Line-Scan Hyperspectral Imaging Techniques for Food Safety and Quality Applications
Abstract
:1. Introduction
2. Line-Scan Hyperspectral Measurement Techniques
2.1. Spectroscopy Techniques
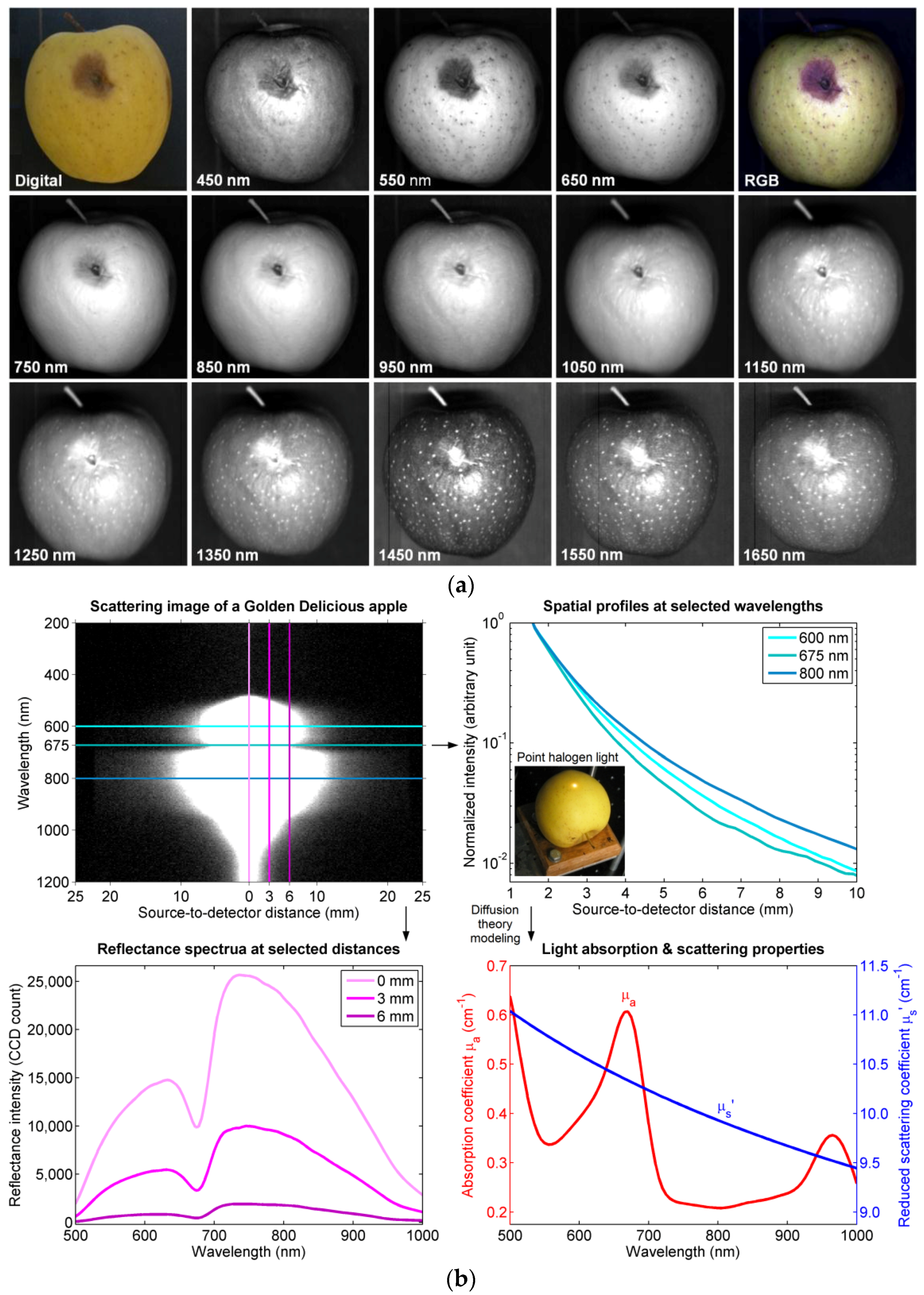
2.1.1. Reflectance and Transmittance Spectroscopy
2.1.2. Fluorescence Spectroscopy
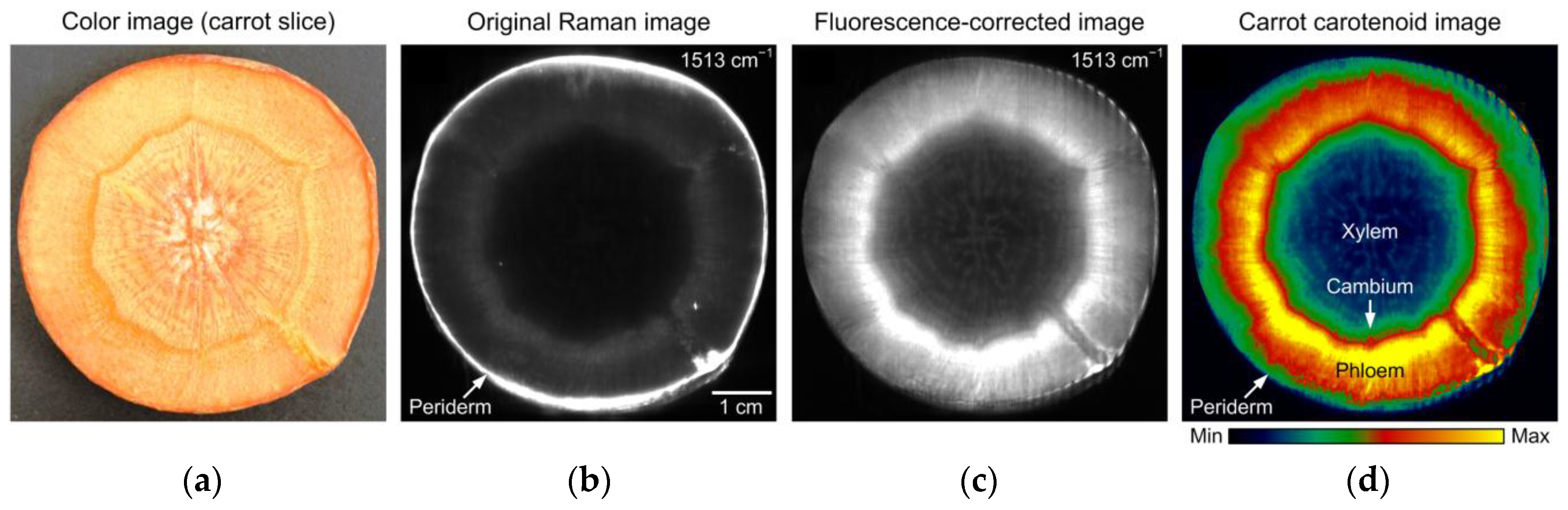
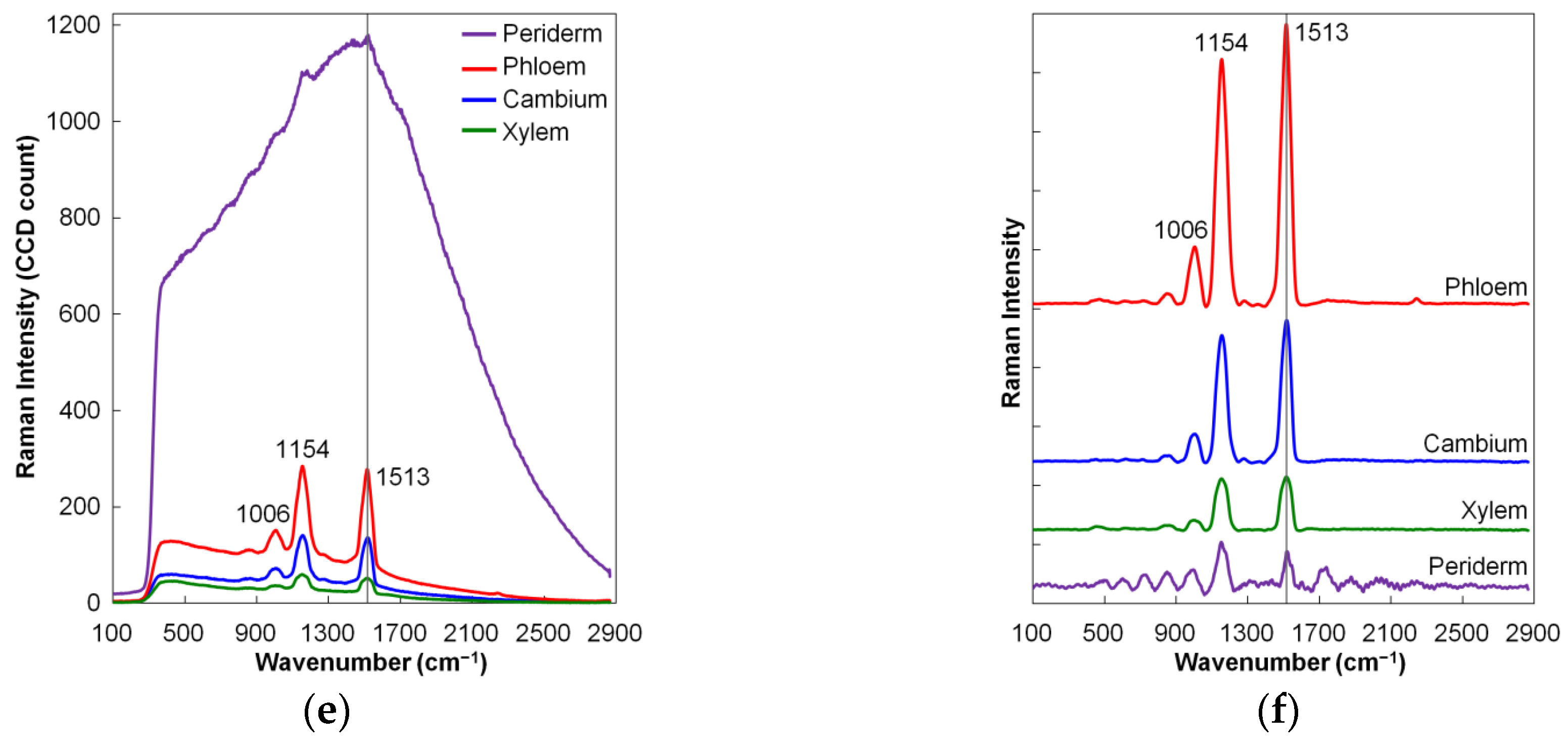
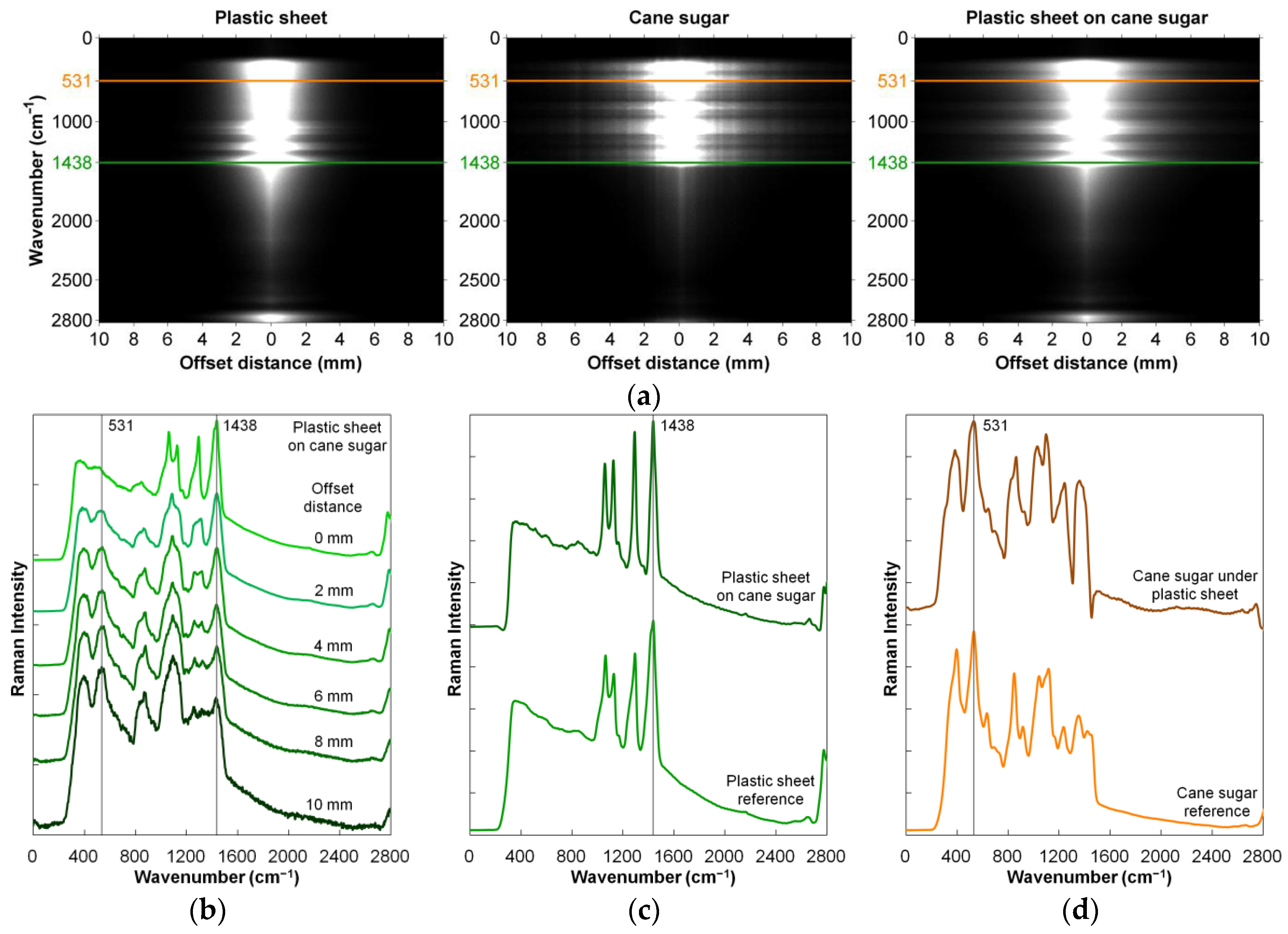
2.1.3. Raman Spectroscopy
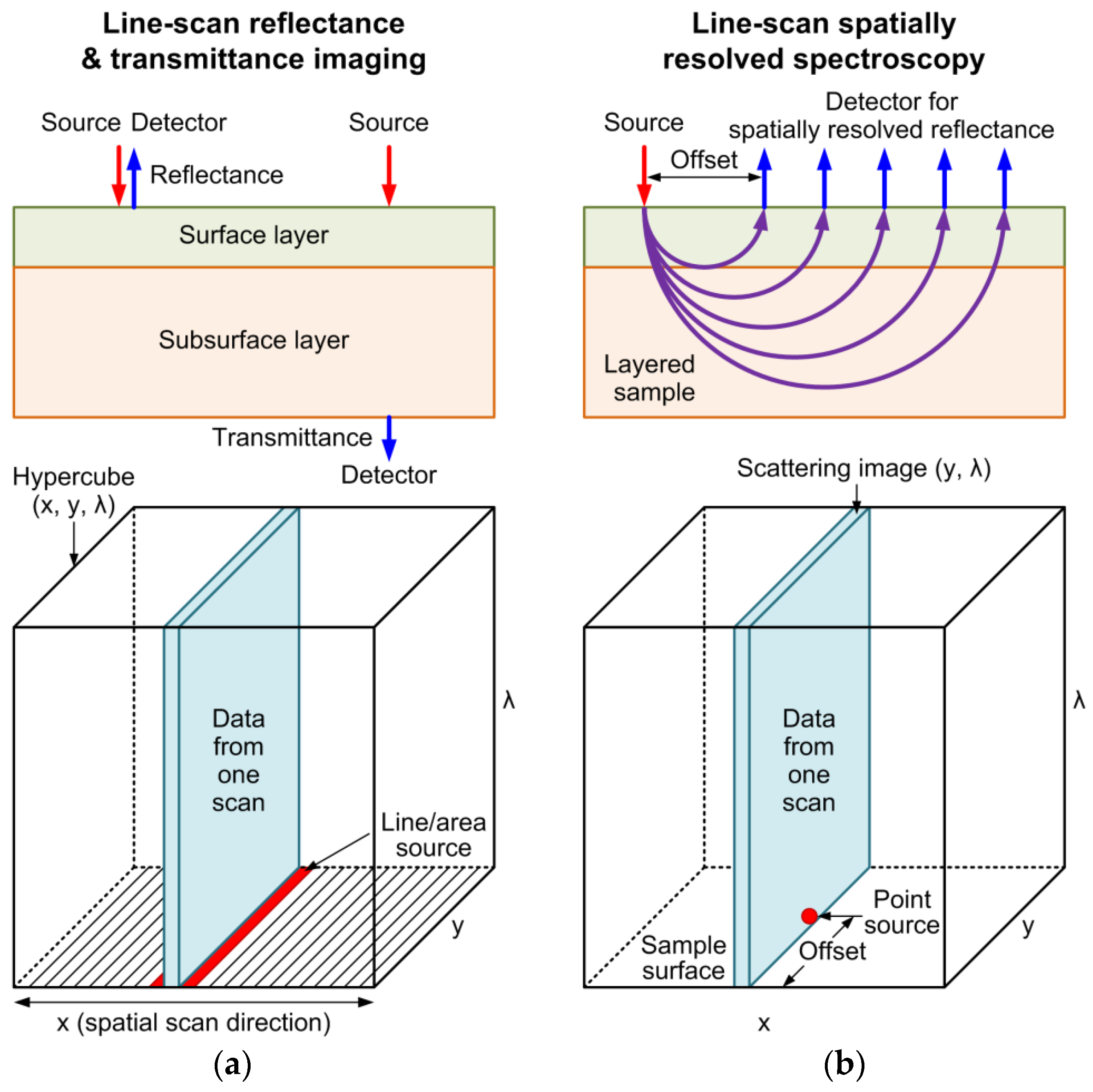
2.2. Line-Scan Measurement Methods
3. Line-Scan Hyperspectral Imaging Instruments
3.1. Major System Components
3.1.1. Light Sources
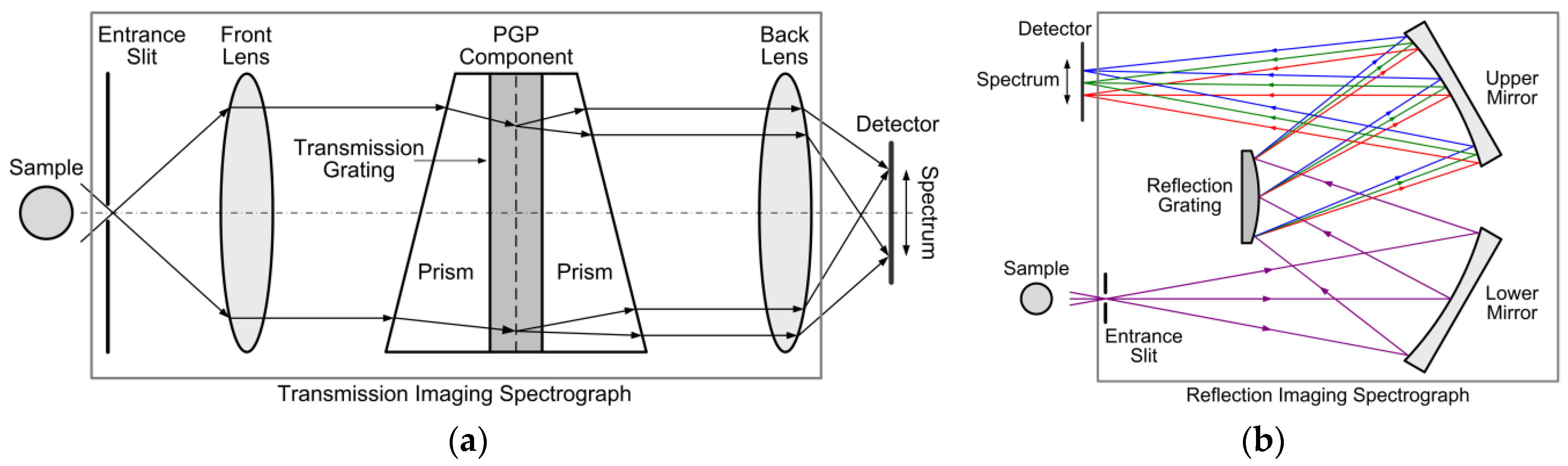
3.1.2. Imaging Spectrographs
3.1.3. Image Sensors
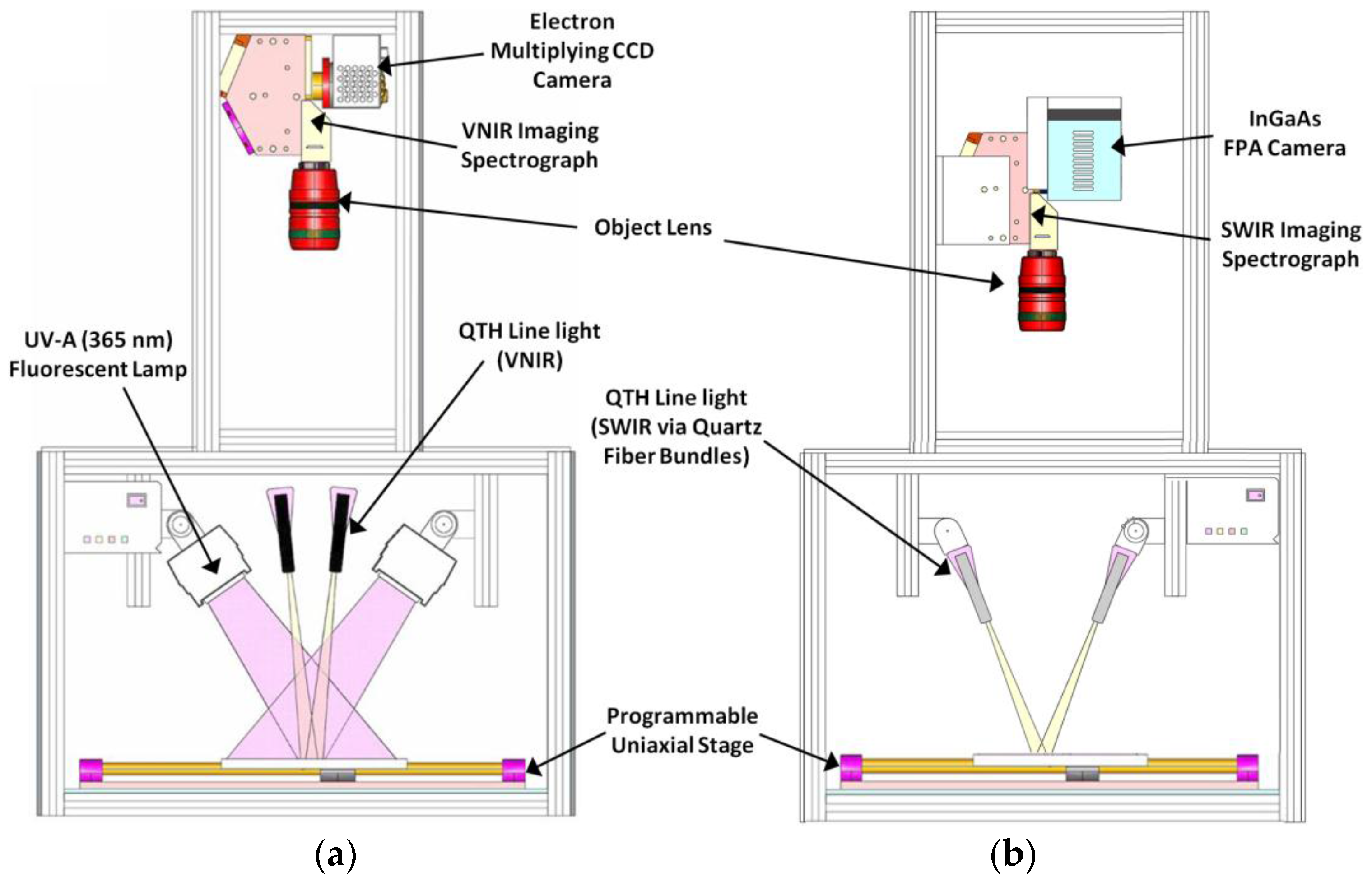
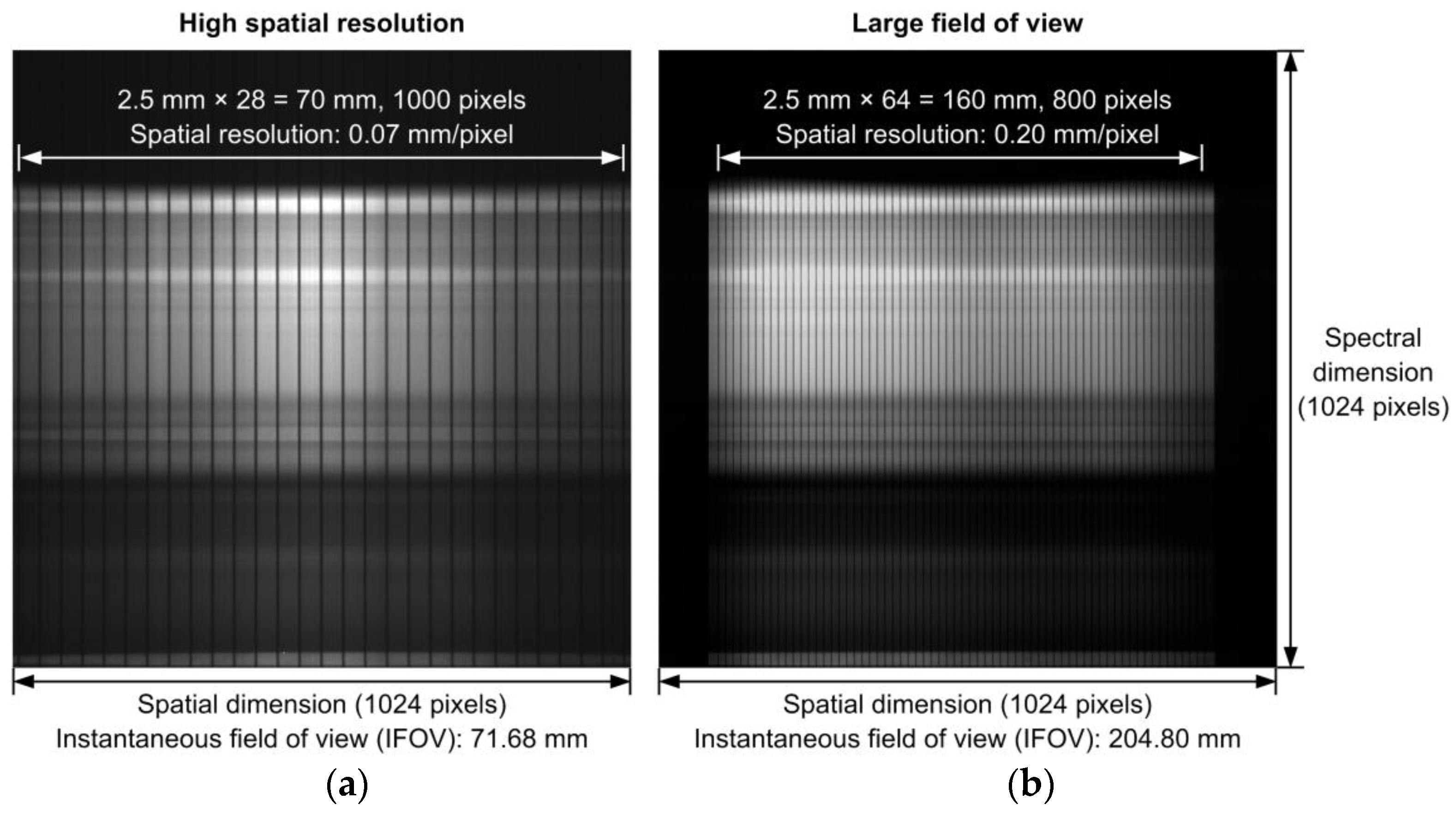
3.2. Line-Scan Hyperspectral Systems and Calibrations
4. Hyperspectral Image Analysis Methods
4.1. Line-Scan Hyperspectral Image Data
4.2. Hyperspectral Image Preprocessing
4.3. Hyperspectral Band Selection
4.4. Hyperspectral Target Identification
5. Food Safety and Quality Applications
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Qin, J.; Chao, K.; Kim, M.S.; Lu, R.; Burks, T.F. Hyperspectral and multispectral imaging for evaluating food safety and quality. J. Food Eng. 2013, 118, 157–171. [Google Scholar] [CrossRef]
- Lu, R.; Chen, Y. Hyperspectral imaging for safety inspection of food and agricultural products. Proc. SPIE 1999, 3544. [Google Scholar] [CrossRef]
- Kim, M.S.; Chen, Y.; Mehl, P.M. Hyperspectral reflectance and fluorescence imaging system for food quality and safety. Trans. ASAE 2001, 44, 721–729. [Google Scholar]
- Huang, H.; Liu, L.; Ngadi, M.O. Recent developments in hyperspectral imaging for assessment of food quality and safety. Sensors 2014, 14, 7248–7276. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chao, K.; Cho, B.; Peng, Y.; Kim, M.S. High-throughput Raman chemical imaging for rapid evaluation of food safety and quality. Trans. ASABE 2014, 57, 1783–1792. [Google Scholar]
- Kim, M.S.; Lefcourt, A.M.; Chen, Y.; Kim, I.; Chan, D.E.; Chao, K. Multispectral detection of fecal contamination on apples based on hyperspectral imagery. Part II. Application of hyperspectral fluorescence imaging. Trans. ASAE 2012, 45, 2039–2047. [Google Scholar]
- McCreery, R.L. Raman Spectroscopy for Chemical Analysis; John Wiley and Sons: New York, NY, USA, 2000. [Google Scholar]
