Rapid Synthesis of Gold Nanoparticles from Quercus incana and Their Antimicrobial Potential against Human Pathogens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Extract
2.2. Synthesis of Nanoparticles
2.2.1. UV–Visible Spectrometric Analysis
2.2.2. Transmission Electron Microscopy (TEM)
2.2.3. Fourier-Transform Infrared Spectroscopy
2.2.4. Identification of Compounds by GC–MS Analysis
2.2.5. Antibacterial Potential of GNPs
2.2.6. Antifungal Potential of GNPs
3. Results and Discussion
3.1. Characterization of Gold Nanoparticles
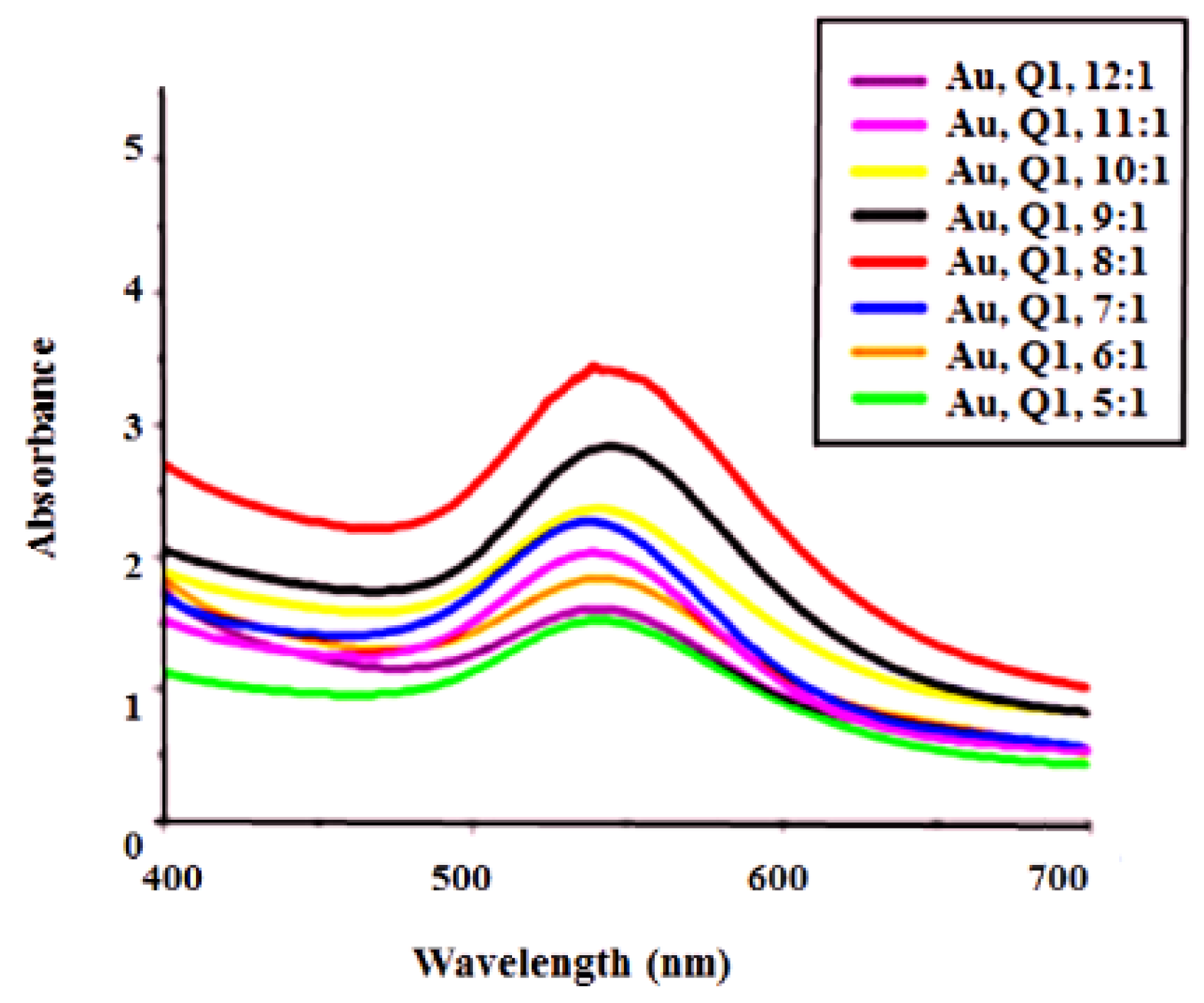
UV–Visible Analysis
3.2. Stability of Gold Nanoparticles
3.2.1. Stability with the Time
3.2.2. Heat Stability
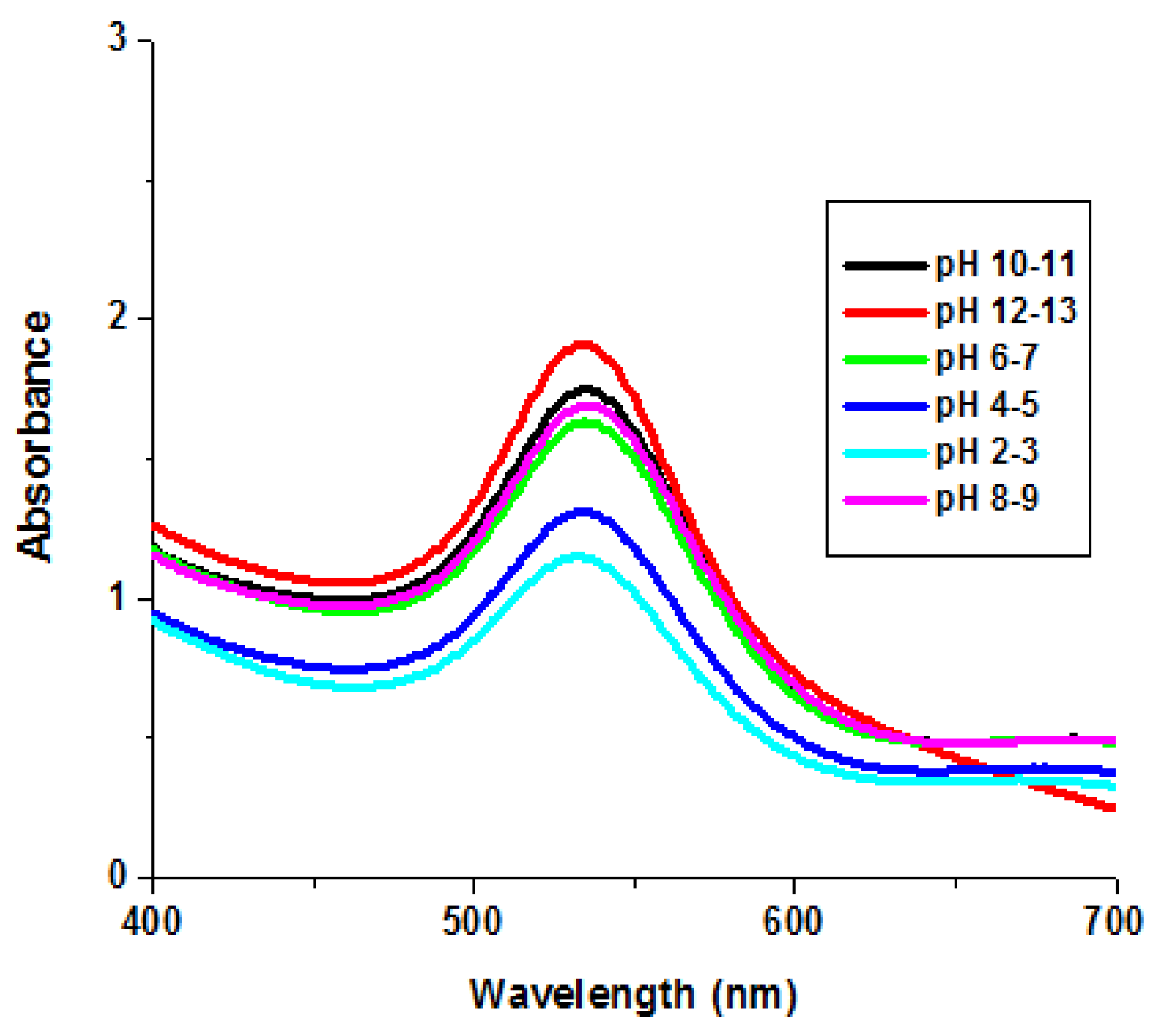
3.2.3. pH Stability of GNPs
3.3. Spectroscopy (FT-IR)
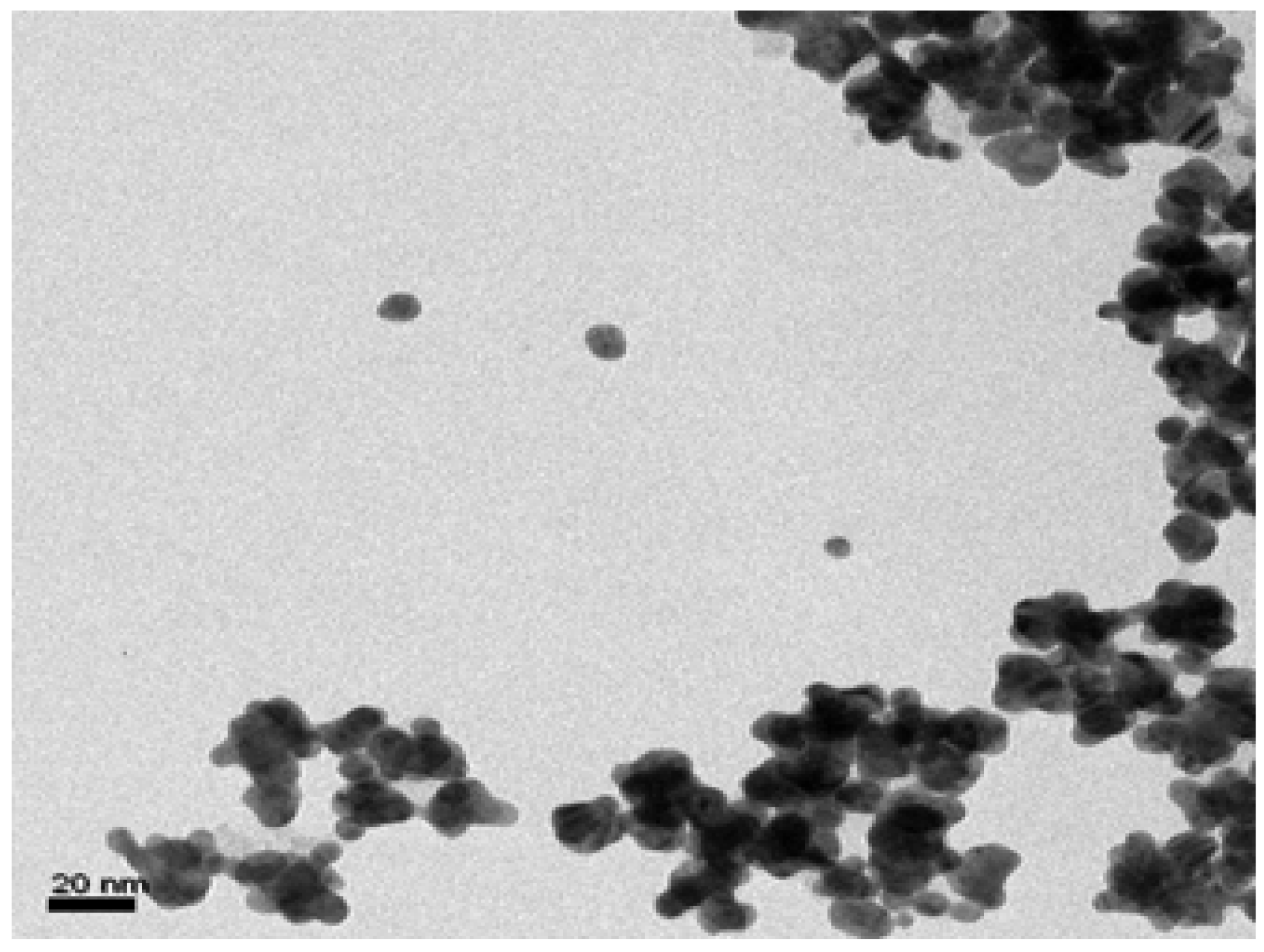
3.4. Transmission Electron Microscopy (TEM)
3.5. GC–MS Analysis of Ethyl Acetate Extract of Quercus incana
3.6. Qualitative Analysis
3.7. Antibacterial Activity
3.8. Antifungal Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| GNPs | Gold Nanoparticles |
| TEM | Transmission Electron Microscopy |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| SPR | Surface Plasmon Resonance |
References
- Sujatha, S.; Tamilselvi, S.; Subha, K.; Panneerselvam, A. Studies on biosynthesis of silver nanoparticles using Mushroom and its antibacterial activities. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 605–614. [Google Scholar]
- Pandiyarajan, T.; Udayabhaskar, R.; Vignesh, S.; James, R.A.; Karthikeyan, B. Synthesis and concentration dependent antibacterial activities of CuO nanoflakes. Mater. Sci. Eng. C 2013, 33, 2020–2024. [Google Scholar] [CrossRef] [PubMed]
- Surya, M. Biocompatible synthesis of silver nanoparticles (AgNPs) using marine Vibrio sp. and study their pharmacological applications. Int. J. Phytopharm. 2015, 5, 65–71. [Google Scholar]
