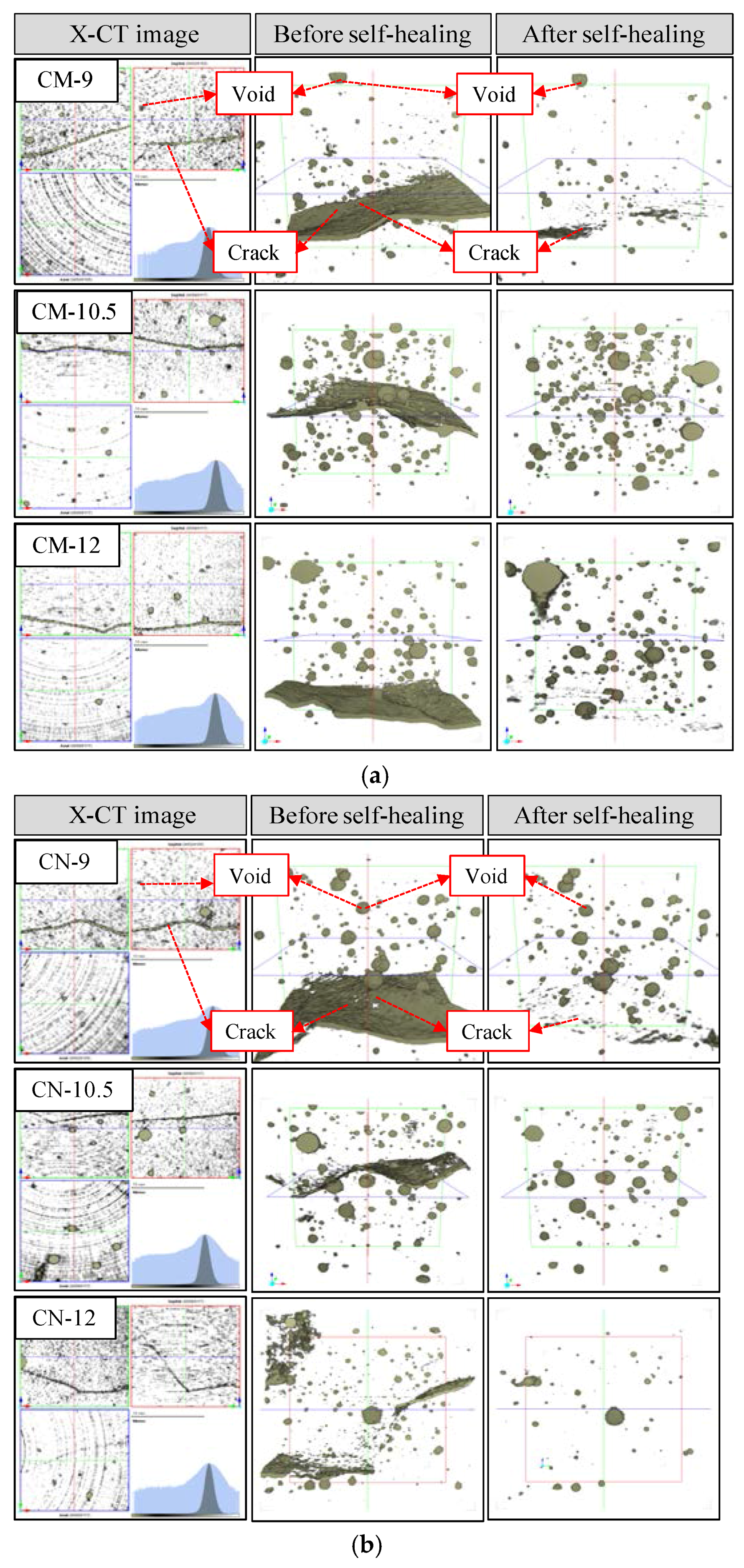
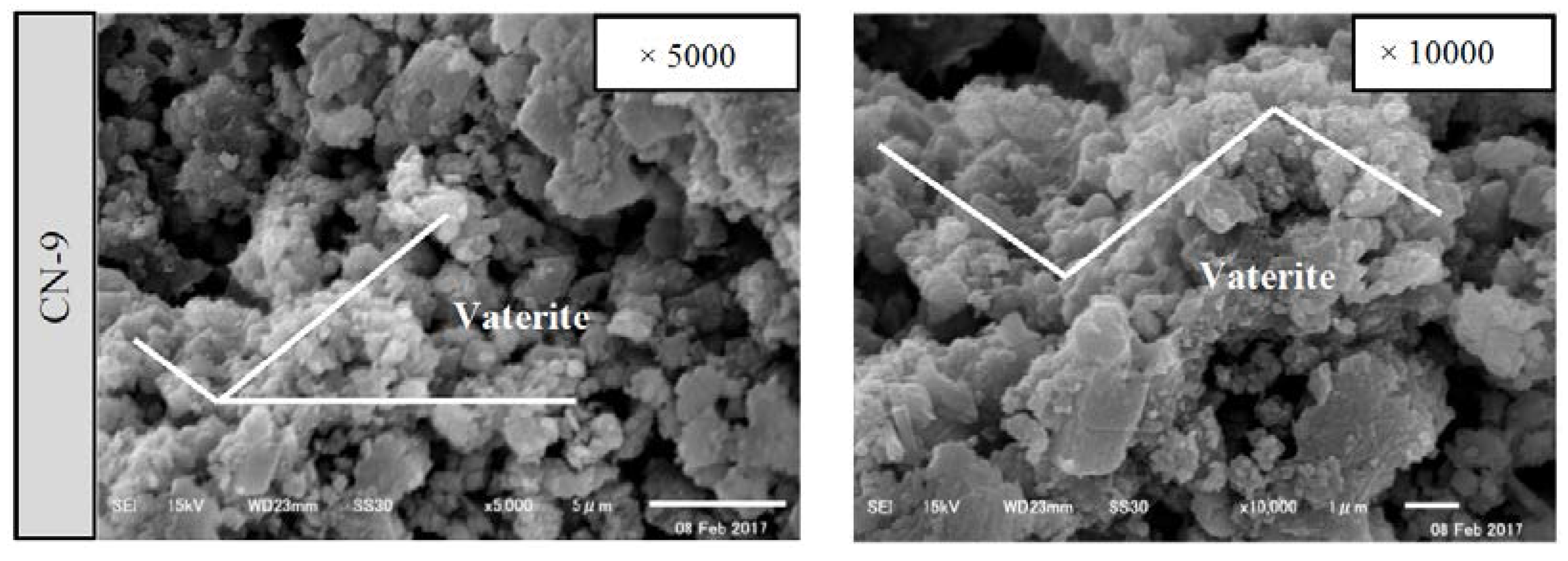
3.1. Surface Section of the Cracks
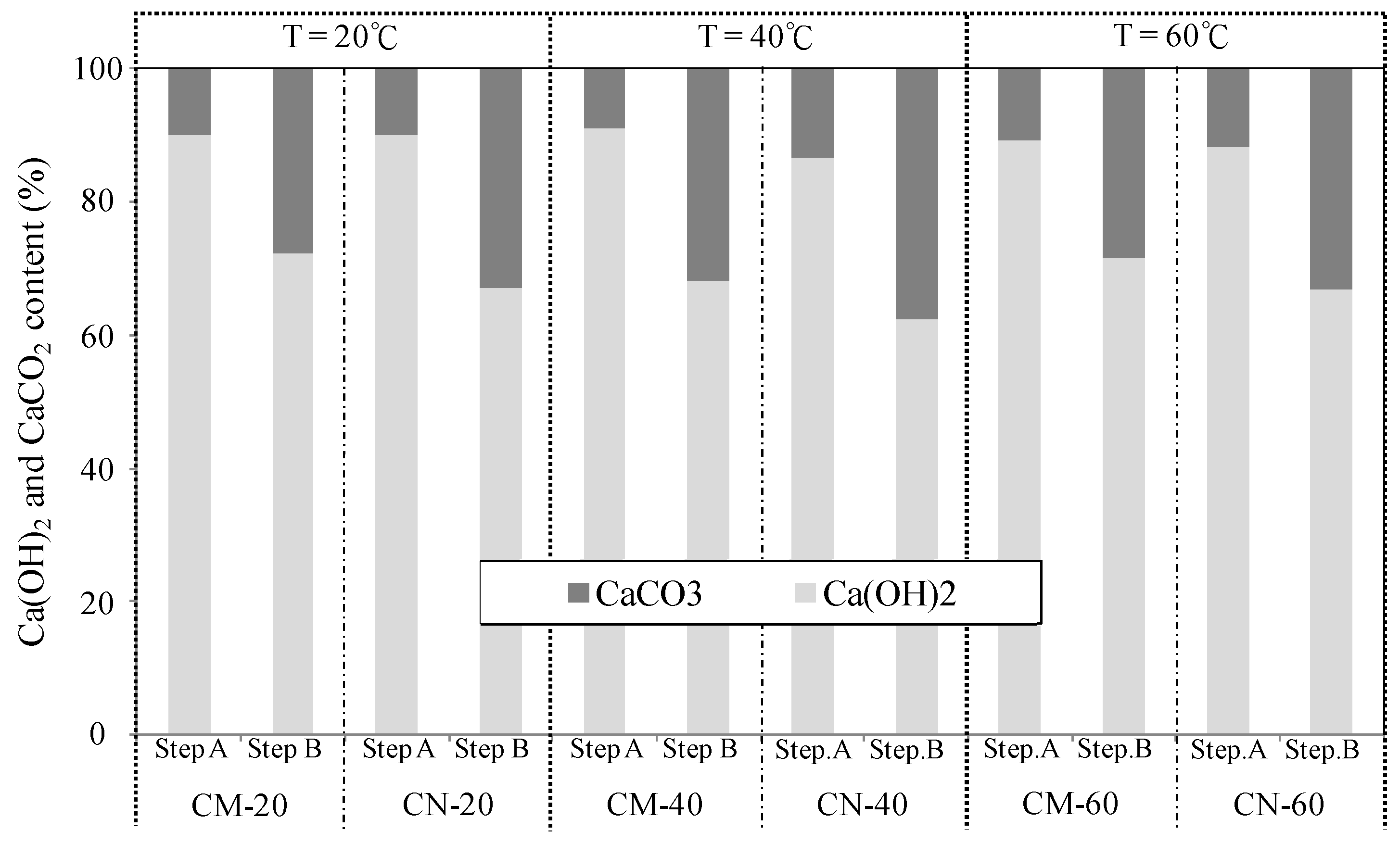
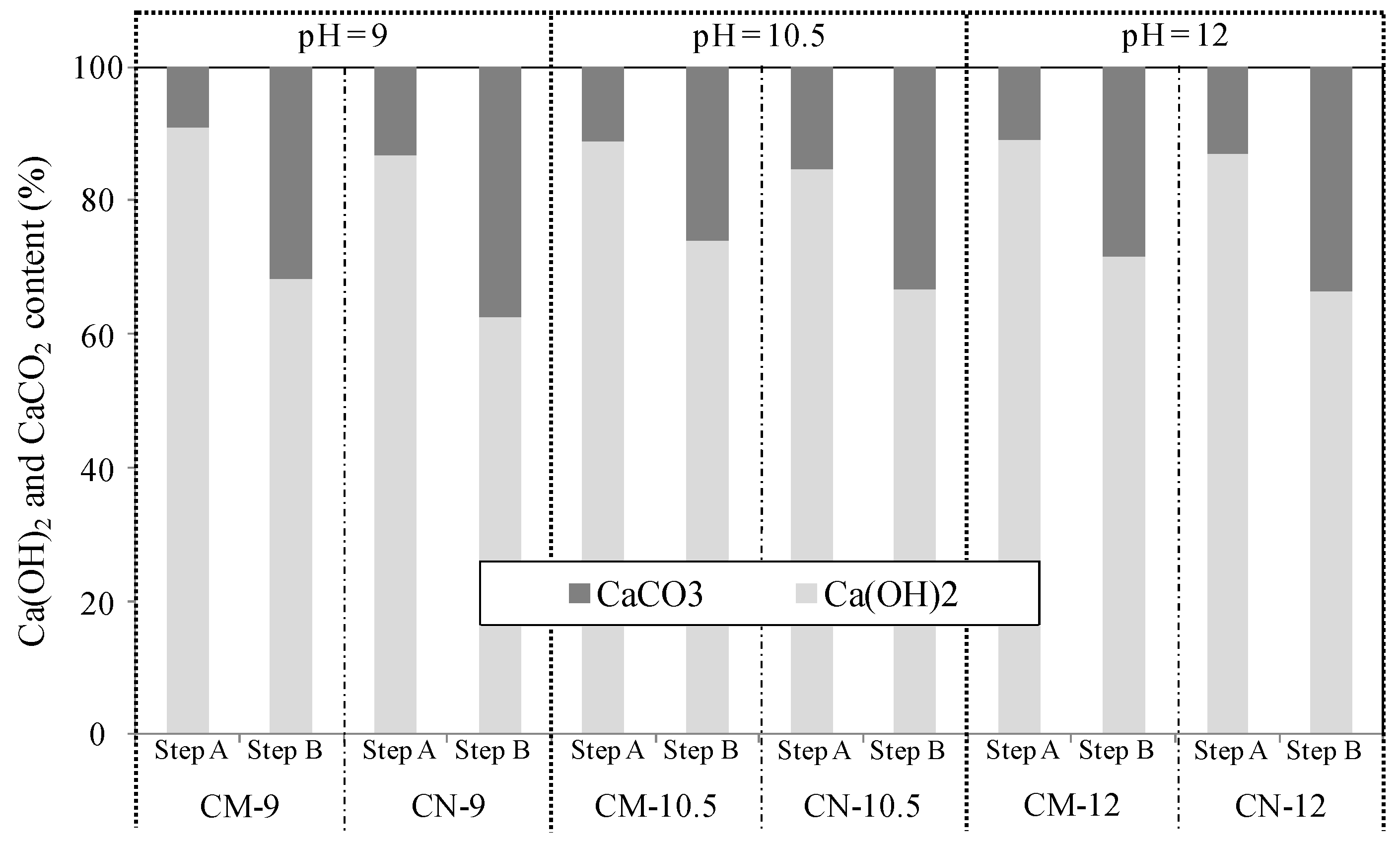
3.2. Evaluation of Self-Healing Performance as a Function of Temperature and pH
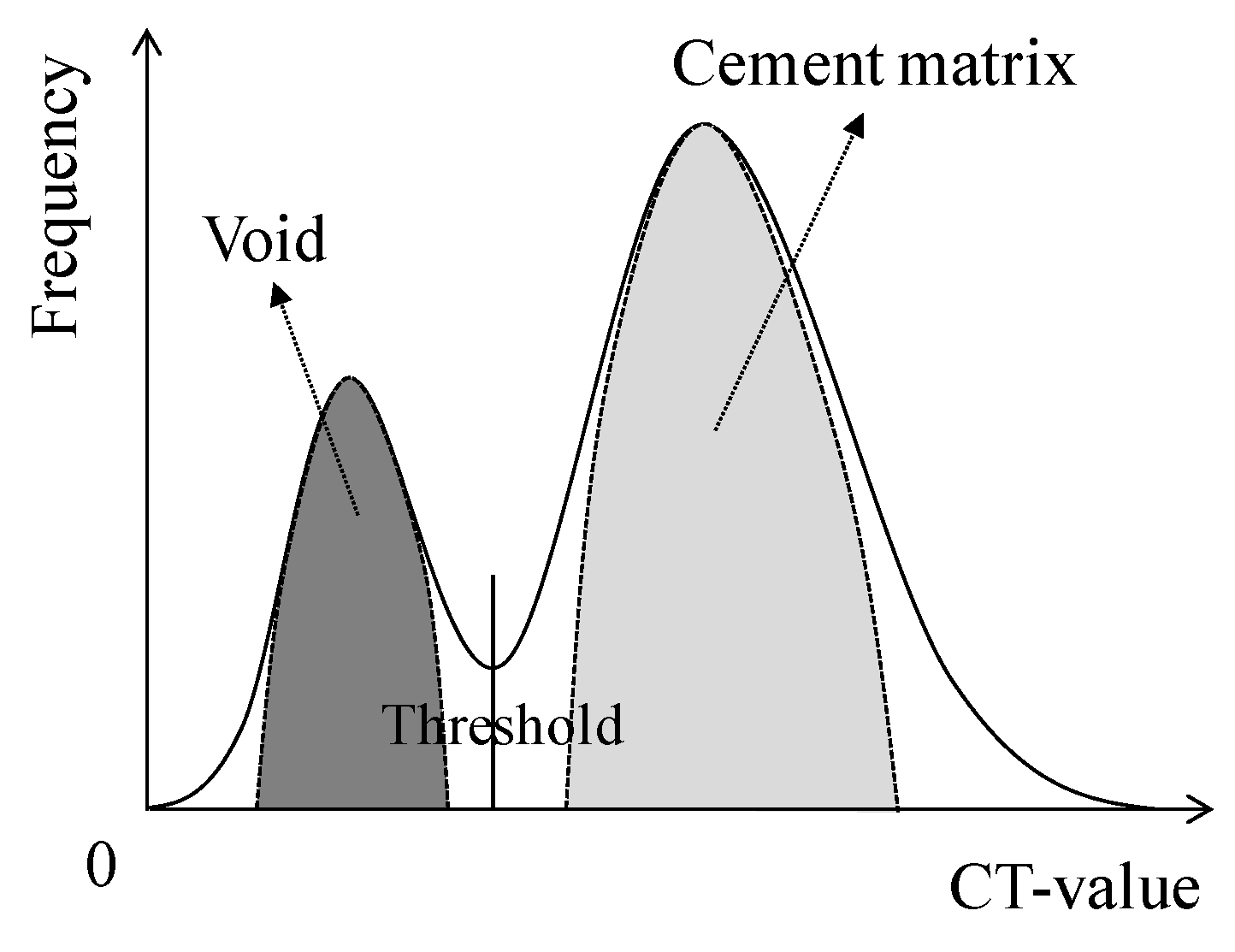
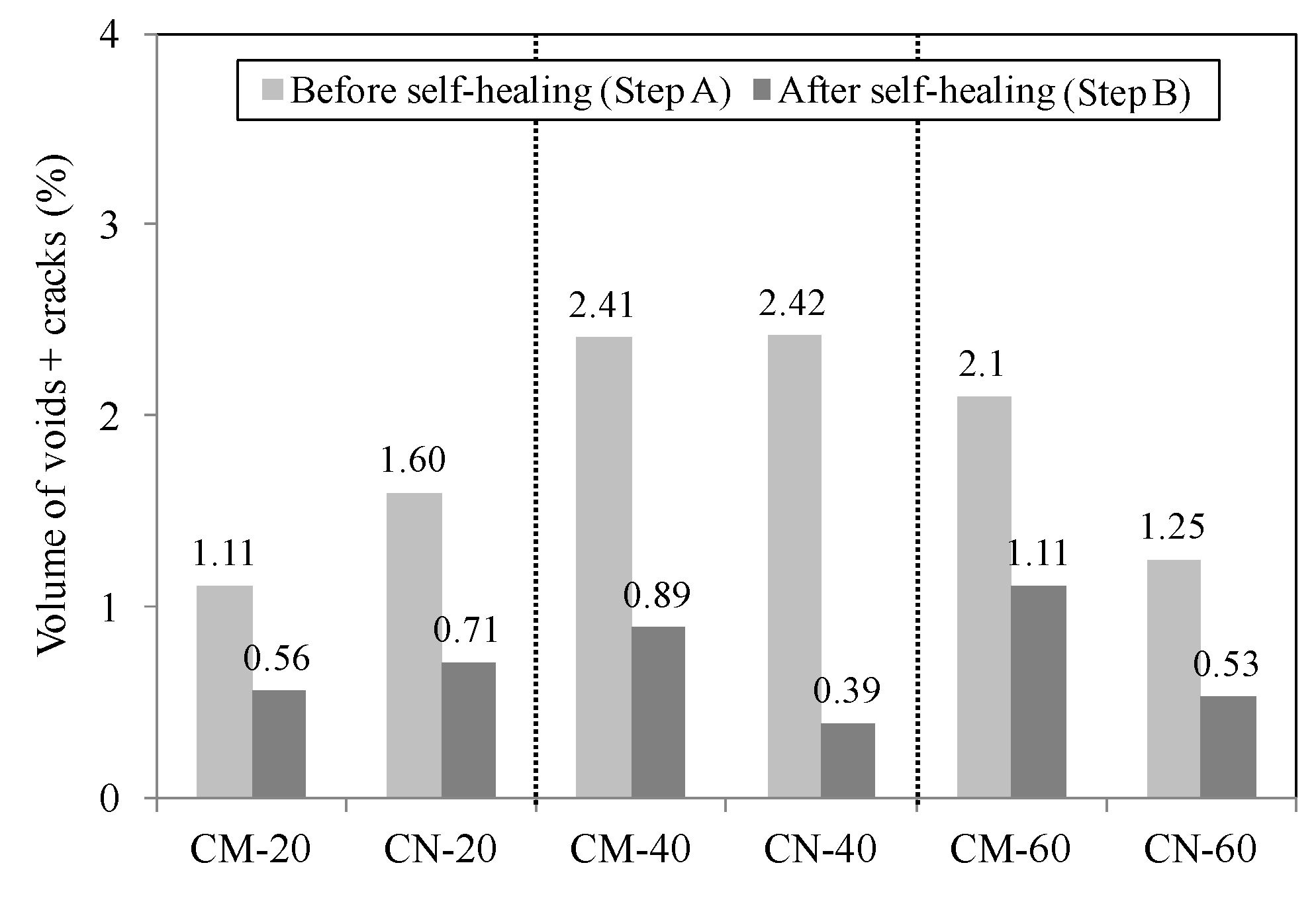
3.2.1. Volume Change in Cracked Section
3.2.2. Chemical Evaluation of Self-Healing Precipitates
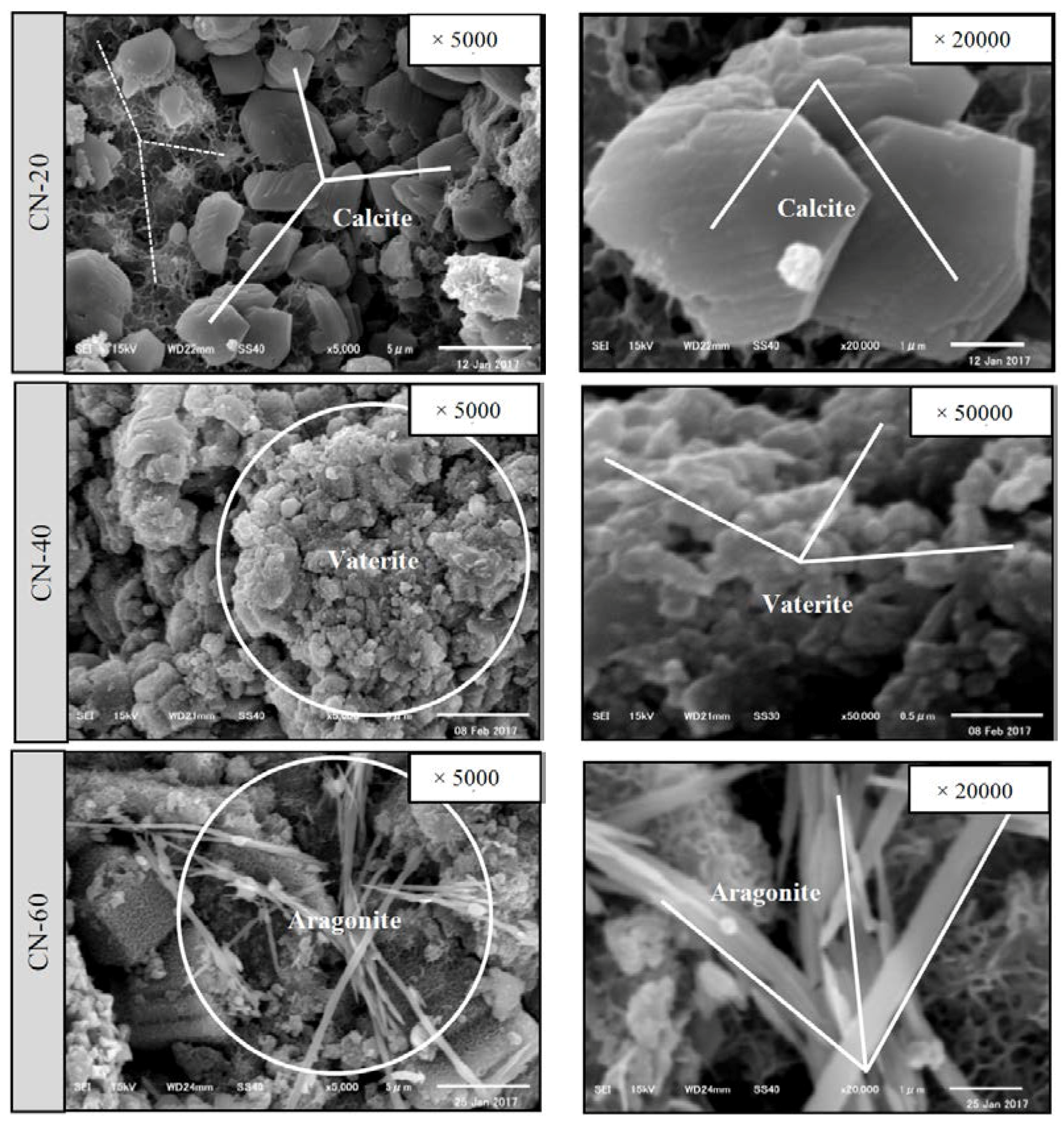
3.2.3. Crystallographic Change in Calcium Carbonate Compound (CaCO3)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Choi, H.S.; Inoue, M.; Kwon, S.M.; Choi, H.G.; Lim, M.K. Effective crack control of concrete by self-healing of cementitious composites using synthetic fiber. J. Mater. 2016, 9, 1–14. [Google Scholar] [CrossRef]
- Japan Concrete Institute. Practical Guideline for Investigation, Repair and Strengthening of Cracked Concrete Structure; Japan Concrete Institute: Tokyo, Japan, 2013. (In Japanese) [Google Scholar]
