Isolation, Identification, and Biotransformation of Teadenol A from Solid State Fermentation of Pu-erh Tea and In Vitro Antioxidant Activity
Abstract
:1. Introduction
2. Experimental Section
2.1. PET Fermentation and Sample Collection
2.2. Determination of Constituents in Tea Samples
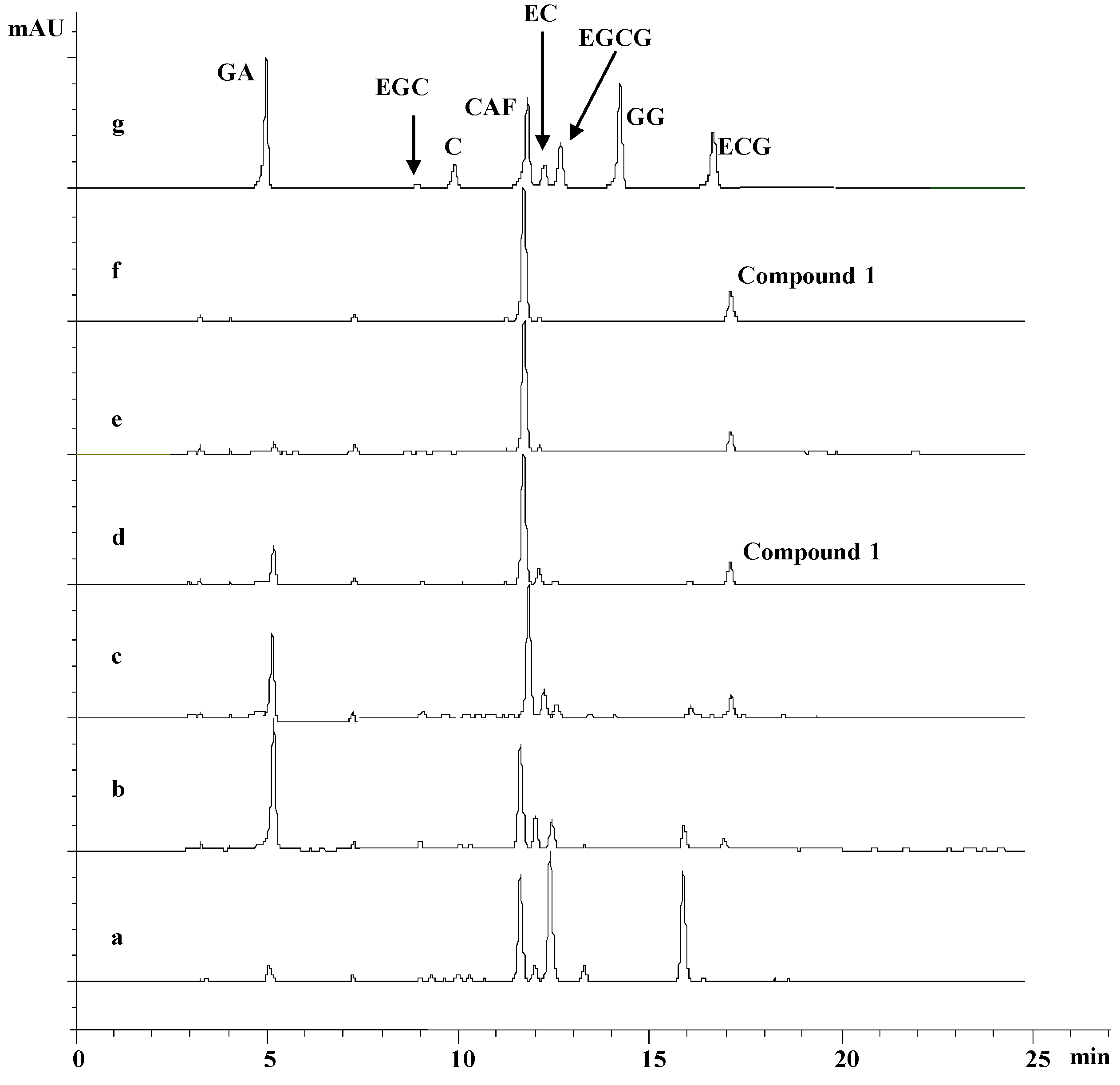
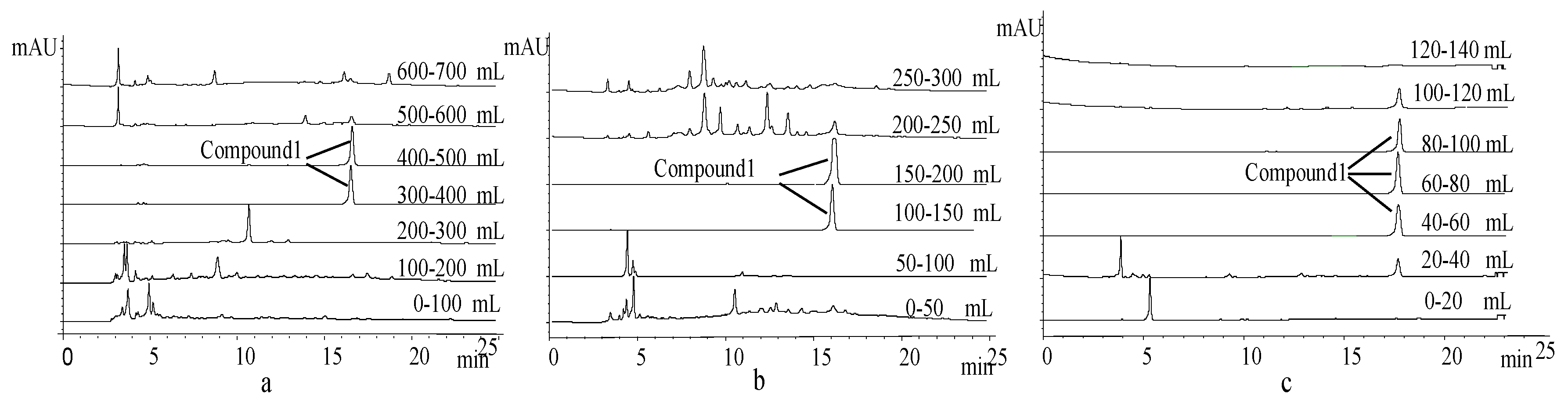
2.3. Extraction and Isolation of 1
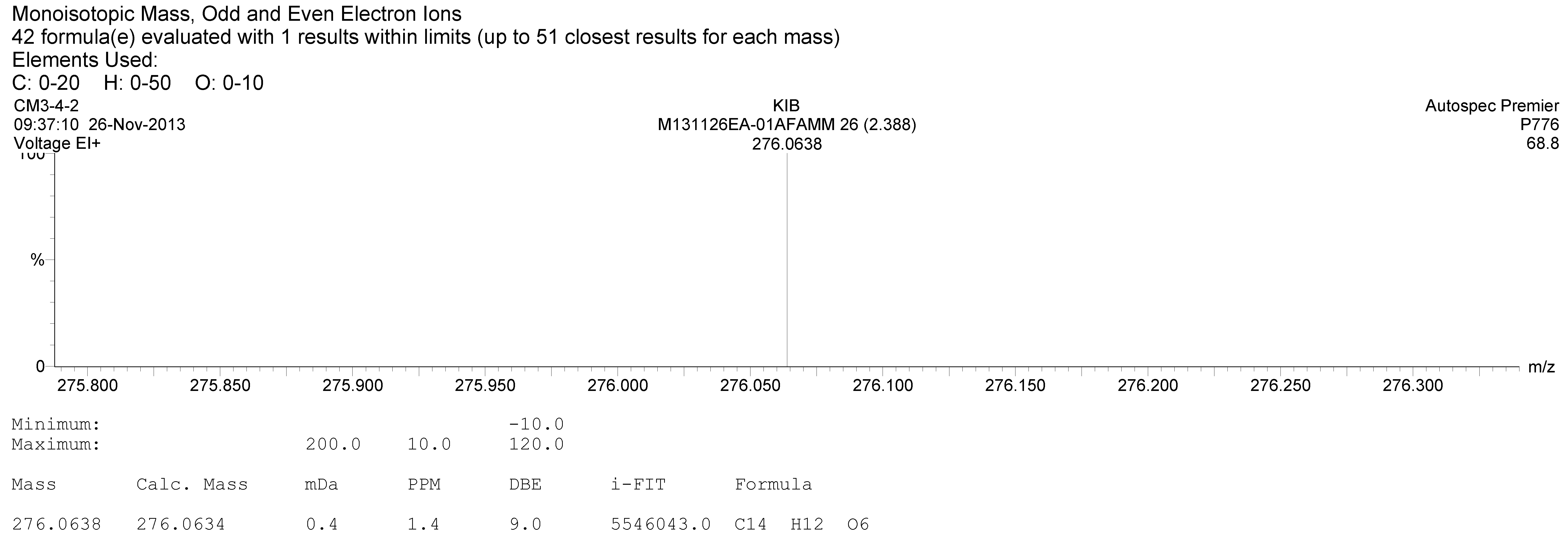
2.4. Structure Elucidation of 1
2.5. In Vitro Antioxidant Activity Assays
2.6. Screening of Compound 1 Producing Microbes
2.7. Determination of Compounds in Commercial PFPT Samples
2.8. Statistical Analyses
2.9. Nucleotide Sequence Accession Numbers
3. Results and Discussion
3.1. Isolation and Identification of Compound 1
3.2. Isolation and Identification of Fungal and Bacterial Strains
3.3. Determination of Teadenol A in Commercial PFPT
3.4. Bioactivity of Teadenol A
Acknowledgments
Author Contributions
Conflict of Interest
Abbreviations
| C | (+)-catechin |
| CAF | Caffeine |
| EC | (−)-epicatechin |
| ECG | (−)-epicatechin 3-O-gallate |
| EGC | (−)-epigallocatechin |
| EGCG | (−)-epigallocatechin 3-O-gallate |
| GA | gallic acid |
| GG | 1,4,6-tri-O-galloyl-β-D-glucose |
| HPLC | high-performance liquid chromatography |
| NA | Nutrient Agar |
| NFPT | non-fermented Pu-erh tea |
| PET | Pu-erh tea |
| PFPT | Post-fermented Pu-erh tea |
| RBMA | Rose Bengal Medium Agar |
| SSF | solid state fermentation |
| TB | theabrownin |
| TF | theaflavin |
| TR | thearubigin |
| UPLC-MS/MS | ultra-performance liquid chromatography tandem mass spectrometry |
References
- Chen, H.Y.; Lin-Shiau, S.Y.; Lin, J.K. Pu-erh tea its manufacturing and health benefits. In Tea and Tea Products: Chemistry and Health-Promoting Properties; Ho, C.T., Lin, J.K., Shahidi, F., Eds.; CRC Press: Boca Raton, FL, USA, 2008; pp. 9–16. [Google Scholar]
- Lv, H.; Zhang, Y.; Lin, Z.; Liang, Y. Processing and chemical constituents of Pu-erh tea: A review. Food Res. Int. 2013, 53, 608–618. [Google Scholar] [CrossRef]