- Kundu, S.; Wang, K.; Liang, H. Size-controlled synthesis and self-assembly of silver nanoparticles within a minute using microwave irradiation. J. Phys. Chem. C 2008, 113, 134–141. [Google Scholar] [CrossRef]
- Tsuji, M.; Hashimoto, M.; Nishizawa, Y.; Tsuji, T. Preparation of gold nanoplates by a microwave-polyol method. Chem. Lett. 2003, 32, 1114–1115. [Google Scholar] [CrossRef]
- Kim, Y.-P.; Oh, E.; Hong, M.-Y.; Lee, D.; Han, M.-K.; Shon, H.K.; Moon, D.W.; Kim, H.-S.; Lee, T.G. Gold nanoparticle-enhanced secondary ion mass spectrometry imaging of peptides on self-assembled monolayers. Anal. Chem. 2006, 78, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Sathy, B.N.; Mony, U.; Koyakutty, M.; Nair, S.V.; Menon, D. Biocompatible magnetite/gold nanohybrid contrast agents via green chemistry for MRI and CT bioimaging. ACS Appl. Mater. Interfaces 2011, 4, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, K.; Basha, S.K.; Kumar, V.G.; Singaravelu, G. Silver, gold and bimetallic nanoparticles production using single-cell protein (Spirulina platensis) Geitler. J. Mater. Sci. 2008, 43, 5115–5122. [Google Scholar] [CrossRef]
- Kowshik, M.; Deshmukh, N.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K. Microbial synthesis of semiconductor CdS nanoparticles, their characterization, and their use in the fabrication of an ideal diode. Biotechnol. Bioeng. 2002, 78, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Lengke, M.F.; Fleet, M.E.; Southam, G. Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver (I) nitrate complex. Langmuir 2007, 23, 2694–2699. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Astruc, D. Gold nanoparticles: Assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Laguna, A. Modern Supramolecular Gold Chemistry: Gold-Metal Interactions and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Zhou, J.; Ralston, J.; Sedev, R.; Beattie, D.A. Functionalized gold nanoparticles: Synthesis, structure and colloid stability. J. Colloid Interface Sci. 2009, 331, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Shipway, A.N.; Katz, E.; Willner, I. Nanoparticle arrays on surfaces for electronic, optical, and sensor applications. ChemPhysChem 2000, 1, 18–52. [Google Scholar] [CrossRef]
- Han, G.; Ghosh, P.; Rotello, V.M. Functionalized gold nanoparticles for drug delivery. Nanomedicine (Lond.) 2007, 2, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Hashmi, A.S.K.; Hutchings, G.J. Gold catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef] [PubMed]
- Quinet, E.; Piccolo, L.; Morfin, F.; Avenier, P.; Diehl, F.; Caps, V.; Rousset, J.-L. On the mechanism of hydrogen-promoted gold-catalyzed CO oxidation. J. Catal. 2009, 268, 384–389. [Google Scholar] [CrossRef]
- Turner, M.; Golovko, V.B.; Vaughan, O.P.; Abdulkin, P.; Berenguer-Murcia, A.; Tikhov, M.S.; Johnson, B.F.; Lambert, R.M. Selective oxidation with dioxygen by gold nanoparticle catalysts derived from 55-atom clusters. Nature 2008, 454, 981–983. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, L.; Stafford, R.; Bankson, J.; Sershen, S.; Rivera, B.; Price, R.; Hazle, J.; Halas, N.; West, J. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, Q.; Austin, L.; Coutts, J.; Knowles, G.; Zou, J.; Chen, H.; Huo, Q. A one-step homogeneous immunoassay for cancer biomarker detection using gold nanoparticle probes coupled with dynamic light scattering. J. Am. Chem. Soc. 2008, 130, 2780–2782. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Yuan, R.; Chai, Y. Biochemical and immunochemical characterization of the antigen–antibody reaction on a non-toxic biomimetic interface immobilized red blood cells of crucian carp and gold nanoparticles. Biosens. Bioelectron. 2007, 22, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Tseng, W.L.; Huang, M.F.; Huang, Y.F.; Chang, H.T. Nanoparticle-filled capillary electrophoresis for the separation of long DNA molecules in the presence of hydrodynamic and electrokinetic forces. Electrophoresis 2005, 26, 3069–3075. [Google Scholar] [CrossRef] [PubMed]
- Kah, J.C.Y.; Kho, K.W.; Lee, C.G.L.; Richard, C.J. Early diagnosis of oral cancer based on the surface plasmon resonance of gold nanoparticles. Int. J. Nanomed. 2007, 2, 785–789. [Google Scholar]
- Dror-Ehre, A.; Mamane, H.; Belenkova, T.; Markovich, G.; Adin, A. Silver nanoparticle–E. coli colloidal interaction in water and effect on E. coli survival. J. Colloid Interface Sci. 2009, 339, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Eby, D.M.; Schaeublin, N.M.; Farrington, K.E.; Hussain, S.M.; Johnson, G.R. Lysozyme catalyzes the formation of antimicrobial silver nanoparticles. ACS Nano 2009, 3, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Sierra, J.F.; Ruiz, F.; Pena, D.C.C.; Martínez-Gutiérrez, F.; Martínez, A.E.; Guillén, A.D.J.P.; Tapia-Pérez, H.; Castañón, G.M. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomedicine 2008, 4, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; You-Sheng, O.-Y.; Chen, Y.-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, Y.; Yan, L.; Peltier, R.; Hui, W.; Yao, X.; Cui, Y.; Chen, X.; Sun, H.; Wang, Z. Interfacial Engineering of Bimetallic Ag/Pt Nanoparticles on Reduced Graphene Oxide Matrix for Enhanced Antimicrobial Activity. ACS Appl. Mater. Interfaces 2016, 8, 8834–8840. [Google Scholar] [CrossRef] [PubMed]