- Dam, J.S.; Pedersen, C.B.; Dalgaard, T.; Fabricius, P.E.; Aruna, P.; Andersson-Engels, S. Fiber-optic probe for noninvasive real-time determination of tissue optical properties at multiple wavelengths. Appl. Opt. 2001, 40, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Hyde, D.E.; Farrell, T.J.; Patterson, M.S.; Wilson, B.C. A diffusion theory model of spatially resolved fluorescence from depth-dependent fluorophore concentrations. Phys. Med. Biol. 2001, 46, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Matousek, P.; Clark, I.P.; Draper, E.R.C.; Morris, M.D.; Goodship, A.E.; Everall, N.; Towrie, M.; Finney, W.F.; Parker, A.W. Subsurface probing in diffusely scattering media using spatially offset Raman spectroscopy. Appl. Spectrosc. 2005, 59, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Lu, R. Measurement of the optical properties of fruits and vegetables using spatially resolved hyperspectral diffuse reflectance imaging technique. Postharvest Biol. Technol. 2008, 49, 355–365. [Google Scholar] [CrossRef]
- Qin, J.; Kim, M.S.; Schmidt, W.F.; Cho, B.; Peng, Y.; Chao, K. A line-scan hyperspectral Raman system for spatially offset Raman spectroscopy. J. Raman Spectrosc. 2016, 47, 437–443. [Google Scholar] [CrossRef]
- Kim, M.S.; Delwiche, S.R.; Chao, K.; Garrido-Varo, A.; Pérez-Marín, D.; Lefcourt, A.M.; Chan, D.E. Visible to SWIR hyperspectral imaging for produce safety and quality evaluation. Sens. Instrum. Food Qual. Saf. 2011, 5, 155–164. [Google Scholar] [CrossRef]
- Qin, J.; Kim, M.S.; Chao, K.; Schmidt, W.F.; Cho, B.; Delwiche, S.R. Line-scan Raman imaging and spectroscopy platform for surface and subsurface evaluation of food safety and quality. J. Food Eng. 2017, 198, 17–27. [Google Scholar] [CrossRef]
- Qin, J.; Lu, R.; Peng, Y. Prediction of apple internal quality using spectral absorption and scattering properties. Trans. ASABE 2009, 52, 499–507. [Google Scholar] [CrossRef]
- Schulze, G.; Jirasek, A.; Yu, M.M.L.; Lim, A.; Turner, R.F.B.; Blades, M.W. Investigation of selected baseline removal techniques as candidates for automated implementation. Appl. Spectrosc. 2005, 59, 545–574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, S.; Liang, Y. Baseline correction using adaptive iteratively reweighted penalized least squares. Analyst 2010, 135, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chao, K.; Kim, M.S.; Kang, S.; Cho, B.; Jun, W. Detection of organic residues on poultry processing equipment surfaces by LED-induced fluorescence imaging. Appl. Eng. Agric. 2011, 27, 153–161. [Google Scholar] [CrossRef]