- Jacobson, S. Effect of cracking and healing on chloride transport in OPC concrete. Cem. Concr. Res. 1996, 26, 869–881. [Google Scholar] [CrossRef]
- Romildo, D.; Filho, T. Free, restrained and drying shrinkage of cement mortar composites reinforced with vegetable fibers. Cem. Concr. Compos. 2005, 27, 537–546. [Google Scholar]
- Wang, K.; Jansen, D.C.; Shah, S.P. Permeability study of cracked concrete. Cem. Concr. Res. 1997, 27, 381–393. [Google Scholar] [CrossRef]
- Khatri, R.P.; Sirivivatnanon, V. Role of permeability in sulfate attack. Cem. Concr. Res. 1997, 27, 1179–1189. [Google Scholar] [CrossRef]
- Neville, A.M. Properties of Concrete; Person Education Limited: London, UK, 1995; p. 328. [Google Scholar]
- Sanjun, M.A. Effectiveness of crack control at early age on the corrosion of steel bars in low modulus sisal and coconut fiber-reinforced mortars. Cem. Concr. Res. 1998, 28, 555–565. [Google Scholar] [CrossRef]
- Jacobsen, S. SEM Observations of the microstructure of frost deteriorated and self-healed concrete. Cem. Concr. Res. 1995, 25, 1781–1790. [Google Scholar] [CrossRef]
