1. Introduction
Fourier domain optical coherence tomography (FDOCT) is a high-speed, high-resolution and non-invasive biomedical optical imaging technique [
1]. It has been applied in the field of biomedical research, clinical diagnostics, and non-destructive material inspection. FDOCT can be categorized into two embodiments. One is based on a wideband laser source and a spectrometer which is called spectral domain OCT (SDOCT), and the other one is based on a frequency sweeping laser source and a balanced photon detector which is called swept source OCT (SSOCT, also called optical frequency domain imaging, OFDI). For both types of the FDOCT, the achievable imaging depth range is an important performance indicator. In some situations, a long ranging depth is highly desirable. One such example is for the endoscopic application where the distance between the distal end of the endoscopic probe and the tissue surface cannot be accurately controlled. Another example is for imaging the whole eye from anterior to retina.
For FDOCT, the ranging depth can be calculated using the formula Δ
z = λ
02/4
nδλ and is fundamentally limited by the center wavelength λ
0 of the light source, the spectral resolution δλ, and the refractive index
n. The spectral resolution is δλ = Δλ/
N when the spectrometer is pixel-limited. In this equation, Δλ represents the spectral bandwidth of the light source and
N is the data number of the digitized axial spectral interference signal that covers the bandwidth. In fact, there is a sensitivity roll-off issue for FDOCT, which poses a further limitation to the practical imaging depth range. For the case of SDOCT, the sensitivity roll-off effect is due to the finite pixel number of the line scanning camera. Due to this effect, the visibility of the spectral interference fringe is maximized when the optical path difference (OPD) is zero and decays as the path length difference increases. Thus, the effective ranging depth is defined as the imaging depth at which the sensitivity has decayed −6 dB from the peak value [
2]. For FDOCT, the complex conjugate ambiguity further limits the imaging depth to one half of the full-range imaging space. Another limitation on the imaging depth of the FDOCT is posed by the finite depth of focus of the objective lens.
For extending the effective imaging depth range, various techniques have been proposed. A novel MEMS-Vertical Cavity Surface Emitting Laser (VCSEL) source-based [
3] SSOCT system was developed for in vivo high-speed imaging of the eye with an unprecedented imaging depth of 50 mm in air and little sensitivity loss. The SDOCT system based on the orthogonal dispersion spectrometer [
4] or the frequency comb approach [
5] was proposed for large ranging depth imaging through enhancing spectral resolution. To solve the complex conjugate ambiguity, many complex Optical Coherence Tomography (OCT) techniques were proposed. Among these complex OCT methods, an approach involving spatial phase modulation induced by the pivot-offset galvo scanner (GS) in the sample arm is regarded as a widely accepted way [
6,
7,
8,
9]. The technique was initially introduced for en face OCT imaging [
10]. In the approach, a slight eccentricity of the laser beam incident upon the galvo scanner causes a path length modulation during lateral scanning, generating the required carrier frequency. Recently, the optimization of galvo scanning for OCT was investigated [
11].
Alternatively, based on implementing multiple reference arms in the FDOCT system the extended ranging depth can also be achieved [
12,
13,
14,
15,
16]. These multiple-reference-arm approaches can be roughly categorized into two sorts: simultaneous acquisition approach and sequential acquisition approach. For the simultaneous acquisition approach, Nezam et al. presented an OFDI system based on two independent interferometer reference arms, each having an acousto-optic frequency shifter operating at a distinct frequency and an independent round-trip delay [
12]. Through combination of a dual-channel OCT system focusing on different segments of the eye, Chuanqing Zhou et al. extended the imaging depth using two OCT setups [
13,
14]. For the sequential acquisition approach, Hui Wang et al. proposed a simple and inexpensive method for increasing the effective imaging depth range of the SDOCT system, which was realized by employing a high-speed fiber optic switch to serially access two reference paths with different offset delays [
2]. Recently, Ruggeriet al. enabled OCT imaging of multi-frames at four depths without sacrificing axial resolution by implementing a galvo scanner-based mechanical optical switch in the reference arm [
15]. They utilized the system to image the whole eye from anterior to retina. More recently, a SDOCT system capable of deep depth imaging using a 3 × 3 fiber coupler, and a mechanical shutter was demonstrated [
16]. To conclude, the simultaneous acquisition approaches can extend the imaging depth of the OCT system without sacrificing the effective imaging rate. However, they use either expensive frequency modulators or two OCT systems, which increase the complexity and cost of the imaging system. Furthermore, due to splitting the light source power into two channels, the simultaneous approaches suffer from a 3dB loss of the imaging sensitivity [
9]. On the other hand, the sequential acquisition approaches extend the ranging depth with no sensitivity penalty, but with the penalty of halving the effective image acquisition rate, and it cannot be applied in some applications that need to image the different depth regions of the sample simultaneously.