- Huang, H.C.; Lin, J.K. Pu-erh tea, green tea, and black tea suppresses hyperlipidemia, hyperleptinemia and fatty acid synthase through activating AMPK in rats fed a high-fructose diet. Food Funct. 2012, 3, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Braud, L.; de Sousa, G.; Peyre, L.; Zeil, J.; Rahmani, R.; Maixent, J. 0261 Hao Ling pu-erh tea attenuates lipid accumulation in primary culture rat hepatocytes. Arch. Cardiovasc. Dis. Suppl. 2014, 6, 14. [Google Scholar] [CrossRef]
- Hou, C.W. Pu-Erh tea and GABA attenuates oxidative stress in kainic acid-induced status epilepticus. J. Biomed. Sci. 2011, 18, 75. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Luo, X.; Zhong, Y.; Yang, W.; Xu, M.; Liu, Y.; Meng, J.; Yao, P.; Yan, H.; Liu, L. Pu-erh black tea extract supplementation attenuates the oxidative DNA damage and oxidative stress in Sprague-Dawley rats with renal dysfunction induced by subchronic 3-methyl-2-quinoxalin benzenevinylketo-1,4-dioxide exposure. Food Chem. Toxicol. 2012, 50, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Qian, Y.; Zhou, Y.L.; Wang, R.; Wang, Q.; Li, G.J. Pu-erh Tea Has In Vitro Anticancer Activity in TCA8113 Cells and Preventive Effects on Buccal Mucosa Cancer in U14 Cells Injected Mice In Vivo. Nutr. Cancer 2014, 1059–1069. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, Q.; Qian, Y.; Zhou, Y.; Wang, R.; Zhao, X. Component analysis of Pu-erh and its anti-constipation effects. Mol. Med. Rep. 2014, 9, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.K.; Foo, K.Y. Recent advances on the beneficial use and health implications of Pu-Erh tea. Food Res. Int. 2013, 53, 619–628. [Google Scholar] [CrossRef]
- Wu, S.; Yen, G.; Wang, B.; Chiu, C.; Yen, W.; Chang, L.; Duh, P. Antimutagenic and antimicrobial activities of pu-erh tea. LWT - Food Sci. Technol. 2007, 40, 506–512. [Google Scholar] [CrossRef]
- Pei, S.; Zhang, Y.; Xu, H.; Chen, X.; Chen, S. Inhibition of the replication of hepatitis B virus in vitro by pu-erh tea extracts. J. Agric. Food Chem. 2011, 59, 9927–9934. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Shao, W.; Yuan, L.; Tu, P.; Ma, Z. Decreasing pro-inflammatory cytokine and reversing the immunosenescence with extracts of Pu-erh tea in senescence accelerated mouse (SAM). Food Chem. 2012, 135, 2222–2228. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Fu, H.; Yang, J.; Liu, G.; Dou, P.; Zhang, L.; Tu, P.; Wang, X. A randomized double-blind placebo-controlled study of Pu’er tea extract on the regulation of metabolic syndrome. Chin. J. Integr. Med. 2011, 17, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Sumi, S.; Tojo, H.; Sumi-Inoue, Y.; I-Chin, H.; Oi, Y.; Fujita, H.; Urata, H. Improvements of mean body mass index and body weight in preobese and overweight Japanese adults with black Chinese tea (Pu-Erh) water extract. Nutr. Res. 2011, 31, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Chou, J.I.; Ueng, K.; Chou, M.; Yang, J.; Lin-Shiau, S.; Hu, M.; Lin, J. Weight Reduction Effect of Puerh Tea in Male Patients with Metabolic Syndrome. Phytother. Res. 2014, 28, 1096–1101. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Zhu, Y.; Tan, J.; Guo, L.; Dai, W.; Lin, Z. Bioactive compounds from Pu-erh tea with therapy for hyperlipidaemia. J. Funct. Foods. 2015, 19, 194–203. [Google Scholar] [CrossRef]
- Peng, C.; Wang, Q.; Liu, H.; Gao, B.; Sheng, J.; Gong, J. Effects of Zijuan pu-erh tea theabrownin on metabolites in hyperlipidemic rat feces by Py-GC/MS. J. Anal. Appl. Pyrol. 2013, 104, 226–233. [Google Scholar] [CrossRef]
- Gong, J.; Peng, C.; Chen, T.; Gao, B.; Zhou, H. Effects of Theabrownin from Pu-erh Tea on the Metabolism of Serum Lipids in Rats: Mechanism of Action. J. Food. Sci. 2010, 75, H182–H189. [Google Scholar] [CrossRef] [PubMed]
- Oi, Y.; Hou, I.C.; Fujita, H.; Yazawa, K. Antiobesity effects of Chinese black tea (Pu-erh tea) extract and gallic acid. Phytother. Res. 2012, 26, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Jeng, K.C.; Chen, C.S.; Fang, Y.P.; Hou, R.C.; Chen, Y.S. Effect of microbial fermentation on content of statin, GABA, and polyphenols in Pu-Erh tea. J. Agric. Food. Chem. 2007, 55, 8787–8792. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.Y.; Wang, X.J.; Huang, Y.W.; Hao, S.M.; Sheng, J. Caffeine is responsible for the bloodglucose-lowering effects of green tea and Puer tea extractsin BALB/c mice. Chin. J. Nat. Med. 2015, 13, 595–601. [Google Scholar] [CrossRef]