- Grace, A.N.; Pandian, K. Antibacterial efficacy of aminoglycosidic antibiotics protected gold nanoparticles—A brief study. Colloids Surfaces A 2007, 297, 63–70. [Google Scholar] [CrossRef]
- Burygin, G.; Khlebtsov, B.; Shantrokha, A.; Dykman, L.; Bogatyrev, V.; Khlebtsov, N. On the enhanced antibacterial activity of antibiotics mixed with gold nanoparticles. Nanoscale Res. Lett. 2009, 4, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, R.; Farooq, U.; Khan, A.; Naz, S.; Khan, S.; Khan, A.; Rauf, A.; Bahadar, H.; Uddin, R. Evaluation of Antioxidant, Free Radical Scavenging, and Antimicrobial Activity of Quercus incana Roxb. Front. Pharmacol. 2015, 6, 277. [Google Scholar] [CrossRef] [PubMed]
- Basavegowda, N.; Sobczak-Kupiec, A.; Malina, D.; Yathirajan, H.; Keerthi, V.; Chandrashekar, N.; Dinkar, S.; Liny, P. Plant mediated synthesis of gold nanoparticles using fruit extracts of Ananas comosus (L.)(Pineapple) and evaluation of biological activities. Adv. Mater. Lett. 2013, 4, 332–337. [Google Scholar] [CrossRef]
- Begum, N.A.; Mondal, S.; Basu, S.; Laskar, R.A.; Mandal, D. Biogenic synthesis of Au and Ag nanoparticles using aqueous solutions of Black Tea leaf extracts. Colloids Surfaces B Biointerfaces 2009, 71, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Jang, H.-K.; Kim, B.S. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochem. 2009, 44, 1133–1138. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X. Synthesis of polysaccharide-stabilized gold and silver nanoparticles: A green method. Carbohydr. Res. 2004, 339, 2627–2631. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.M.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arabian J. Chem. 2014, 7, 1131–1139. [Google Scholar] [CrossRef]
- Toderas, F.; Iosin, M.; Astilean, S. Luminescence properties of gold nanorods. Nuclear Instrum. Methods Phys. Res. Sect. B 2009, 267, 400–402. [Google Scholar] [CrossRef]
