Understanding Hydrothermal Dechlorination of PVC by Focusing on the Operating Conditions and Hydrochar Characteristics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
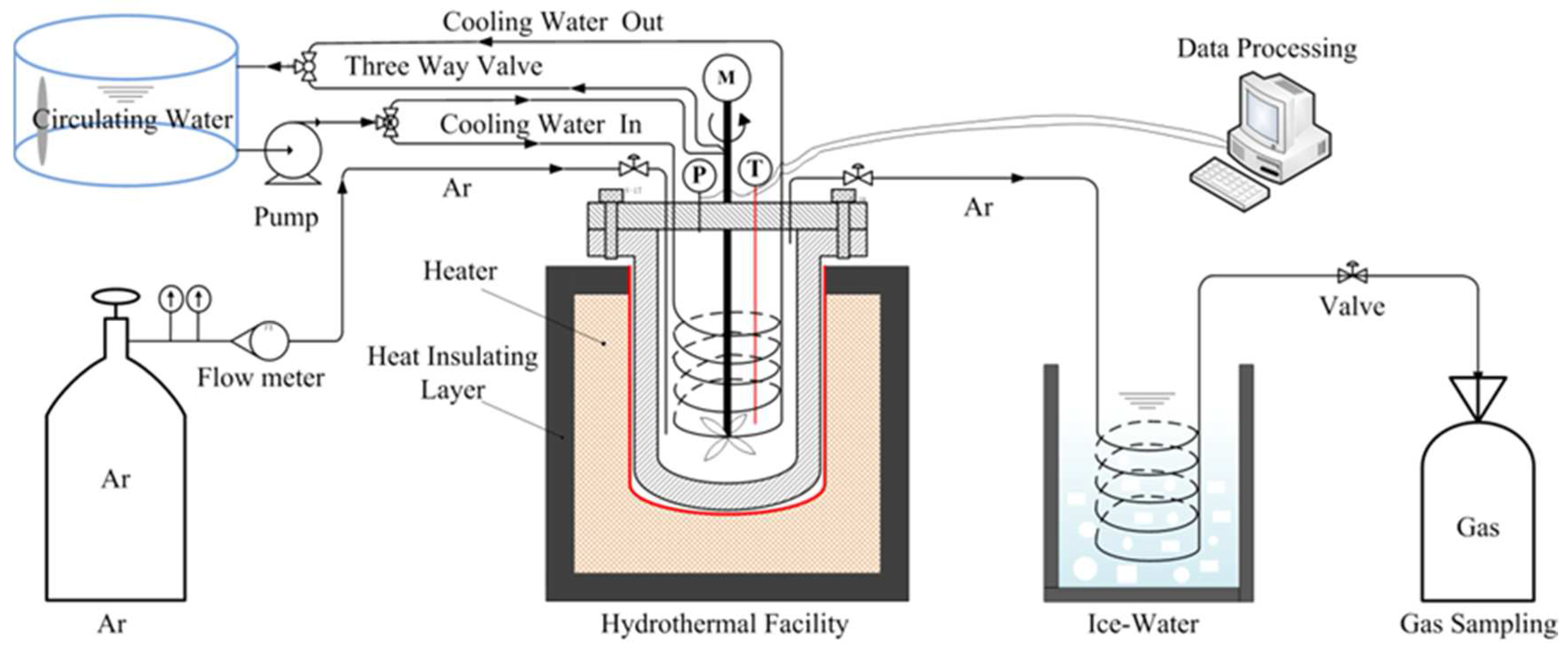
2.2. Hydrothermal Dechlorination
2.3. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3. Results
3.1. Effect of Operation Conditions on HT Dechlorination Behavior
3.1.1. Effect of Temperature on HT Dechlorination of PVC
3.1.2. Effect of NaOH Dosage on HT Dechlorination of PVC
3.1.3. Effect of Residence Time on HT Dechlorination of PVC
3.2. Effect of HT Dechlorination on Functional Group Existing in PVC
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Poerschmann, J.; Weiner, B.; Woszidlo, S.; Koehler, R.; Kopinke, F.D. Hydrothermal carbonization of poly (vinyl chloride). Chemosphere 2014, 119C, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Al-Salem, S.M.; Lettieri, P.; Baeyens, J. Recycling and recovery routes of plastic solid waste (PSW): A review. Waste Manag. 2009, 29, 2625–2643. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, Y.; Kato, K.; Takahashi, K.; Sato, Y.; Nishi, S. Basic study on treatment of waste polyvinyl chloride plastics by hydrothermal decomposition in subcritical and supercritical regions. J. Supercrit. Fluid 2004, 31, 185–193. [Google Scholar] [CrossRef]
- Yu, J.; Sun, L.; Ma, C.; Qiao, Y.; Yao, H. Thermal degradation of PVC: A review. Waste Manag. 2016, 48, 300–314. [Google Scholar] [CrossRef] [PubMed]
- López, A.; de Marco, I.; Caballero, B.M.; Laresgoiti, M.F.; Adrados, A. Dechlorination of fuels in pyrolysis of PVC containing plastic wastes. Fuel Process. Technol. 2011, 92, 253–260. [Google Scholar] [CrossRef]
- Yoshioka, T.; Akama, T.; Uchida, M.; Okuwaki, A. Analysis of Two Stages Dehydrochlorination of Poly (vinyl chloride) Using TG-MS. Chem. Lett. 2000, 2000, 322–323. [Google Scholar] [CrossRef]
- Yoshioka, T.; Saitoh, N.; Okuwaki, A. Temperature dependence on the activation energy of dechlorination in thermal degradation of polyvinylchloride. Chem. Lett. 2005, 34, 70–71. [Google Scholar] [CrossRef]