In this paper, we present a simultaneous acquisition approach for extending the imaging depth range of a SDOCT system. The method, which is termed spatial frequency domain multiplexing method, is based on two pivot-offset galvo scanners in two separate reference arms. Galvo scanners are popular devices that offer a good scanning speed, a good field of scan, high precision, and repeatability [
17]. Thus, the presented approach can image the different depth regions of the sample simultaneously without any expensive acousto-optic or electro-optic modulation device. In the following sections, we theoretically derive the parameter choice criteria for the high signal/crosstalk ratio (SCR) reconstruction and demonstrate the extended imaging depth performances of the system experimentally.
3. Experiments and Results
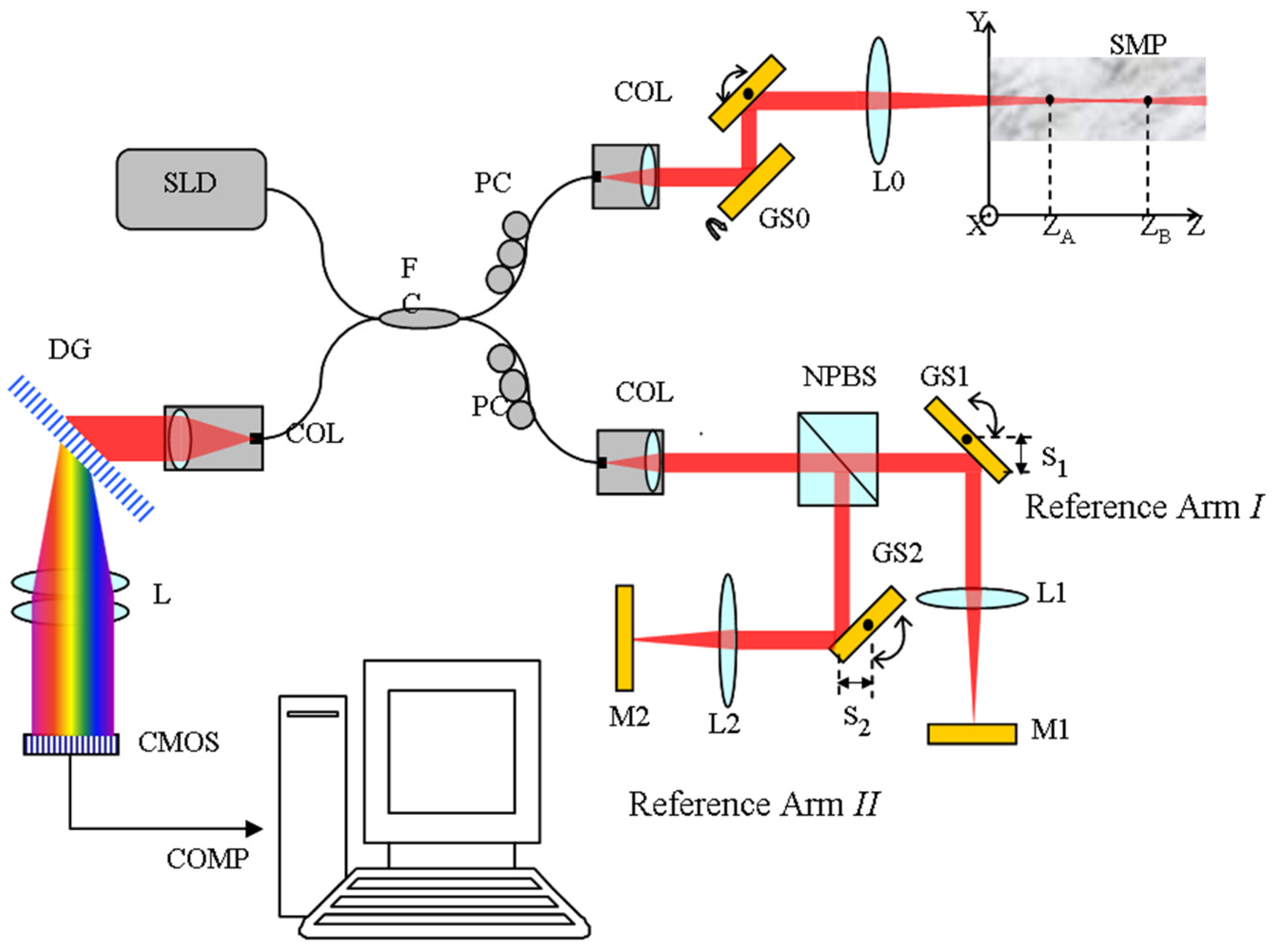
For experimental verification of the method, we constructed a fiber-based spatial frequency domain multiplexing SDOCT system as depicted in