- Edvardsen, C. Water permeability and autogenous healing of cracks in concrete. ACI Mater. J. 1999, 96, 448–454. [Google Scholar]
- Nishiwaki, T.; Koda, M.; Yamada, M.; Mihashi, H.; Kikuta, T. Experimental study on self-healing capability of FRCC using different types of synthetic fibers. J. Adv. Concr. Technol. 2012, 10, 195–206. [Google Scholar] [CrossRef]
- Homma, D.; Mihashi, H.; Nishiwaki, T. Self-healing capability of fiber reinforced cementitious composites. J. Adv. Concr. Technol. 2009, 7, 217–228. [Google Scholar] [CrossRef]
- Gruyaert, E.; Van Tittelboom, K.; Sucaet, J.; Anrijs, J.; Van Vlierberghe, S.; Dubruel, P.; De Geest, B.G.; Remon, J.P.; De Belie, N. Capsules with evolving brittleness to resist the preparation of self-healing concrete. J. Mater. Const. 2016, 66, 1–13. [Google Scholar]
- Hilloulin, B.; Van Tittelboom, K.; Gruyaert, E.; De Belie, N.; Loukili, A. Design of polymeric capsules for self-healing concrete. Cem. Concr. Compos. 2015, 55, 298–307. [Google Scholar] [CrossRef]