- Chang, C.I. An information theoretic-based approach to spectral variability, similarity and discriminability for hyperspectral image analysis. IEEE Trans. Inf. Theory 2000, 46, 1927–1932. [Google Scholar] [CrossRef]
- Windig, W.; Guilment, J. Interactive self-modeling mixture analysis. Anal. Chem. 1991, 63, 1425–1432. [Google Scholar] [CrossRef]
- Qin, J.; Kim, M.S.; Chao, K.; Schmidt, W.F.; Dhakal, S.; Cho, B.; Peng, Y.; Huang, M. Subsurface inspection of food safety and quality using line-scan spatially offset Raman spectroscopy technique. Food Control 2017, 75, 246–254. [Google Scholar] [CrossRef]
- Lee, W.S.; Alchanatis, V.; Yang, C.; Hirafuji, M.; Moshou, D.; Li, C. Sensing technologies for precision specialty crop production. Comput. Electron. Agric. 2010, 74, 2–33. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Q.; Huang, D. A review of imaging techniques for plant phenotyping. Sensors 2014, 14, 20078–20111. [Google Scholar] [CrossRef] [PubMed]
- Lu, R. Detection of bruises on apples using near-infrared hyperspectral imaging. Trans. ASAE 2003, 46, 523–530. [Google Scholar]
- Qin, J.; Chao, K.; Kim, M.S.; Cho, B. Line-scan macro-scale Raman chemical imaging for authentication of powdered foods and ingredients. Food Bioprocess Technol. 2016, 9, 113–123. [Google Scholar] [CrossRef]
- Huang, M.; Wan, X.; Zhang, M.; Zhu, Q. Detection of insect-damaged vegetable soybeans using hyperspectral transmittance image. J. Food Eng. 2013, 116, 45–49. [Google Scholar] [CrossRef]
- Wold, J.; Kermit, M.; Segtnan, V. Chemical imaging of heterogeneous muscle foods using near-infrared hyperspectral imaging in transmission mode. Appl. Spectrosc. 2016, 70, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Ariana, D.P.; Lu, R. Quality evaluation of pickling cucumbers using hyperspectral reflectance and transmittance imaging: Part I. Development of a prototype. Sens. Instrum. Food Qual. Saf. 2008, 2, 144–151. [Google Scholar] [CrossRef]
- Zhu, F.; Yao, H.; Hruska, Z.; Kincaid, R.; Brown, R.; Bhatnagar, D.; Cleveland, T. Integration of fluorescence and reflectance visible near-infrared (VNIR) hyperspectral images for detection of aflatoxins in corn kernels. Trans. ASABE 2016, 59, 785–794. [Google Scholar]