Figure 1. The imaging system employs a super luminescent diode light source (SLD-371-HP2, Superlum Inc., Carrigtwohill, Ireland) with a center wavelength at 837 nm and a full width at half maximum (FWHM) of 54 nm. The beam diameter
d in the sample arm is 1.8 mm, which will produce a 44 μm 1/e
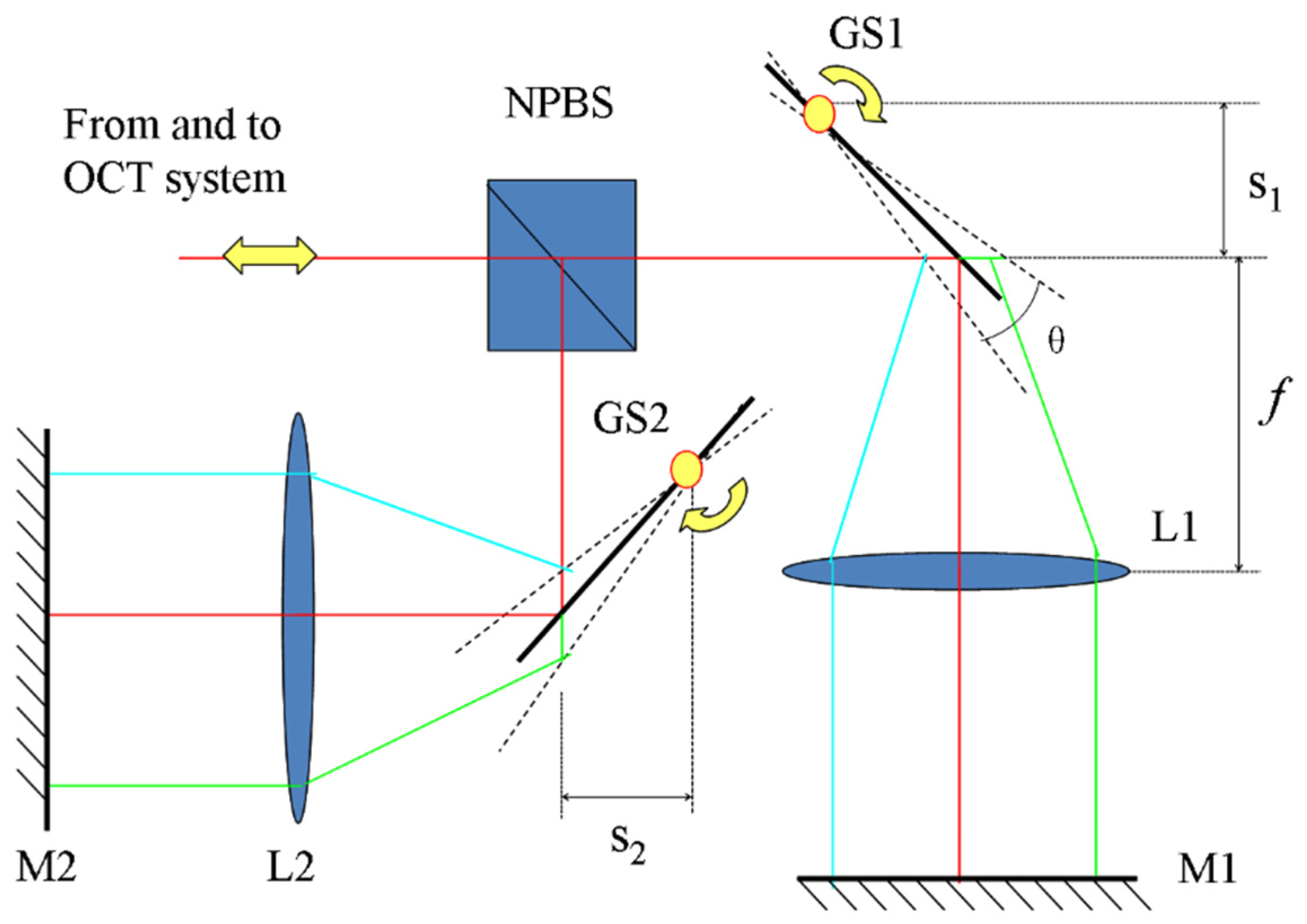
2 diameter focal spot on the sample with a confocal parameter of 3.7 mm. A 50/50 fiber coupler splits the light beam into the sample arm and the reference arms. In the sample arm, the light beam is deflected by a 2D galvo scanner and focused via a 75 mm achromatic lens L0 to the sample. Within the reference arm, a wide bandwidth non-polarizing beam splitter (NPBS, Daheng Optics Inc., Beijing, China) is used to divide the reference optical power into the two separate reference arms. The fiber collimator on a translation stage in the reference arm is used to adjust the overall delay of the two channels. The single-pass OPD between Reference Arms I and II is set to 3 mm. The GS1 and GS2 are the
X and
Y mirror of a pair of large beam 2D GS system (GVS012, Thorlabs Inc., Newton, NJ, USA), respectively. The focus lengths of achromatic lens L1and L2 are both 30 mm. The plane mirror M1 and M2 are the wide bandwidth dielectric coated mirrors covering the wavelength range of 800 to 900 nm. The spectral interference signal is detected by a custom-built spectrometer, which consists of a collimator (
f = 60 mm, OZ optics Inc., Ottawa, ON, Canada), a transmissive diffraction grating (1800 lines/mm), a camera lens (
f =105 mm, Nikon Inc., Tokyo, Japan), and a line-scan CMOS camera (sp2048-70km, Basler AG Inc., Ahrensburg, German). The spectral resolution of the spectrometer is 0.065 nm, allowing for a theoretical effective imaging depth range of 3.5 mm.