- Jonkers, H.M. Bacteria-based self-healing concrete. Heron 2011, 56, 1–12. [Google Scholar]
- Fukuda, D.; Nara, Y.; Kobayashi, Y.; Maruyama, M.; Koketsu, M.; Hayashi, D.; Ogawa, H.; Kaneko, K. Investigation of self-sealing in high-strength and ultra-low-permeability concrete in water using micro-focus X-ray CT. Cem. Concr. Res. 2012, 42, 1494–1500. [Google Scholar] [CrossRef]
- Benoit, H.; Jean-Baptiste, L.; Elisabeth, L.; Odile, A.; Ahmed, L.; Frédéric, G.; Olivier, D.; Vincent, T. Monitoring of autogenous crack healing in cementitious materials by the nonlinear modulation of ultrasonic coda waves, 3D microscopy and X-ray microtomography. Constr. Build. Mater. 2016, 123, 143–152. [Google Scholar]
- Kishi, T.; Ahn, T.-H.; Hosoda, A.; Suzuki, S.; Takaoka, H. Self-healing behaviour by cementitious recrystallization of cracked concrete incorporating expansive agent. In Proceedings of the First International Conference on Self-Healing Materials, Noordwijk aan Zee, The Netherlands, 18–20 April 2007; pp. 1–10. [Google Scholar]