- Lu, R.; Peng, Y. Hyperspectral scattering for assessing peach fruit firmness. Biosyst. Eng. 2006, 93, 161–171. [Google Scholar] [CrossRef]
- Kim, M.S.; Chen, Y.; Cho, B.; Chao, K.; Yang, C.; Lefcourt, A.M.; Chan, D.E. Hyperspectral reflectance and fluorescence line-scan imaging for online defect and fecal contamination inspection of apples. Sens. Instrum. Food Qual. Saf. 2007, 1, 151–159. [Google Scholar] [CrossRef]
- Chao, K.; Yang, C.; Kim, M.S.; Chan, D.E. High throughput spectral imaging system for wholesomeness inspection of chicken. Appl. Eng. Agric. 2008, 24, 475–485. [Google Scholar] [CrossRef]
- Kim, M.S.; Lefcourt, A.M.; Chao, K.; Chen, Y.; Kim, I.; Chan, D.E. Multispectral detection of fecal contamination on apples based on hyperspectral imagery. Part I. Application of visible and near-infrared reflectance imaging. Trans. ASAE 2002, 45, 2027–2037. [Google Scholar]
- Park, B.; Lawrence, K.C.; Windham, W.R.; Buhr, R.J. Hyperspectral imaging for detecting fecal and ingesta contaminants on poultry carcasses. Trans. ASAE 2002, 45, 2017–2026. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Wang, C.; Chan, D.E.; Kim, M.S. Development of a simple algorithm for the detection of chilling injury in cucumbers from visible/near-infrared hyperspectral imaging. Appl. Spectrosc. 2005, 59, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Ngadi, M.O.; Wang, N.; Gariepy, C.; Prasher, S.O. Pork quality and marbling level assessment using a hyperspectral imaging system. J. Food Eng. 2007, 83, 10–16. [Google Scholar] [CrossRef]
- ElMasry, G.; Wold, J.P. High-speed assessment of fat and water content distribution in fish fillets using online imaging spectroscopy. J. Agric. Food Chem. 2008, 56, 7672–7677. [Google Scholar] [CrossRef] [PubMed]
- Gowen, A.A.; O’Donnell, C.P.; Taghizadeh, M.; Cullen, P.J.; Frias, J.M.; Downey, G. Hyperspectral imaging combined with principal component analysis for bruise damage detection on white mushrooms (Agaricus bisporus). J. Chemom. 2008, 22, 259–267. [Google Scholar] [CrossRef]
- Naganathan, G.K.; Grimes, L.M.; Subbiah, J.; Calkins, C.R.; Samal, A.; Meyer, G.E. Visible/near-infrared hyperspectral imaging for beef tenderness prediction. Comput. Electron. Agric. 2008, 64, 225–233. [Google Scholar] [CrossRef]
- Qin, J.; Burks, T.F.; Ritenour, M.A.; Bonn, W.G. Detection of citrus canker using hyperspectral reflectance imaging with spectral information divergence. J. Food Eng. 2009, 93, 183–191. [Google Scholar] [CrossRef]