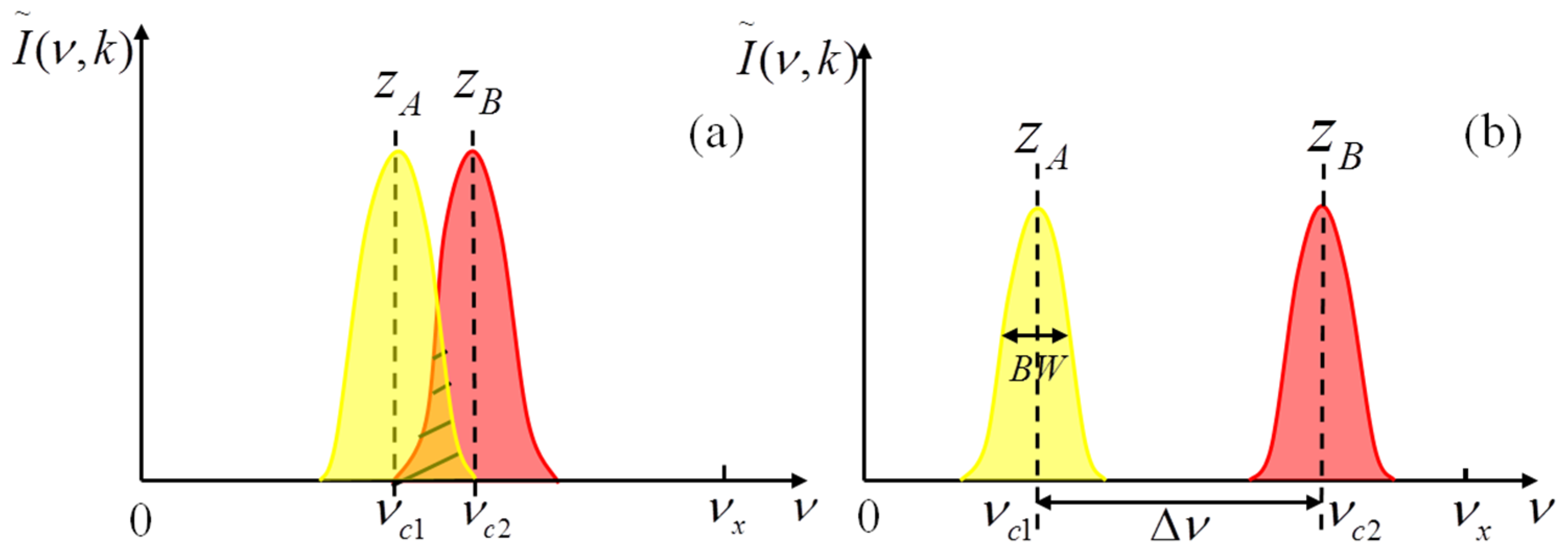
In view of avoiding aliasing terms and according to Equation (9), the number of A-scans per B-scan is set to 500, and the lateral scanning range is set to 1.3 mm. The highest positive spatial frequency calculated by Equation (6) is 192 mm
−1. According to the values of the sample beam diameter
d, the center wavelength, the focus length of the sample objective and Equation (7), the theoretical BW of the spatial frequency content is calculated to be 22.5 mm
−1. In order to suppress the crosstalk of the two spatial frequency contents of the two depths signal, the difference of the two center spatial carrier frequencies must be greater than 67.5 mm
−1 according to Equation (8). Considering that the optimal conjugate suppression ratio occurs when
is
[
9], each of the two center spatial carrier frequencies needs to be close to the central region of the positive spatial frequency range. Thus, the two center spatial carrier frequencies
νc1 and
νc2 are set to 56.5 and 123.5 mm
−1, respectively. According to Equation (4), the phase shift
and
between successive axial scans are calculated to be
and
. The angular velocity of the galvo scanners is set to 0.93 rad/s. The exposure time of the CMOS camera is set to 18 μs. According to Equation (3) and the above parameters, the pivot-offset distances on the GS1 and GS2 mirrors are calculated to be 1.8 and 3.9 mm, respectively. The pivot-offset distance is adjusted via the diagonal translation of the scanners to keep the light beam at the center of the focusing optics.
To experimentally characterize the extension of the imaging depth range of the spatial frequency domain multiplexing SDOCT system, the point spread function is measured by using a plane mirror at various depths in the sample arm.