- Yuan, G.; Chen, D.; Yin, L.; Wang, Z.; Zhao, L.; Wang, J.Y. High efficiency chlorine removal from polyvinyl chloride (PVC) pyrolysis with a gas-liquid fluidized bed reactor. Waste Manag. 2014, 34, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, T.; Luo, X.; Han, D.; Wang, Z.; Wu, J. TG/FTIR analysis on co-pyrolysis behavior of PE, PVC and PS. Waste Manag. 2014, 34, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.T.; Shen, Y.F.; Ge, S.F.; Chen, Z.Q.; Yoshikawa, K. Clean solid biofuel production from high moisture content waste biomass employing hydrothermal treatment. Appl. Energy 2014, 131, 345–367. [Google Scholar] [CrossRef]
- Prawisudha, P.; Namioka, T.; Yoshikawa, K. Coal alternative fuel production from municipal solid wastes employing hydrothermal treatment. Appl. Energy 2012, 90, 298–304. [Google Scholar] [CrossRef]
- Lv, B.; Zhao, G.; Li, D.; Liang, C. Dechlorination and oxidation for waste poly (vinylidene chloride) by hydrothermal catalytic oxidation on Pd/AC catalyst. Polym. Degrad. Stabil. 2009, 94, 1047–1052. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.M.; Fühner, C.; Bens, O.; Kern, J. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Hashimoto, K.; Suga, S.; Wakayama, Y.; Funazukuri, T. Hydrothermal dechlorination of PVC in the presence of ammonia. J. Mater. Sci. 2007, 43, 2457–2462. [Google Scholar] [CrossRef]
- Hwang, I.H.; Aoyama, H.; Matsuto, T.; Nakagishi, T.; Matsuo, T. Recovery of solid fuel from municipal solid waste by hydrothermal treatment using subcritical water. Waste Manag. 2012, 32, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ma, S.; Gao, J. Study on the Pressurized Hydrolysis Dechlorination of PVC. Energy Fuel 2002, 16, 1251–1255. [Google Scholar] [CrossRef]
- Kuhlmann, B.; Arnett, E.M.; Siskin, M. Classical Organic Reactions in Pure Superheated Water. J. Org. Chem. 1994, 59, 3098–3101. [Google Scholar] [CrossRef]
- Endo, K.; Emori, N. Dechlorination of poly (vinyl chloride) without anomalous units under high pressure and at high temperature in water. Polym. Degrad. Stabil. 2001, 74, 113–117. [Google Scholar] [CrossRef]
- Shun-Myung, S.; Yoshioka, T.; Okuwaki, A. Dehydrochlorination behavior of flexible PVC pellets in NaOH solutions at elevated temperature. J. Appl. Polym. Sci. 1998, 67, 2171–2177. [Google Scholar]