- Yoon, S.C.; Lawrence, K.C.; Siragusa, G.R.; Line, J.E.; Park, B.; Feldner, P.W. Hyperspectral reflectance imaging for detecting a foodborne pathogen: Campylobacter. Trans. ASABE 2009, 52, 651–662. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Q.; Cai, J.; Ouyang, Q. Automated tea quality classification by hyperspectral imaging. Appl. Opt. 2009, 48, 3557–3564. [Google Scholar] [CrossRef] [PubMed]
- Delwiche, S.R.; Kim, M.S.; Dong, Y. Fusarium damage assessment in wheat kernels by Vis/NIR hyperspectral imaging. Sens. Instrum. Food Qual. Saf. 2011, 5, 63–71. [Google Scholar] [CrossRef]
- Nguyen Do Trong, N.; Tsuta, M.; Nicolaï, B.M.; De Baerdemaeker, J.; Saeys, W. Prediction of optimal cooking time for boiled potatoes by hyperspectral imaging. J. Food Eng. 2011, 105, 617–624. [Google Scholar] [CrossRef]
- Baiano, A.; Terracone, C.; Peri, G.; Romaniello, R. Application of hyperspectral imaging for prediction of physico-chemical and sensory characteristics of table grapes. Comput. Electron. Agric. 2012, 87, 142–151. [Google Scholar] [CrossRef]
- Rajkumar, P.; Wang, N.; EImasry, G.; Raghavan, G.S.V.; Gariepy, Y. Studies on banana fruit quality and maturity stages using hyperspectral imaging. J. Food Eng. 2012, 108, 194–200. [Google Scholar] [CrossRef]
- Fu, X.; Kim, M.S.; Chao, K.; Qin, J.; Lim, J.; Lee, H.; Garrido-Varo, A.; Pérez-Marín, D.; Ying, Y. Detection of melamine in milk powders based on NIR hyperspectral imaging and spectral similarity analyses. J. Food Eng. 2014, 124, 97–104. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Makino, Y.; Oshita, S. Rapid and non-destructive detection of chicken adulteration in minced beef using visible near-infrared hyperspectral imaging and machine learning. J. Food Eng. 2016, 170, 8–15. [Google Scholar] [CrossRef]
- Qin, J.; Lu, R. Detection of pits in tart cherries by hyperspectral transmission imaging. Trans. ASAE 2005, 48, 1963–1970. [Google Scholar] [CrossRef]
- Coelho, P.A.; Soto, M.E.; Torres, S.N.; Sbarbaro, D.G.; Pezoa, J.E. Hyperspectral transmittance imaging of the shell-free cooked clam Mulinia edulis for parasite detection. J. Food Eng. 2013, 117, 408–416. [Google Scholar] [CrossRef]
- Song, D.; Song, L.; Sun, Y.; Hu, P.; Tu, K.; Pan, L.; Yang, H.; Huang, M. Black heart detection in white radish by hyperspectral transmittance imaging combined with chemometric analysis and a successive projections algorithm. Appl. Sci. 2016, 6, 249. [Google Scholar] [CrossRef]