- Deng, Y.T.; Lin-Shiau, S.Y.; Shyur, L.F.; Lin, J.K. Pu-erh tea polysaccharides decrease blood sugar by inhibition of alpha-glucosidase activity in vitro and in mice. Food. Funct. 2015, 6, 1539–1546. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Chen, S.; Chen, H.; Wang, Y.; Wang, Y.; Hochstetter, D.; Xu, P. Studies on the bioactivity of aqueous extract of pu-erh tea and its fractions: in vitro antioxidant activity and alpha-glycosidase inhibitory property, and their effect on postprandial hyperglycemia in diabetic mice. Food Chem. Toxicol. 2013, 53, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, L.; Wang, S.; Shi, S.; Jiang, Y.; Li, N.; Tu, P. 8-C N-ethyl-2-pyrrolidinone substituted flavan-3-ols as the marker compounds of Chinese dark teas formed in the post-fermentation process provide significant antioxidative activity. Food Chem. 2014, 152, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.P.; Fan, C.; Dong, W.M.; Gao, B.; Yuan, W.; Gong, J.S. Free radical scavenging and anti-oxidative activities of an ethanol-soluble pigment extract prepared from fermented Zijuan Pu-erh tea. Food Chem. Toxicol. 2013, 59, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, G.; Li, C.; Zhang, M.; Zhao, H.; Sheng, J.; Shi, W. Pu-erh tea reduces nitric oxide levels in rats by inhibiting inducible nitric oxide synthase expression through toll-like receptor 4. Int. J. Mol. Sci. 2012, 13, 7174–7185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Wang, C.F.; Shen, S.M.; Wang, G.L.; Liu, P.; Liu, Z.M.; Wang, Y.Y.; Du, S.S.; Liu, Z.L.; Deng, Z.W. Antioxidant phenolic compounds from Pu-erh tea. Molecules 2012, 17, 14037–14045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, D.; Chen, W.; Tan, X.; Wang, P. Impact of fermentation degree on the antioxidant activity of pu-erh tea in vitro. J. Food Biochem. 2012, 36, 262–267. [Google Scholar] [CrossRef]