- Abdelhalim, M.A.K.; Mady, M.M.; Ghannam, M.M. Physical properties of different gold nanoparticles: Ultraviolet-visible and fluorescence measurements. J. Nanomed. Nanotechnol. 2012, 3, 133. [Google Scholar] [CrossRef]
- Pandey, S.; Oza, G.; Mewada, A.; Sharon, M. Green synthesis of highly stable gold nanoparticles using Momordica charantia as nano fabricator. Arch. Appl. Sci. Res. 2012, 4, 1135–1141. [Google Scholar]
- Vimalavady, A.; Kadavul, K. Phytocomponents identified on the various extracts of stem of Hugonia mystax L.(Linaceae). Eur. J. Exp. Biol. 2013, 3, 73–80. [Google Scholar]
- Laghari, A.Q.; Memon, S.; Nelofar, A.; Laghari, A.H. Structurally diverse alkaloids from Tecomella undulata G. Don flowers. J. King Saud Univ. Sci. 2014, 26, 300–304. [Google Scholar] [CrossRef]
- Jones, T.H.; Gorman, J.S.; Snelling, R.R.; Delabie, J.H.; Blum, M.S.; Garraffo, H.M.; Jain, P.; Daly, J.W.; Spande, T.F. Further alkaloids common to ants and frogs: Decahydroquinolines and a quinolizidine. J. Chem. Ecol. 1999, 25, 1179–1193. [Google Scholar] [CrossRef]
- Kumar, V.; Yadav, S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- William, E.C. Trease and Evans Pharmacology; Harcourt Brace and company. Asia. Pvt. Ltd.: Singapore, 1997. [Google Scholar]
- Harborne, J.B. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Chitravadivu, C.; Manian, S.; Kalaichelvi, K. Qualitative analysis of selected medicinal plants, Tamilnadu, India. Middle East J. Sci. Res. 2009, 4, 144–146. [Google Scholar]