- Kim, I.; Kim, M.S.; Chen, Y.; Kong, S.G. Detection of skin tumors on chicken carcasses using hyperspectral fluorescence imaging. Trans. ASAE 2004, 47, 1785–1792. [Google Scholar] [CrossRef]
- Noh, H.; Lu, R. Hyperspectral laser-induced fluorescence imaging for assessing apple fruit quality. Postharvest Biol. Technol. 2007, 43, 193–201. [Google Scholar] [CrossRef]
- Zhu, B.; Jiang, L.; Jin, F.; Qin, L.; Vogel, A.; Tao, Y. Walnut shell and meat differentiation using fluorescence hyperspectral imagery with ICA-kNN optimal wavelength selection. Sens. Instrum. Food Qual. Saf. 2007, 1, 123–131. [Google Scholar] [CrossRef]
- Jun, W.; Kim, M.S.; Lee, K.; Millner, P.; Chao, K. Assessment of bacterial biofilm on stainless steel by hyperspectral fluorescence imaging. Sens. Instrum. Food Qual. Saf. 2009, 3, 41–48. [Google Scholar] [CrossRef]
- Yao, H.; Hruska, Z.; Kincaid, R.; Brown, R.; Cleveland, T.; Bhatnagar, D. Correlation and classification of single kernel fluorescence hyperspectral data with aflatoxin concentration in corn kernels inoculated with Aspergillus flavus spores. Food Addit. Contam. Part A 2010, 27, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.; Kim, M.S.; Baek, I.; Kim, D.; Lee, W.; Kim, J.; Bae, H.; Kim, Y. Detection of cuticle defects on cherry tomatoes using hyperspectral fluorescence imagery. Postharvest Biol. Technol. 2013, 76, 40–49. [Google Scholar] [CrossRef]
- Mo, C.; Kim, M.S.; Lim, J.; Lee, K.; Kim, G.; Cho, B. Multispectral fluorescence imaging technique for discrimination of cucumber seed viability. Trans. ASABE 2015, 58, 959–968. [Google Scholar]
- Mustafic, A.; Jiang, Y.; Li, C. Cotton contamination detection and classification using hyperspectral fluorescence imaging. Text. Res. J. 2016, 86, 1574–1584. [Google Scholar] [CrossRef]
- Yoon, S.C.; Lawrence, K.C.; Smith, D.P.; Park, B.; Windham, W.R. Embedded bone fragment detection in chicken fillets using transmittance image enhancement and hyperspectral reflectance imaging. Sens. Instrum. Food Qual. Saf. 2008, 2, 197–207. [Google Scholar] [CrossRef]
- Wang, H.; Li, C.; Wang, M. Quantitative determination of onion internal quality using reflectance, interactance, and transmittance modes of hyperspectral imaging. Trans. ASABE 2013, 56, 1623–1635. [Google Scholar]