- Lane, D.J. 16S/23S rRNA sequencing. In Nucleic acid techniques in bacterial systematics; Stackebrandt, E., Goodfellow, M.D., Eds.; Wiley: Chichester, UK, 1991; pp. 125–175. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes--application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Kim, O.S.; Cho, Y.J.; Lee, K.; Yoon, S.H.; Kim, M.; Na, H.; Park, S.C.; Jeon, Y.S.; Lee, J.H.; Yi, H.; et al. Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, R.A.; Amano, M.; Yanagita, T.; Tanaka, T.; Kouno, I.; Kawamura, D.; Ishimaru, K. New phenolic compounds from Camellia sinensis L. leaves fermented with Aspergillus sp. J. Nat. Med. 2011, 65, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, R.A.; Haraguchi, N.; Nakayama, H.; Furukawa, Y.; Tanaka, T.; Kouno, I.; Kawamura, D.; Ishimaru, K. HPLC and HPLC-TOFMS analyses of tea catechins and teadenols. Jpn. J. Food Chem. Saf. 2011, 18, 116–121. [Google Scholar]
- Yanagita, T.; Ishimaru, K.; Tanaka, T.; Koba, K.; Miyazaki, H.; Aoki, N.; Kawamura, D. Polyphenol derivative and method for producing the same. US8558016, 15 October 2013. [Google Scholar]
- Ukkola, O.; Santaniemi, M. Adiponectin: A link between excess adiposity and associated comorbidities? J. Mol. Med. 2002, 80, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Spranger, J.; Kroke, A.; Möhlig, M.; Bergmann, M.M.; Ristow, M.; Boeing, H.; Pfeiffer, A.F. Adiponectin and protection against type 2 diabetes mellitus. The Lancet 2003, 361, 226–228. [Google Scholar] [CrossRef]
- Matsuzawa, Y.; Funahashi, T.; Kihara, S.; Shimomura, I. Adiponectin and metabolic syndrome. Arterioscler..Thromb. Vasc. Biol. 2004, 24, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.O.; Ermolieff, J.; Jirousek, M.R. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nat. Rev. Drug Discov. 2002, 1, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, B.J. Protein-tyrosine phosphatase 1B (PTP1B): A novel therapeutic target for type 2 diabetes mellitus, obesity and related states of insulin resistance. Curr. Drug Targets Immune Endocr. Metab. Disord. 2001, 1, 265–275. [Google Scholar] [CrossRef]
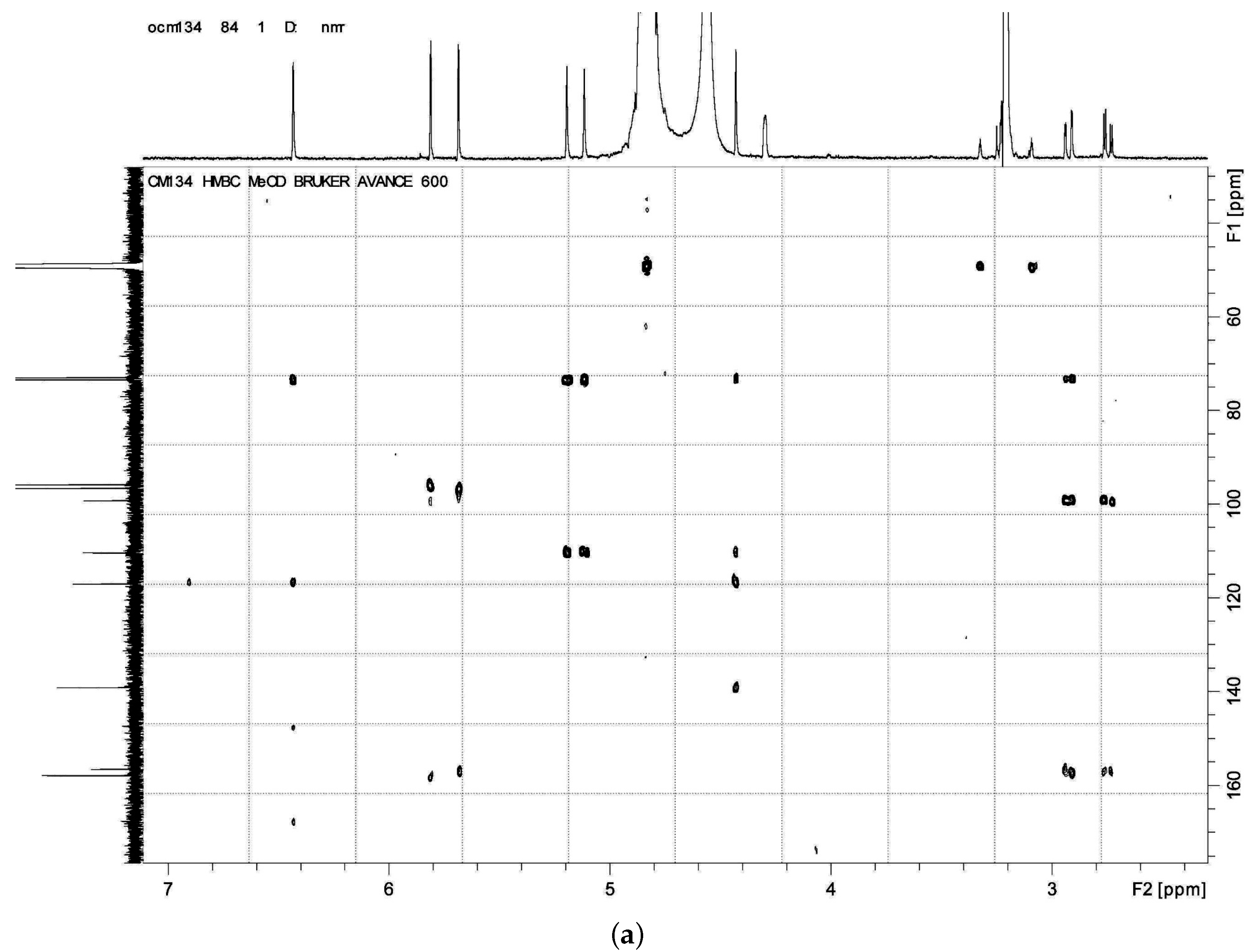
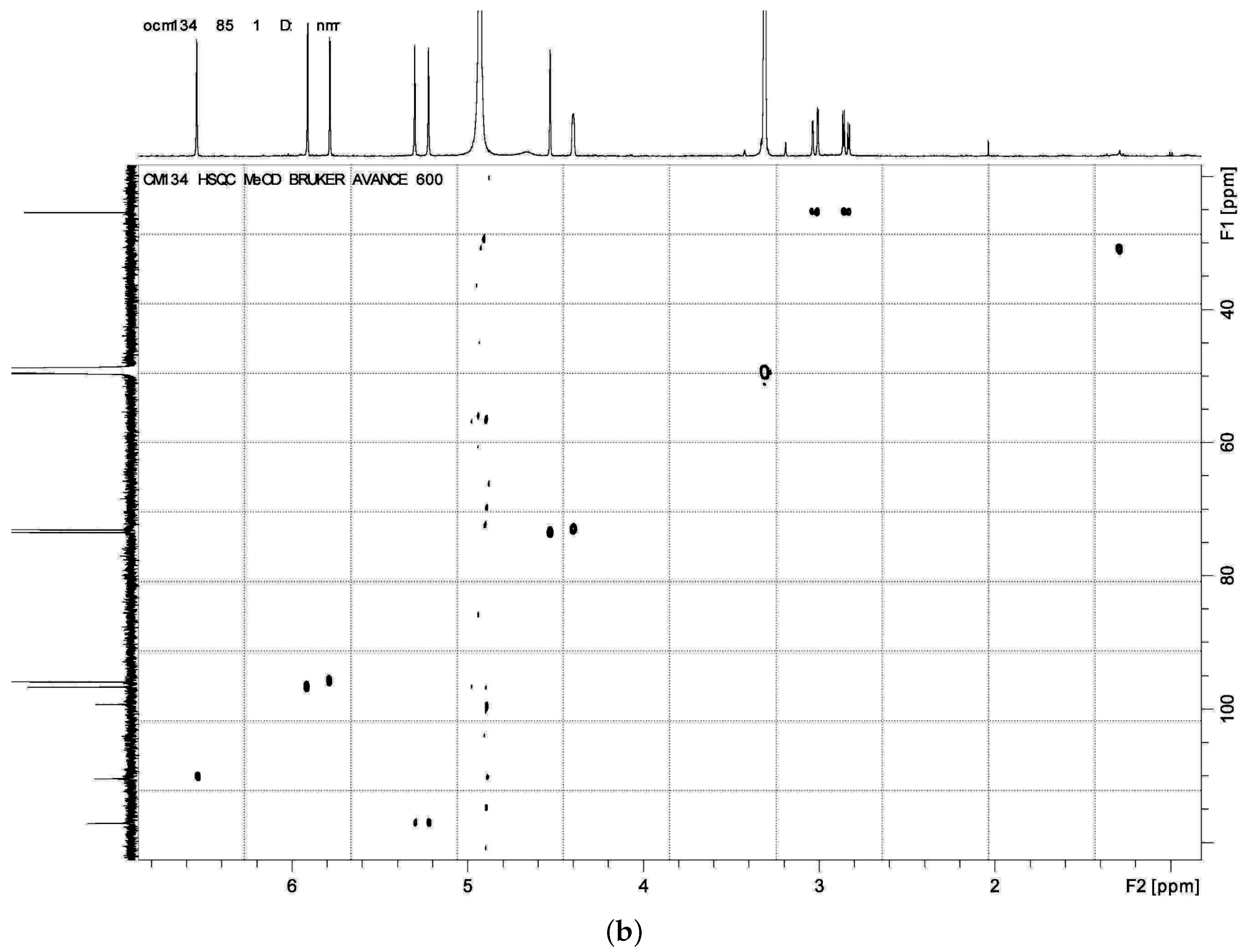
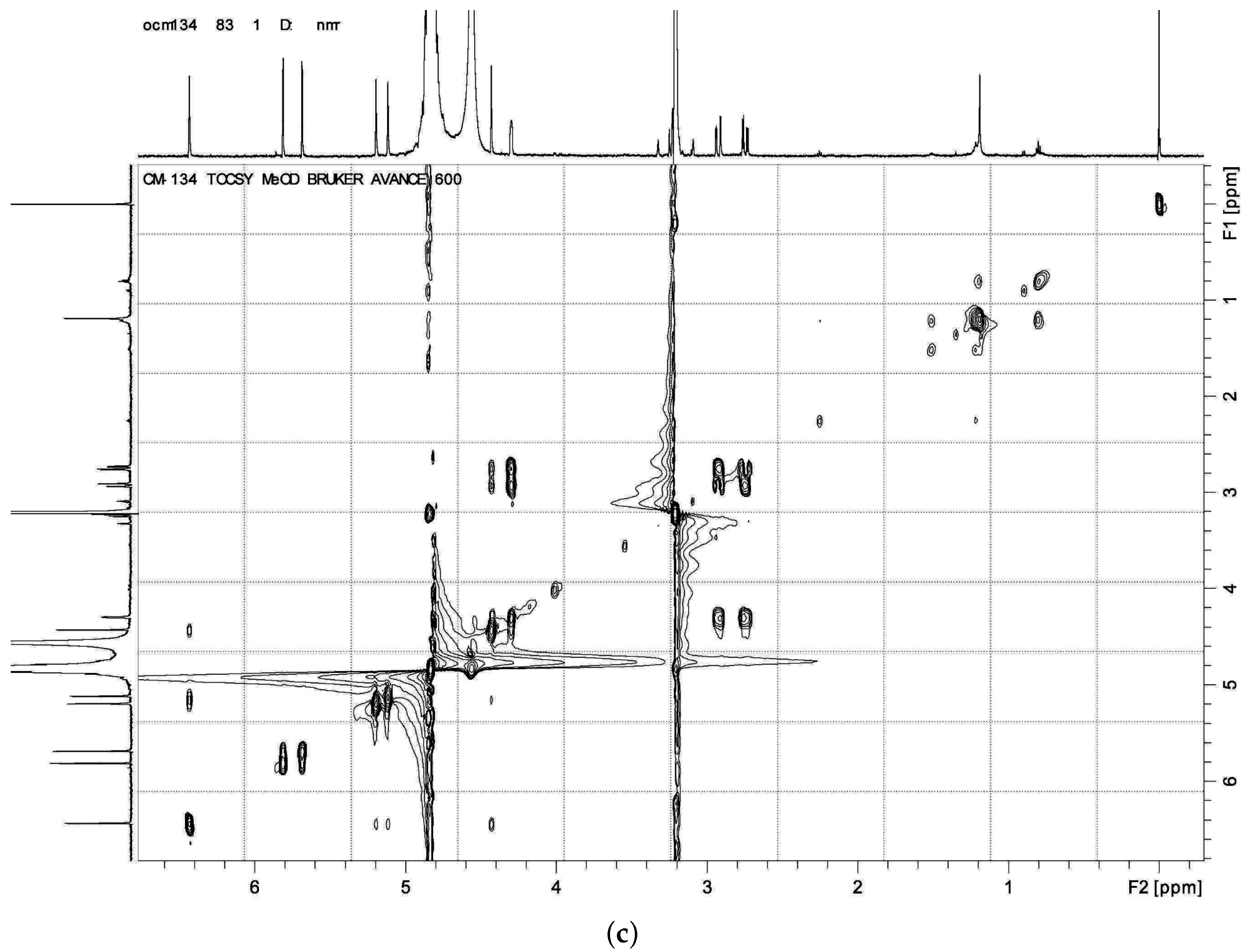
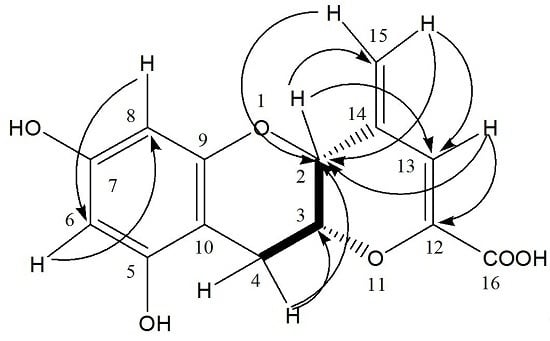
 HMBC,
HMBC,  1H - 1H COSY).