- Jamil, M.; Ul Haq, I.; Mirza, B.; Qayyum, M. Isolation of antibacterial compounds from Quercus dilatata L. through bioassay guided fractionation. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Nagaraj, B.; Malakar, B.; Divya, T.; Krishnamurthy, N.; Liny, P.; Dinesh, R.; Iconaru, S.; Ciobanu, C. Synthesis of plant mediated gold nanoparticles using flower extracts of Carthamus tinctorius L. (safflower) and evaluation of their biological activities. Dig. J. Nanomater. Biostruct. 2012, 7, 1289–1296. [Google Scholar]
- Lutterodt, G.; Ismail, A.; Basheer, R.; Baharudin, H.M. Antimicrobial effects of Psidium guajava extract as one mechanism of its antidiarrhoeal action. Malays. J. Med. Sci. 1999, 6, 17–20. [Google Scholar] [PubMed]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [PubMed]





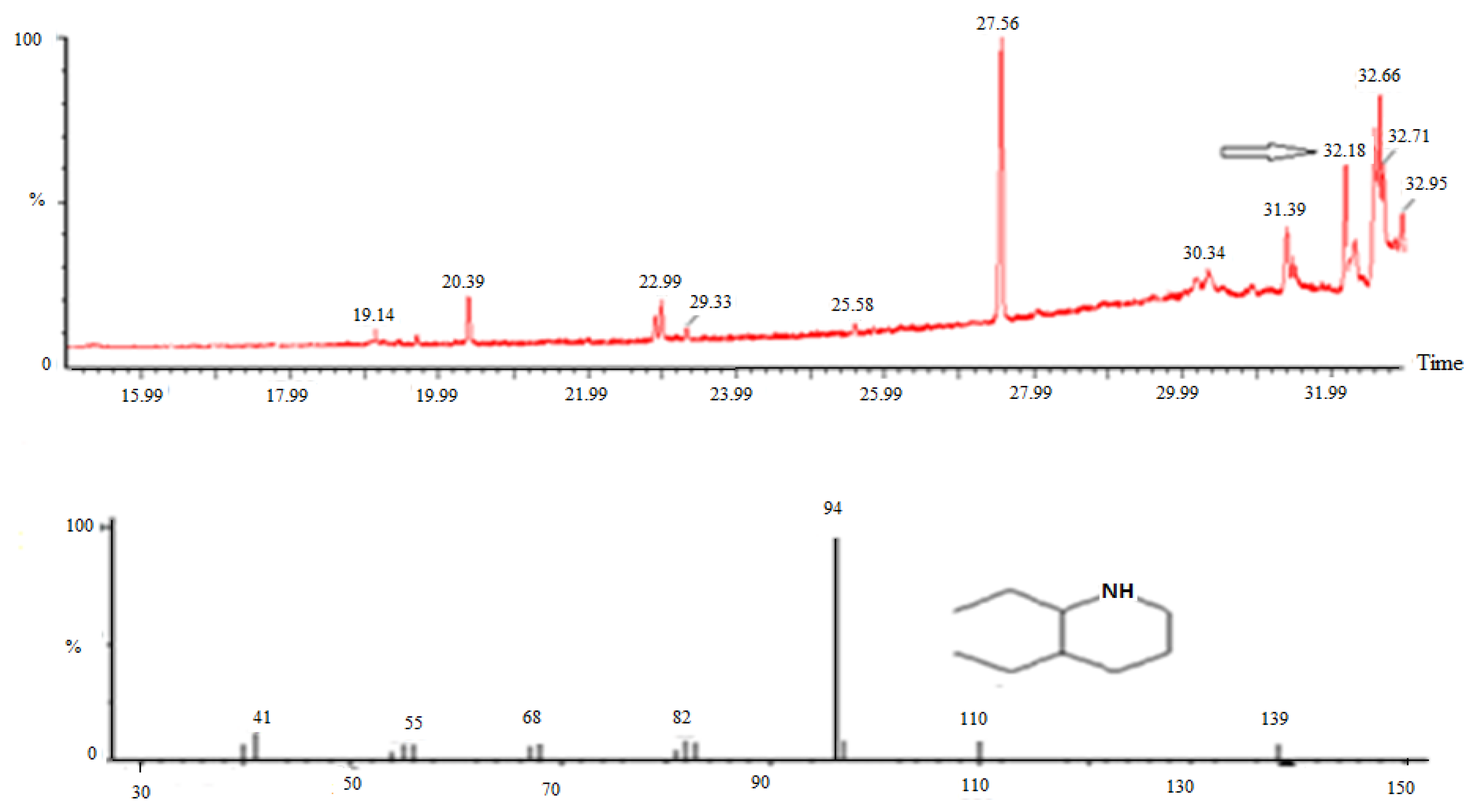
| S No. | Rt (min) | Compound | Molecular Formula | Molecular Weight | NIST ID |
|---|---|---|---|---|---|
| 1 | 20.39 | Cyclopentyl carboxylic acid | C6H10O2 | 114 | 3217 |
| 2 | 22.99 | 3-Dimethylaminocrylonitrile | C5H8N2 | 96 | 53,996 |
| 3 | 25.56 | 4-AminoFurazan-3-carbhydroamic acid | C3H5O2N5 | 143 | 262,446 |
| 4 | 27.56 | Dibutyl phthalate | C16H22O4 | 278 | 228,847 |
| 5 | 30.34 | 3,5-Dimethyldihydropyran-2,6-dione | C7H16O3 | 142 | 18,795 |
| 6 | 31.39 | 7,11-Hexadecadien-1-ol, acetate, | C18H32O2 | 280 | 130,865 |
| 7 | 32.18 | Quinoline, decahydro cis | C9H17N | 139 | 54,094 |
| 8 | 32.66 | (1-Methoxy-pentyl)-cyclopropane | C9H18O | 142 | 46,868 |
| Phytochemical | Result | Method | References |
|---|---|---|---|
| Alkaloids | + | Mayer’s test | [46] |
| Carbohydrates | + | Molish’s test | [46] |
| Glycoside | + | Bortrager test | [46] |
| Proteins and amino acids | + | Biuret test, Ninhydrin test | [47] |
| Flavonoids | + | Alkaline reagent test | [47] |
| Phenolic compounds | + | Lead acetate test | [47] |
| Bacterial Isolate | GNPs Zone of Inhibition (mm) | Plant extract Zone of Inhibition (mm) | Ciprofloxacin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 25 µg | 50 µg | 75 µg | 1 mg | 25 µg | 50 µg | 75 µg | 1 mg | 5 µg/mL | |