- Snoeck, D.; de Belie, N. Repeated autogenous healing in strain-hardening cementitious composites by using superabsorbent polymers. J. Mater. Civil Eng. 2015, 28, 1–11. [Google Scholar] [CrossRef]
- Snoeck, D.; Dewanckele, J.; Cnudde, V.; de Belie, N. X-ray computed microtomography to study autogenous healing of cementitious materials promoted by superabsorbent polymers. Cem. Concr. Compos. 2016, 65, 83–93. [Google Scholar] [CrossRef]
- Yang, Y.; Lepech, M.D.; Yang, E.-H.; Li, V.C. Autogenous healing of engineered cementitious composites under wet-dry cycles. Cem. Concr. Res. 2009, 39, 382–390. [Google Scholar] [CrossRef]
- Choi, H.-S.; Inoue, M.; Choi, H.-G.; Lim, M.-K.; Nishiwaki, T.; Kawajiri, S. The fundamental study of the crack control by self-healing of PVA fiber reinforced cementitious composites. J. Civil Eng. Archit. Res. 2016, 9(3), 1680–1688. [Google Scholar]
- Choi, H.-S.; Inoue, M.; Kamada, M.; Iwasawa, M. Microcrack control of cementitious materials by self-healing. In Proceedings of the 7th International Conference of Asian Concrete Federation 2016, Hanoi, Vietnam, 30 October–2 November 2016; pp. 1–8. [Google Scholar]