- Leiva-Valenzuela, G.A.; Lu, R.; Aguilera, J.M. Assessment of internal quality of blueberries using hyperspectral transmittance and reflectance images with whole spectra or selected wavelengths. Innov. Food Sci. Emerg. Technol. 2014, 24, 2–13. [Google Scholar] [CrossRef]
- Noh, H.; Peng, Y.; Lu, R. Integration of hyperspectral reflectance and fluorescence imaging for assessing apple maturity. Trans. ASAE 2007, 50, 963–971. [Google Scholar] [CrossRef]
- Qin, J.; Lu, R. Measurement of the absorption and scattering properties of turbid liquid foods using hyperspectral imaging. Appl. Spectrosc. 2007, 61, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, J.; Wang, W.; Li, Y.; Wu, J.; Huang, H.; Gao, X.; Jiang, W. Potential prediction of the microbial spoilage of beef using spatially resolved hyperspectral scattering profiles. J. Food Eng. 2011, 102, 163–169. [Google Scholar] [CrossRef]




| Technique | Product | Application | Wavelength | Reference |
|---|---|---|---|---|
| Reflectance imaging | Apple | Feces detection | 450–851 nm | [33] |
| Chicken | Feces and ingesta detection | 400–900 nm | [34] | |
| Apple | Bruise detection | 900–1700 nm | [24] | |
| Cucumber | Chilling injury detection | 447–951 nm | [35] | |
| Pork | Quality evaluation | 430–1000 nm | [36] | |
| Chicken | Wholesomeness inspection | 430–750 nm | [32] | |
| Fish | Moisture and fat evaluation | 460–1040 nm | [37] | |
| Mushroom | Bruise detection | 400–1000 nm | [38] | |
| Beef | Tenderness evaluation | 400–1000 nm | [39] | |
| Citrus | Canker disease detection | 450–930 nm | [40] | |
| Pathogen | Campylobacter detection | 400–900 nm | [41] | |
| Tea | Quality classification | 408–1117 nm | [42] | |
| Wheat kernel | Fusarium damage detection | 400–1700 nm | [43] | |
| Potato | Cooking time prediction | 400–1000 nm | [44] | |
| Grape | Quality evaluation | 400–1000 nm | [45] | |
| Banana | Quality and maturity evaluation | 400–1000 nm | [46] | |
| Milk powder | Melamine detection | 990–1700 nm | [47] | |
| Beef | Chicken adulterant detection | 400–1000 nm | [48] | |
| Transmittance imaging | Cherry | Pit detection | 450–1000 nm | [49] |
| Clam | Parasite detection | 400–1000 nm | [50] | |
| Soybean | Insect damage detection | 400–1000 nm | [26] | |
| Radish | Black heart detection | 400–1000 nm | [51] | |
| Pork and salmon | Fat mapping | 760–1040 nm | [27] | |
| Fluorescence imaging | Apple | Feces detection | 425–775 nm | [6] |
| Chicken | Skin tumor detection | 425–710 nm | [52] | |
| Apple | Quality evaluation | 500–1040 nm | [53] | |