1H - 1H COSY).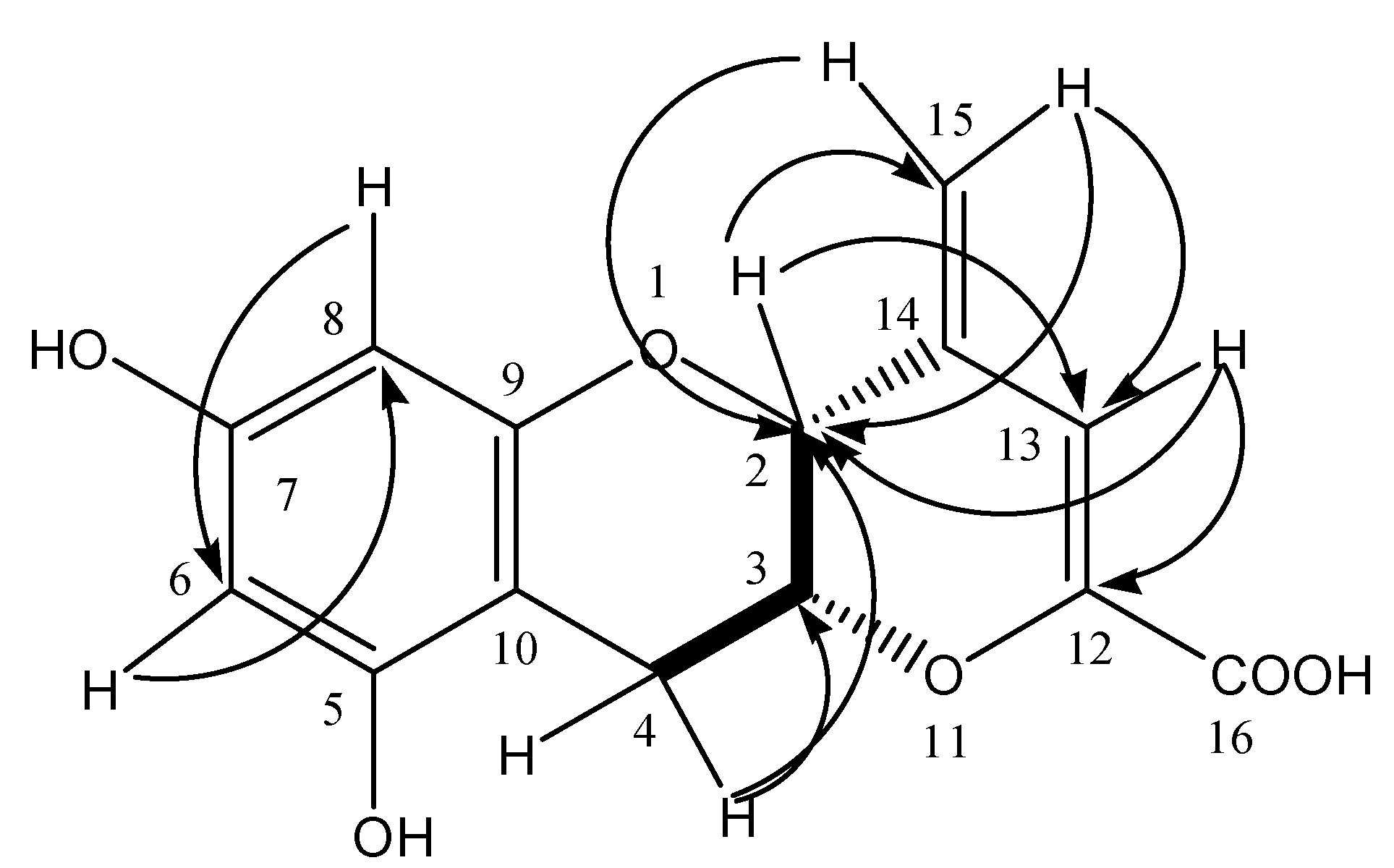
| No. | PFPT Name | GA | EGC | C | CA | EC | EGCG | GG | ECG | Teaden-ol A |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean Content (mg/g) (n = 6) | ||||||||||
| 1 | Jijin ziyin | 39.16 ± 1.1 | 10.85 ± 0.3 | 8.46 ± 0.5 | 39.35 ± 0.1 | 1.80 ± 0.0 | 22.47 ± 0.0 | 10.39 ± 0.0 | 9.52 ± 0.0 | 0.25 ± 0.0 |
| 2 | Zichun | 53.56 ± 2.1 | 9.02 ± 0.1 | 5.35 ± 0.1 | 46.06 ± 0.9 | 1.37 ± 0.1 | 18.48 ± 0.3 | 9.09 ± 0.3 | 4.57 ± 0.1 | 0.20 ± 0.0 |
| 3 | Shunde Jijin | 121.81 ± 1.6 | 25.06 ± 0.8 | 15.9 ± 0.5 | 52.87 ± 0.6 | 5.02 ± 0.1 | 43.39 ± 1.4 | 13.91 ± 0.4 | 1.90 ± 0.1 | 1.15 ± 0.0 |
| 4 | Gongting pu-er | 13.23 ± 0.1 | 1.52 ± 0.0 | 0.59 ± 0.2 | 34.88 ± 0.4 | 0.35 ± 0.1 | 0.32 ± 0.0 | 0.20 ± 0.0 | 0.67 ± 0.1 | 0.17 ± 0.0 |
| 5 | Nanye repaocha | 11.62 ± 0.8 | 4.60 ± 0.1 | 1.85 ± 0.1 | 30.29 ± 0.8 | 2.34 ± 0.2 | 1.68 ± 0.2 | 0.46 ± 0.1 | 1.25 ± 0.1 | 0.40 ± 0.0 |
| 6 | Mabang | 1.55 ± 0.1 | 3.67 ± 0.3 | 1.51 ± 0.1 | 12.17 ± 0.2 | 1.01 ± 0.3 | 0.72 ± 0.0 | 0.47 ± 0.1 | 0.60 ± 0.1 | 1.00 ± 0.2 |
| 7 | Huilong shengtaiqizibing | 124.50 ± 1.1 | 12.24 ± 0.0 | 11.23 ± 0.0 | 55.70 ± 0.0 | 2.74 ± 0.0 | 4.29 ± 0.1 | 1.23 ± 0.0 | 7.87 ± 0.0 | 1.43 ± 0.1 |
| 8 | Pinzang bing | 6.33 ± 0.2 | 3.80 ± 0.5 | 1.18 ± 0.2 | 36.78 ± 0.5 | 1.18 ± 0.4 | 0.98 ± 0.5 | 0.26 ± 0.1 | 0.19 ± 0.0 | 1.18 ± 0.2 |
| 9 | Menghai qizibing | 8.09 ± 0.1 | 6.93 ± 0.0 | 2.14 ± 0.0 | 32.24 ± 0.1 | 2.23 ± 0.0 | 0.91 ± 0.0 | 0.54 ± 0.0 | 1.37 ± 0.2 | 1.17 ± 0.2 |
| 10 | Lizhi hong | 22.80 ± 0.5 | 54.64 ± 1.0 | 26.16 ± 0.6 | 55.83 ± 0.6 | 1.60 ± 0.0 | 78.07 ± 0.3 | 20.12 ± 1.0 | 10.78 ± 0.5 | 1.28 ± 0.0 |
| 11 | Nannuo yihao | 4.30 ± 0.5 | 1.50 ± 0.2 | 0.61 ± 0.1 | 30.31 ± 0.5 | 0.34 ± 0.1 | 0.25 ± 0.0 | 0.69 ± 0.1 | 0.47 ± 0.0 | 5.03 ± 0.2 |
| 12 | Nanfangjiamu paka | 21.62 ± 1.2 | 11.18 ± 1.1 | 2.41 ± 0.1 | 40.45 ± 0.9 | 2.91 ± 0.2 | 1.74 ± 0.1 | 0.59 ± 0.1 | 1.86 ± 0.1 | 1.25 ± 0.2 |
| 13 | Puxiuqizibing | 23.96 ± 1.6 | 9.98 ± 0.1 | 7.65 ± 0.2 | 34.12 ± 0.1 | 1.15 ± 0.1 | 17.34 ± 0.1 | 8.46 ± 0.1 | 1.61 ± 0.1 | 3.15 ± 0.3 |
| 14 | Menghaitie bing | 18.11 ± 0.4 | 3.59 ± 0.1 | 2.15 ± 0.2 | 36.04 ± 0.1 | 0.47 ± 0.0 | 8.34 ± 0.1 | 3.76 ± 0.0 | 1.75 ± 0.1 | 6.20 ± 0.3 |
| 15 | Xiangyupu-er | 60.02 ± 49.5 | 3.27 ± 0.0 | 3.99 ± 0.2 | 44.66 ± 0.1 | 0.54 ± 0.0 | 12.30 ± 0.0 | 6.23 ± 0.1 | 3.16 ± 0.1 | 4.10 ± 0.0 |
| 16 | Nannuoyihao (shucha) | 33.78 ± 0.4 | 4.82 ± 0.2 | 3.50 ± 0.4 | 50.99 ± 1.5 | 1.18 ± 0.1 | 15.81 ± 0.6 | 6.99 ± 0.1 | 3.72 ± 0.5 | 8.15 ± 0.1 |
| 17 | Qingyungongma-o | 44.34 ± 1.6 | 6.94 ± 0.6 | 2.91 ± 0.1 | 37.88 ± 1.9 | 1.04 ± 0.1 | 13.34 ± 0.9 | 6.69 ± 0.1 | 2.13 ± 0.3 | 4.38 ± 0.1 |
| 18 | Chunqiaomuch | 32.90 ± 0.4 | 9.92 ± 0.5 | 7.55 ± 0.1 | 43.67 ± 1.2 | 1.73 ± 0.0 | 17.83 ± 0.4 | 7.20 ± 0.1 | 4.31 ± 0.4 | 1.31 ± 0.0 |
| 19 | Banzhangwang | 22.80 ± 0.5 | 6.86 ± 0.1 | 4.58 ± 0.4 | 40.77 ± 0.6 | 1.60 ± 0.0 | 18.82 ± 0.5 | 9.65 ± 0.6 | 5.96 ± 0.2 | 7.44 ± 0.3 |
| 20 | Daixiangyubing | 36.82 ± 1.6 | 9.00 ± 0.2 | 5.13 ± 0.1 | 52.79 ± 0.5 | 1.73 ± 0.1 | 24.49 ± 0.9 | 13.53 ± 0.2 | 5.00 ± 0.3 | 1.28 ± 0.1 |
| 21 | Lanxiangguiqi | 20.67 ± 0.6 | 9.49 ± 0.6 | 6.83 ± 0.6 | 53.14 ± 1.2 | 1.18 ± 0.0 | 21.65 ± 0.5 | 10.52 ± 1.6 | 2.05 ± 0.1 | 5.93 ± 0.0 |