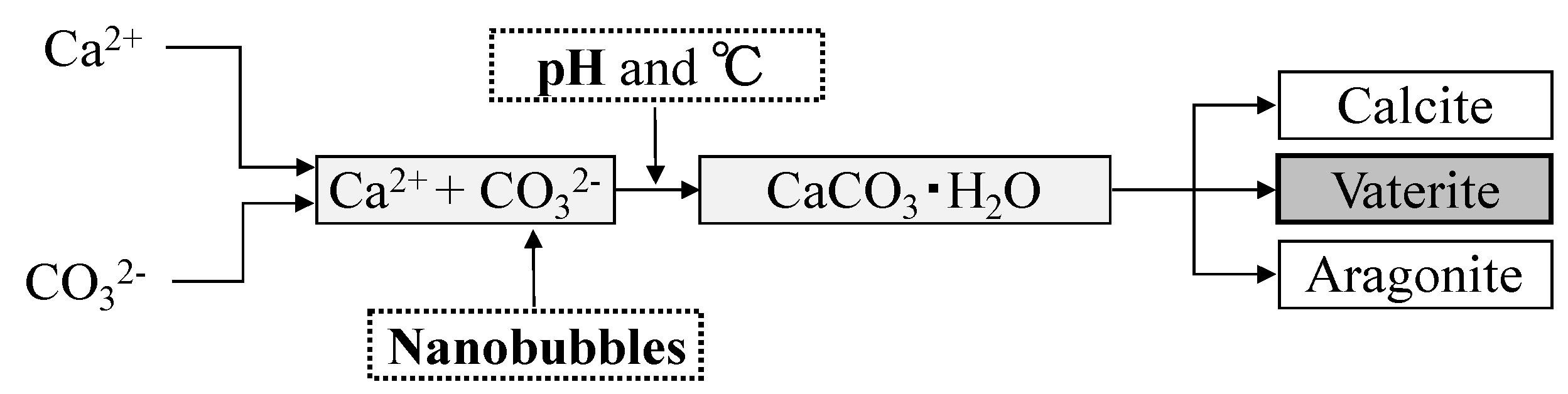
- Matsumoto, M. Polymorph control of calcium carbonate by reactive crystallization using microbubble technique. Chem. Eng. Res. Des. 2010, 88, 1624–1630. [Google Scholar] [CrossRef]
- Kojima, Y. Controls of polymorphism and morphology of calcium carbonate compounds formed by crystallizing amorphous calcium carbonate hydrate. J. Ceram. Soc. Jpn. 1994, 12, 1128–1136. (In Japanese) [Google Scholar] [CrossRef]
- Huang, H.; Ye, G. Self-healing of cracks in cement paste affected by additional Ca2+ ions in the healing agent. J. Intell. Mater. Sys. Struct. 2014, 27, 1–12. [Google Scholar] [CrossRef]
- Kim, H.-S. Fundamental study on recycling of low-quality recycled fine aggregate using carbonated nanobubble water. In Proceedings of the First International Conference on Concrete Sustainability, Tokyo, Japan, 27–29 May 2013. [Google Scholar]