| B. subtilis | 6 | 8 | 9 | 17 | 0 | 0 | 0 | 8 | 13 |
| S. aureus | 0 | 4 | 6 | 12 | 0 | 0 | 0 | 11 | 22 |
| S. setubal | 3 | 5 | 7 | 15 | 0 | 0 | 0 | 8 | 24 |
| P. pickettii | 0 | 0 | 3 | 9 | 0 | 0 | 0 | 0 | 20 |
| Fungal Strain | GNPs Zone of Inhibition (mm) | Plant extract Zone of Inhibition (mm) | Nystatin | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 25 µg | 50 µg | 75 µg | 1 mg | 25 µg | 50 µg | 75 µg | 1 mg | 5 µg/mL | |
| Aspergillus flavus | 0 | 0 | 5 | 9 | 0 | 0 | 0 | 4 | 11 |
| Aspergillus niger | 0 | 3 | 5 | 7 | 0 | 0 | 0 | 6 | 17 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarwar, R.; Farooq, U.; Raza Shah, M.; Khan, S.; Riaz, N.; Naz, S.; Ibrar, A.; Khan, A. Rapid Synthesis of Gold Nanoparticles from Quercus incana and Their Antimicrobial Potential against Human Pathogens. Appl. Sci. 2017, 7, 29. https://doi.org/10.3390/app7010029
Sarwar R, Farooq U, Raza Shah M, Khan S, Riaz N, Naz S, Ibrar A, Khan A. Rapid Synthesis of Gold Nanoparticles from Quercus incana and Their Antimicrobial Potential against Human Pathogens. Applied Sciences. 2017; 7(1):29. https://doi.org/10.3390/app7010029
Chicago/Turabian StyleSarwar, Rizwana, Umar Farooq, Muhammad Raza Shah, Sara Khan, Nadia Riaz, Sadia Naz, Aliya Ibrar, and Ajmal Khan. 2017. "Rapid Synthesis of Gold Nanoparticles from Quercus incana and Their Antimicrobial Potential against Human Pathogens" Applied Sciences 7, no. 1: 29. https://doi.org/10.3390/app7010029
APA StyleSarwar, R., Farooq, U., Raza Shah, M., Khan, S., Riaz, N., Naz, S., Ibrar, A., & Khan, A. (2017). Rapid Synthesis of Gold Nanoparticles from Quercus incana and Their Antimicrobial Potential against Human Pathogens. Applied Sciences, 7(1), 29. https://doi.org/10.3390/app7010029