| 22 | Pu-er sancha | 11.75 ± 0.2 | 1.90 ± 0.0 | 0.42 ± 0.0 | 32.52 ± 0.0 | 0.62 ± 0.0 | 0.33 ± 0.0 | 0.15 ± 0.0 | 1.25 ± 0.9 | 6.98 ± 0.4 |
| Position | δC | δH | HMBC (1H–13C) | NOE (1H–1H) |
|---|---|---|---|---|
| 2 | 73.5 | 4.52 (1H, s) | C-13, 14, 15 | H-3, 4, 13, 15 |
| 3 | 72.9 | 4.39 (1H, m) | - | - |
| 4 | 25.4 | 2.85 (1H, dd, J = 4.8, 17.4 Hz) | C-5, 10 | H-2, 3 |
| 3.02 (1H, dd, J = 1.8, 17.4 Hz) | C-2, 3, 5, 10 | H-2, 3 | ||
| 5 | 157.8 | - | - | - |
| 6 | 96.7 | 5.91 (1H, d, J = 2.4) | C-5, 8, 10 | H-8 |
| 7 | 157.9 | |||
| 8 | 95.9 | 5.78 (1H, d, J = 2.4) | C-6, 9, 10 | H-6 |
| 9 | 156.6 | - | - | - |
| 10 | 99.3 | - | - | - |
| 12 | 148.1 | - | - | - |
| 13 | 109.8 | 6.51 (1H, s) | C-2, 12, 16 | H-2, 15 |
| 14 | 139.3 | - | - | - |
| 15 | 116.6 | 5.19 (1H, s) | C-2, 13 | H-13 |
| 16 | 168.3 | 5.27 (1H, s) | C-2, 13 | H-13 |
| No. | Isolates (GenBank Accession No.) | Length (bp) | Results of EzTaxon | |
|---|---|---|---|---|
| Closest Match (GenBank Accession No.) | Similarity (%) | |||
| 1 | 1-3-b-5 (KR149614) | 1373 | Achromobacter xylosoxidans DSM 10346(T) (Y14908) | 99.854 |
| 2 | 2-3-b-2 (KR149617) | 1365 | Achromobacter xylosoxidans NBRC 15126(T) (CP006958) | 99.93 |
| 3 | 1-1-b-5 (KR149606) | 1385 | Bacillus amyloliquefaciens subsp. plantarum FZB42(T) (CP000560) | 99.928 |
| 4 | 3-3-b-3 (KR149598) | 1345 | Bordetella avium 197N (AM167904) | 99.18 |
| 5 | 1-1-b-3 (KR149624) | 1385 | Enterobacter asburiae JCM 6051(T) (AB004744) | 99.35 |
| 6 | 1-1-b-4 (KR149608) | 1375 | Enterobacter asburiae JCM 6051(T) (AB004744) | 99.345 |
| 7 | 1-1-b-6 (KR149611) | 1367 | Enterobacter asburiae JCM 6051(T) (AB004744) | 99.415 |
| 8 | 1-2-b-1 (KR149610) | 1376 | Enterobacter hormaechei ATCC 49162(T) (AFHR01000079) | 99.345 |
| 9 | 2-4-b-4 (KR149622) | 1298 | Microbacterium sediminis YLB-01(T) (HQ219727) | 98.54 |
| 10 | 1-4-b-7 (KR149612) | 1322 | Ochrobactrum pseudintermedium ADV31(T) (DQ365921) | 99.849 |
| 11 | 2-3-b-5 (KR149618) | 1323 | Ochrobactrum pseudintermedium ADV31(T) DQ365921 | 99.697 |
| 12 | 1-1-b-8 (KR149609) | 1384 | Pantoea dispersa LMG 2603(T) (DQ504305) | 100 |
| 13 | 3-3-b-2 (KR149596) | 1373 | Pseudomonas aeruginosa JCM 5962(T) (BAMA01000316) | 99.93 |
| 14 | 3-4-b-2 (KR149600) | 1370 | Pseudomonas aeruginosa JCM 5962(T) (BAMA01000316) | 99.93 |
| 15 | 3-4-b-1 (KR149599) | 1375 | Pseudomonas aeruginosa LMG 1242(T) (Z76651) | 99.854 |
| 16 | 3-5-b-5 (KR149604) | 1363 | Pseudomonas aeruginosa LMG 1242(T) (Z76651) | 99.853 |
| 17 | 1-4-b-9 (KR149607) | 1376 | P seudomonas aeruginosa LMG 1242(T) (Z76651) | 99.854 |
| 18 | 1-5-b-2-1 (KR149613) | 1372 | Pseudomonas aeruginosa LMG 1242(T) (Z76651) | 99.854 |
| 19 | 1-4-b-1 (KR149615) | 1371 | Pseudomonas aeruginosa LMG 1242(T) (Z76651) | 99.854 |
| 20 | 1-3-b-2 (KR149616) | 1379 | Pseudomonas aeruginosa LMG 1242(T) (Z76651) | 99.855 |
| 21 | 2-4-b-1 (KR149619) | 1369 | Pseudomonas aeruginosa LMG 1242(T) (Z76651) | 99.854 |
| 22 | 2-4-b-2 (KR149620) | 1330 | Pseudomonas aeruginosa LMG 1242(T) (Z76651) | 99.85 |
| 23 | 2-4-b-5 (KR149623) | 1387 | Pseudomonas aeruginosa LMG 1242(T) (Z76651) | 99.856 |