- Taniguchi, S.; Otani, J.; Nishizaki, I. A study on the new evaluation method for asphalt pavement materials using X-ray CT scanner. Jpn. Soc. Civil Eng. J. Pavement Eng. 2010, 15, 41–48. (In Japanese) [Google Scholar]
- Wada, N.; Takao, U. Effect of cation (Sr, Pb and Ba) on calcium carbonate polymorphs under diffusional conditions. Gypsum Lime 1993, 245, 211–219. (In Japanese) [Google Scholar]
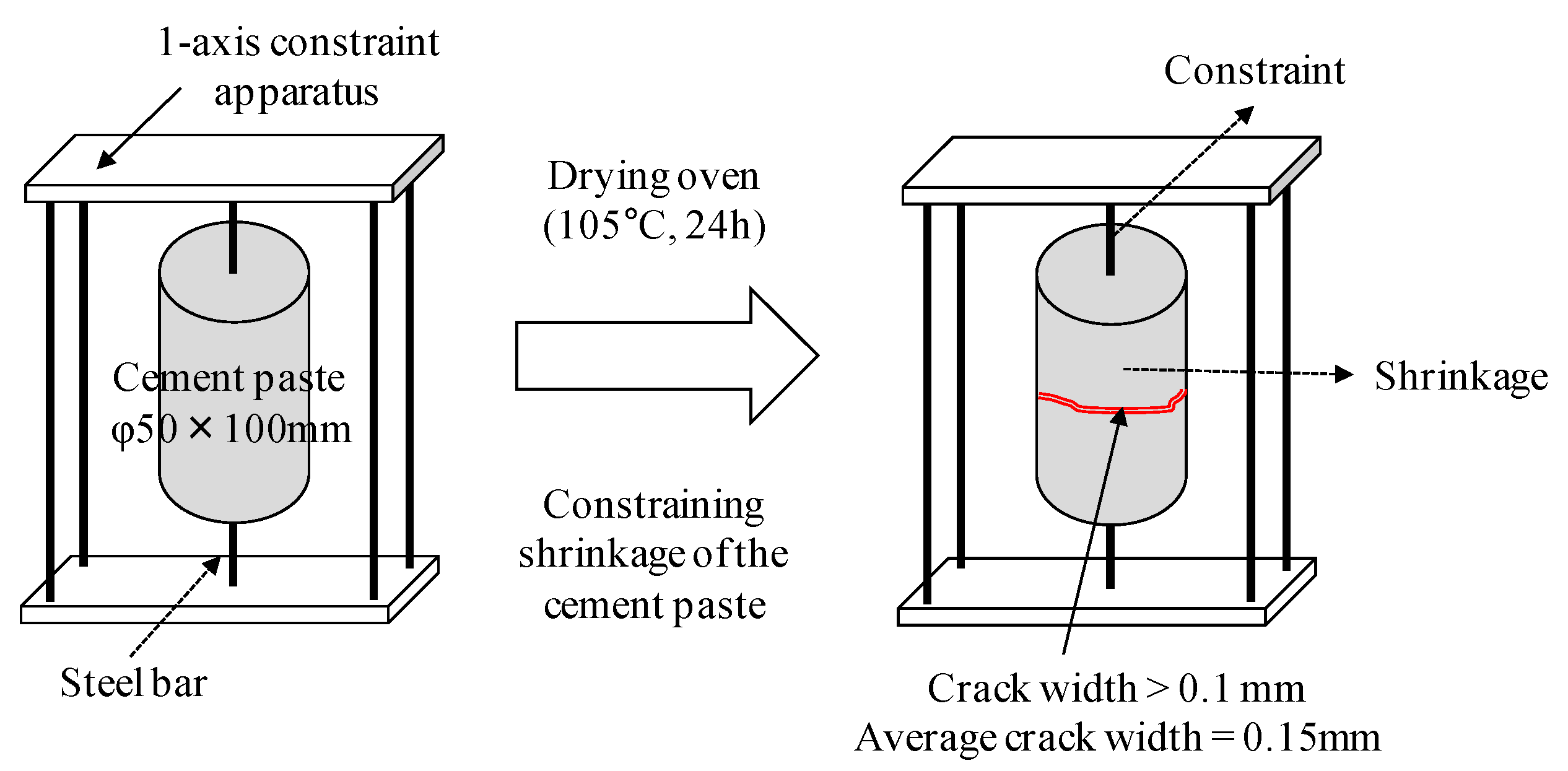





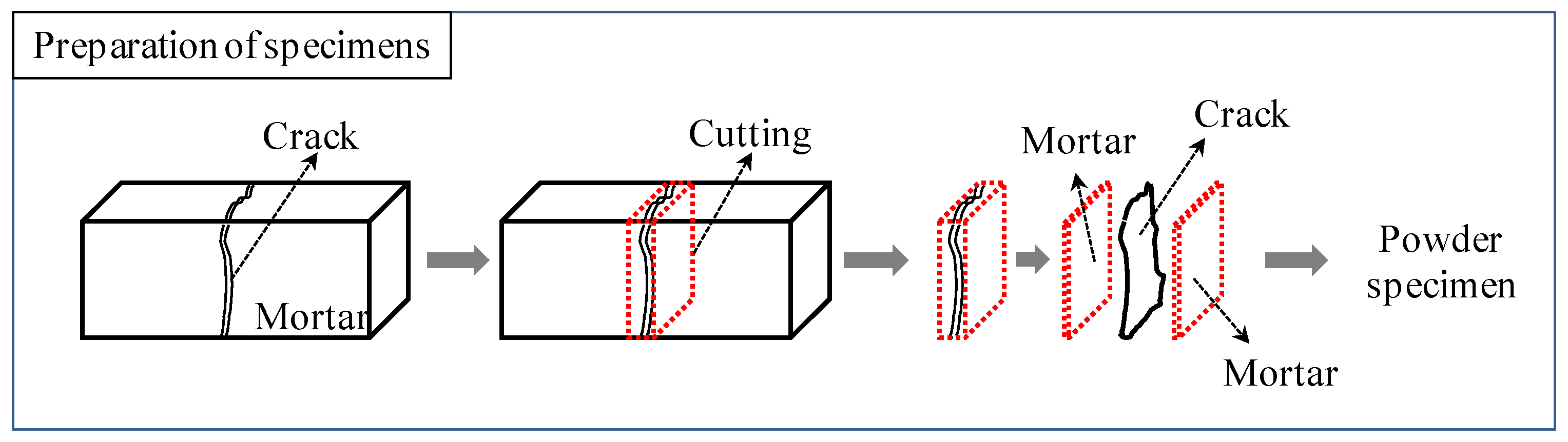

| Sample: Hardened cement paste (water/cement ratio: 40%) | ||
| Crack: Fine cracks generated by drying (105 °C, 24 h) | ||
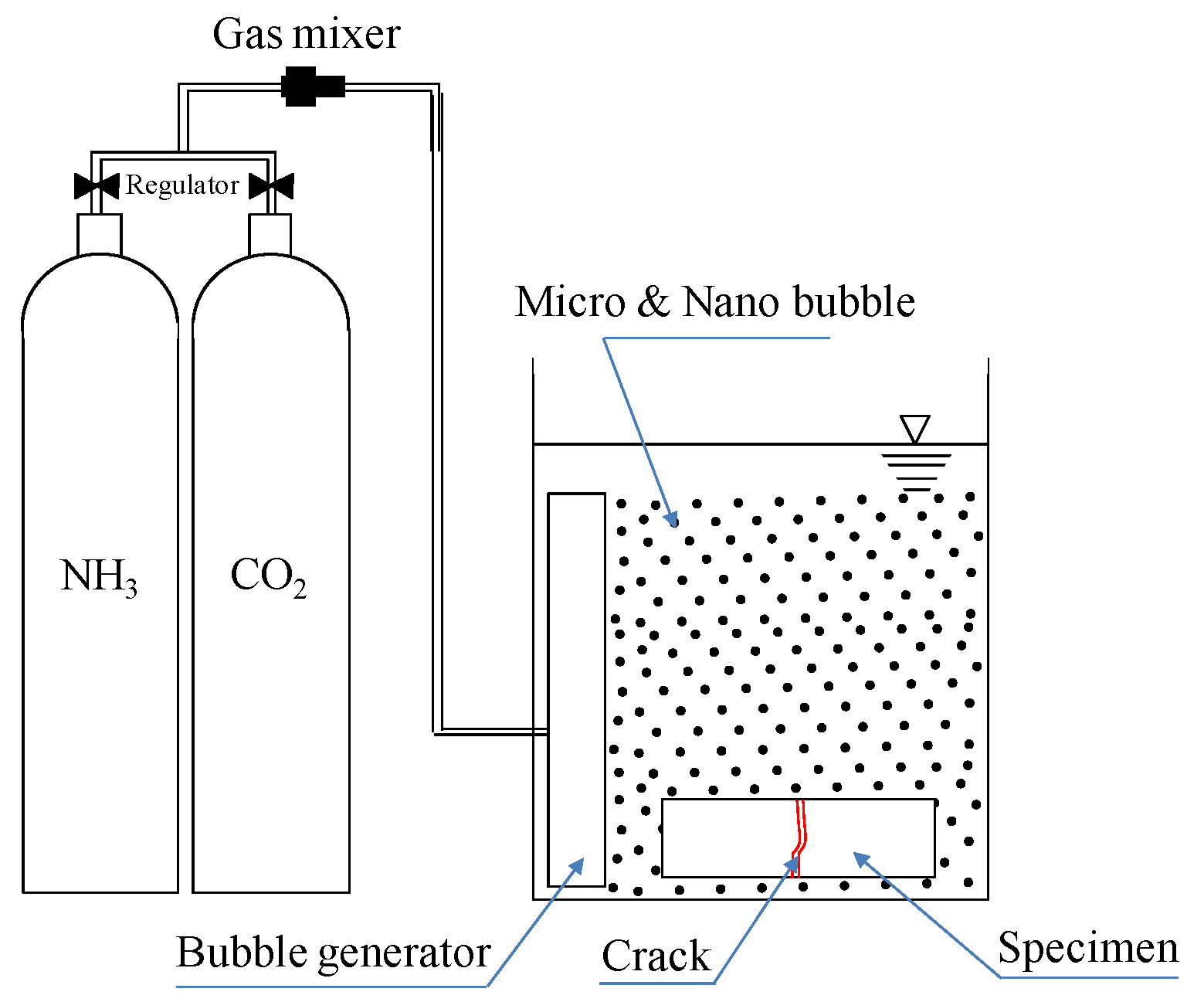
| Self-healing conditions [24,25] | Temperature: 20, 40, and 60 °C (constant pH of 9.0) | Ca(OH)2 + microbubbles (CM) Ca(OH)2 + nanobubbles (CN) |
| pH: 9.0, 10.5, and 12.0 (constant temperature of 40 °C) | ||
| Self-healing period: 7 cycles (7 days) (1 cycle: Bubbles supplied for 4 h+soaking in saturated Ca(OH)2 solution for 20 h) | ||
| Step | Experimental Sequence | Subject and Method of Evaluation | |