| Walnut | Shell and meat differentiation | 425–775 nm | [54] | |
| Bacterial biofilm | Equipment surface inspection | 416–700 nm | [55] | |
| Corn kernel | Aflatoxin detection | 400–600 nm | [56] | |
| Chicken residues | Equipment surface inspection | 500–700 nm | [18] | |
| Cherry tomato | Cuticle defect detection | 400–700 nm | [57] | |
| Cucumber seed | Seed viability identification | 425–700 nm | [58] | |
| Cotton | Foreign matter detection | 425–700 nm | [59] | |
| Reflectance and transmittance imaging | Cucumber | Quality evaluation | 400–1000 nm | [28] |
| Chicken | Bone fragment detection | 364–1024 nm | [60] | |
| Onion | Internal quality evaluation | 400–1000 nm | [61] | |
| Blueberry | Internal quality evaluation | 400–1000 nm | [62] | |
| Reflectance and fluorescence imaging | Apple | Defect and feces detection | 400–1000 nm | [31] |
| Apple | Maturity and quality evaluation | 500–1000 nm | [63] | |
| Corn kernel | Aflatoxin detection | 400–878 nm | [29] | |
| Raman imaging | Food powders | Adulterant detection | 102–2865 cm−1 | [25] |
| Carrot | Carotenoid mapping | 102–2865 cm−1 | [14] | |
| Spatially resolved spectroscopy | Peach | Firmness evaluation | 500–1000 nm | [30] |
| Milk | Fat content evaluation | 530–900 nm | [64] | |
| Apple | Internal quality evaluation | 500–1000 nm | [15] | |
| Beef | Microbial spoilage detection | 400–1100 nm | [65] | |
| Sugar | Through-package detection | 102–2865 cm−1 | [21] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, J.; Kim, M.S.; Chao, K.; Chan, D.E.; Delwiche, S.R.; Cho, B.-K. Line-Scan Hyperspectral Imaging Techniques for Food Safety and Quality Applications. Appl. Sci. 2017, 7, 125. https://doi.org/10.3390/app7020125
Qin J, Kim MS, Chao K, Chan DE, Delwiche SR, Cho B-K. Line-Scan Hyperspectral Imaging Techniques for Food Safety and Quality Applications. Applied Sciences. 2017; 7(2):125. https://doi.org/10.3390/app7020125
Chicago/Turabian StyleQin, Jianwei, Moon S. Kim, Kuanglin Chao, Diane E. Chan, Stephen R. Delwiche, and Byoung-Kwan Cho. 2017. "Line-Scan Hyperspectral Imaging Techniques for Food Safety and Quality Applications" Applied Sciences 7, no. 2: 125. https://doi.org/10.3390/app7020125
APA StyleQin, J., Kim, M. S., Chao, K., Chan, D. E., Delwiche, S. R., & Cho, B.-K. (2017). Line-Scan Hyperspectral Imaging Techniques for Food Safety and Quality Applications. Applied Sciences, 7(2), 125. https://doi.org/10.3390/app7020125