- Yoshinaga, T.; Yamaye, M.; Kito, T.; Ichiki, T.; Ogata, M.; Chen, J.; Fujino, H.; Tanimura, T.; Yamanobe, T. Alkaline dechlorination of poly (vinyl chloride) in organic solvents under mild conditions. Polym. Degrad. Stabil. 2004, 86, 541–547. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kameda, T.; Imai, S.; Noritsune, M.; Okuwaki, A. Dechlorination of poly (vinylidene chloride) in NaOH/ethylene glycol as a function of NaOH concentration, temperature, and solvent. Polym. Degrad. Stabil. 2008, 93, 1979–1984. [Google Scholar] [CrossRef]
- Yoshioka, T.; Furukawa, K.; Okuwaki, A. Chemical recycling of rigid-PVC by oxygen oxidation in NaOH solutions at elevated temperatures. Polym. Degrad. Stabil. 2000, 67, 285–290. [Google Scholar] [CrossRef]
- Yoshioka, T.; Kameda, T.; Imai, S.; Okuwaki, A. Dechlorination of poly (vinyl chloride) using NaOH in ethylene glycol under atmospheric pressure. Polym. Degrad. Stabil. 2008, 93, 1138–1141. [Google Scholar] [CrossRef]
- Yuan, G.; Keane, M.A. Liquid phase hydrodechlorination of chlorophenols over Pd/C and Pd/Al2O3: A consideration of HC1/catalyst interactions and solution pH effects. Appl. Catal. B Environ. 2004, 52, 301–314. [Google Scholar] [CrossRef]
- Weiner, B.; Baskyr, I.; Poerschmann, J.; Kopinke, F.D. Potential of the hydrothermal carbonization process for the degradation of organic pollutants. Chemosphere 2013, 92, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, T.; Kameda, T.; Ieshige, M.; Okuwaki, A. Dechlorination behaviour of flexible poly (vinyl chloride) in NaOH/EG solution. Polym. Degrad. Stabil. 2008, 93, 1822–1825. [Google Scholar] [CrossRef]
- Stromberg, R.R.; Straus, S.; Achhammer, B.G. Infrared spectra of thermally degraded poly (vinyl chloride). J. Res. Natl. Bur. Stand. 1958, 60, 147. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Zhao, P.; Lei, M.; Li, Z. Understanding Hydrothermal Dechlorination of PVC by Focusing on the Operating Conditions and Hydrochar Characteristics. Appl. Sci. 2017, 7, 256. https://doi.org/10.3390/app7030256
Li T, Zhao P, Lei M, Li Z. Understanding Hydrothermal Dechlorination of PVC by Focusing on the Operating Conditions and Hydrochar Characteristics. Applied Sciences. 2017; 7(3):256. https://doi.org/10.3390/app7030256
Chicago/Turabian StyleLi, Tian, Peitao Zhao, Meng Lei, and Zhaozhi Li. 2017. "Understanding Hydrothermal Dechlorination of PVC by Focusing on the Operating Conditions and Hydrochar Characteristics" Applied Sciences 7, no. 3: 256. https://doi.org/10.3390/app7030256