|---|---|---|---|
| Surface and Internal Sections of Cracks | Self-Healing Substances | ||
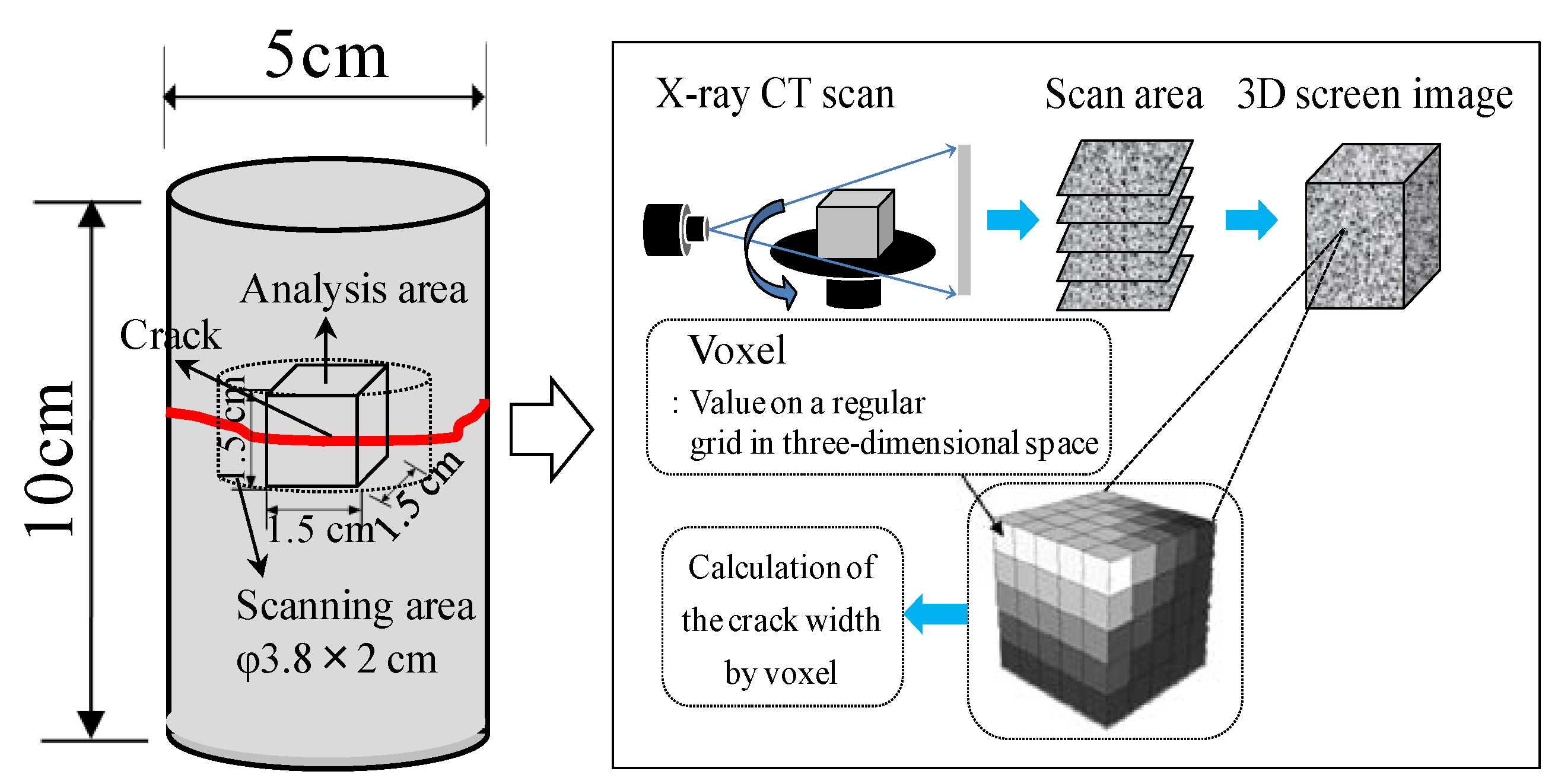
| A | Prior to self-healing | Microscope, X-ray CT | Raman spectroscopic analysis, TG-DTA, SEM analysis |
| B | After self-healing | ||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Choi, H.; Inoue, M.; Sengoku, R. Control of the Polymorphism of Calcium Carbonate Produced by Self-Healing in the Cracked Part of Cementitious Materials. Appl. Sci. 2017, 7, 546. https://doi.org/10.3390/app7060546
Choi H, Choi H, Inoue M, Sengoku R. Control of the Polymorphism of Calcium Carbonate Produced by Self-Healing in the Cracked Part of Cementitious Materials. Applied Sciences. 2017; 7(6):546. https://doi.org/10.3390/app7060546
Chicago/Turabian StyleChoi, Heesup, Hyeonggil Choi, Masumi Inoue, and Risa Sengoku. 2017. "Control of the Polymorphism of Calcium Carbonate Produced by Self-Healing in the Cracked Part of Cementitious Materials" Applied Sciences 7, no. 6: 546. https://doi.org/10.3390/app7060546