| 24 | 3-3-b-4 (KR149597) | 1377 | Pseudomonas beteli ATCC 19861(T) (AB021406) | 99.564 |
| 25 | 3-5-b-2 (KR149602) | 1358 | Pseudomonas plecoglossicida FPC951(T) (AB009457) | 99.853 |
| 26 | 3-4-b-5 (KR149601) | 1368 | Sphingobacterium thalpophilum DSM 11723(T) (AJ438177) | 99.415 |
| 27 | 2-4-b-3 (KR149621) | 1373 | Sphingobacterium thalpophilum DSM 11723(T) (AJ438177) | 99.854 |
| 28 | 2-5-b-1 (KR149605) | 1356 | Staphylococcus sciuri subsp. sciuri DSM 20345(T) (AJ421446) | 100 |
| 29 | 3-5-b-4 (KR149603) | 1385 | Staphylococcus sciuri subsp. sciuri DSM 20345(T) (AJ421446) | 100 |
| 30 | 1-5-f-10 (KR149625) | 506 | Acremonium falciforme (DQ094533) | 99.33 |
| 31 | y-2-b-2 (KR149637) | 546 | Aspergillus fumigatus_1 (AF455542) | 100 |
| 32 | 3-2-f-4(KR149634) | 534 | Aspergillus fumigatus_1 (JN226940) | 100 |
| 33 | 2-5-f-1 (KR149635) | 545 | Aspergillus fumigatus_1 (JN226940) | 100 |
| 34 | 2-1-f-1 (KR14964) | 542 | Aspergillus niger_1 (EF660199) | 99.8 |
| 35 | y-2-f-4 (KR149644) | 531 | Aspergillus niger_1 (EF660199) | 99.6 |
| 36 | 2-4-f-2 (KR149630) | 529 | Aspergillus niger_1 (EU821308) | 100 |
| 37 | 3-1-f-4(KR149642) | 529 | Aspergillus niger_1 (EU821308) | 100 |
| 38 | 1-5-f-15 (KR149631) | 547 | Aspergillus sclerotiorum_1 (GQ398087) | 100 |
| 39 | y-2-f-7 (KR149626) | 504 | Aspergillus sydowii_1 JN986787 | 100 |
| 40 | 2-5-f-4 (KR149638) | 554 | Aspergillus tamarii_1 (KC621084) | 100 |
| 41 | 3-5-f-1 (KR149640) | 553 | Aspergillus tamarii_2 (DQ411548) | 100 |
| 42 | 1-4-f-9 (KR149628) | 529 | Aspergillus tamarii_2 (EU021614) | 100 |
| 43 | 2-4-f-4 (KR149636) | 538 | Aspergillus tamarii_2 (EU021614) | 100 |
| 44 | 3-2-f-1 ( KR149627) | 537 | Aspergillus tubingensis (EU821292) | 100 |
| 45 | 3-1-f-2 (KR149633) | 538 | Aspergillus tubingensis (EU821292) | 100 |
| 46 | y-2-b-1 (KR149641) | 533 | Aspergillus tubingensis (EU821292) | 100 |
| 47 | 3-5-f-2 (KR149632) | 510 | Earliella scabrosa_2 (EU661875) | 98.88 |
| 48 | 1-5-f-9 (KR149639) | 706 | Lichtheimia corymbifera_9 (FJ719392) | 100 |
| 49 | 2-2-f-3 (KR149629) | 635 | Rhizopus microsporus_1 (AF115729) | 100 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.-q.; Zhang, G.-j.; Ma, Y.; Chen, M.; Chen, S.-h.; Duan, S.-m.; Wan, J.-q.; Hashimoto, F.; Lv, H.-p.; Li, J.-h.; et al. Isolation, Identification, and Biotransformation of Teadenol A from Solid State Fermentation of Pu-erh Tea and In Vitro Antioxidant Activity. Appl. Sci. 2016, 6, 161. https://doi.org/10.3390/app6060161
Su X-q, Zhang G-j, Ma Y, Chen M, Chen S-h, Duan S-m, Wan J-q, Hashimoto F, Lv H-p, Li J-h, et al. Isolation, Identification, and Biotransformation of Teadenol A from Solid State Fermentation of Pu-erh Tea and In Vitro Antioxidant Activity. Applied Sciences. 2016; 6(6):161. https://doi.org/10.3390/app6060161
Chicago/Turabian StyleSu, Xiao-qin, Gao-ju Zhang, Yan Ma, Mao Chen, Sheng-hu Chen, Shuang-mei Duan, Jin-qiong Wan, Fumio Hashimoto, Hai-peng Lv, Jia-hua Li, and et al. 2016. "Isolation, Identification, and Biotransformation of Teadenol A from Solid State Fermentation of Pu-erh Tea and In Vitro Antioxidant Activity" Applied Sciences 6, no. 6: 161. https://doi.org/10.3390/app6060161