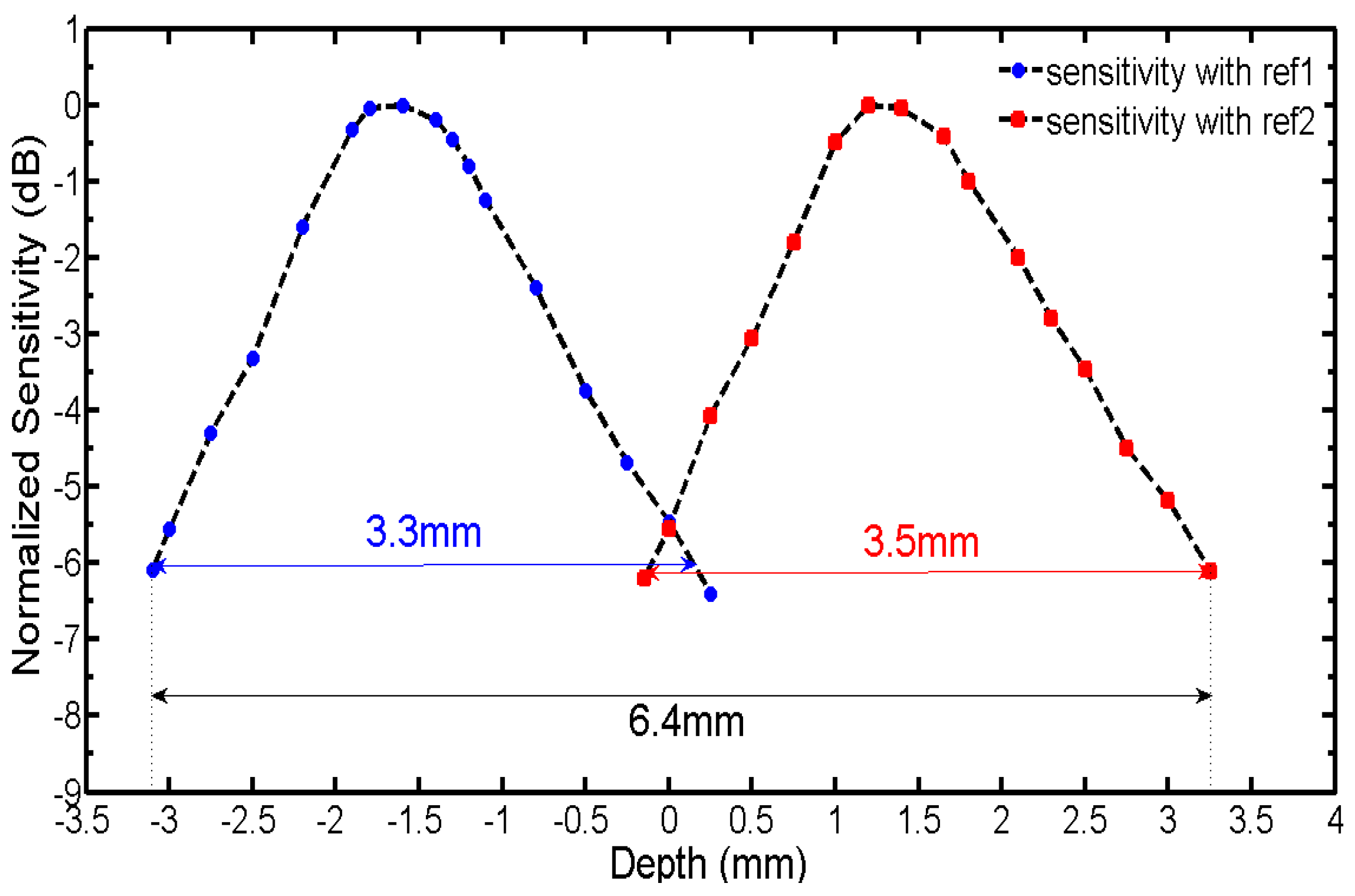
Figure 5 shows the experimentally measured normalized sensitivity as a function of depth for the two reference arms. The measured value of the absolute sensitivity is 59.7 dB. From
Figure 5, the separation between the two sensitivity peaks can be used to calibrate the actual relative OPD between the two reference arms, and it can be shown that the actual relative OPD is about 3 mm. The benefit from the use of the spatial frequency domain multiplexing SDOCT system is also demonstrated, and the effective imaging depth range for the proposed system is increased from about 3.4 to 6.4 mm, compared with a conventional full-range SDOCT without depth range enhancement. The depth enhancement factor is 1.9. In this demonstration, the two sensitivity curves are crossed at the −5.5 dB from the sensitivity peaks. Therefore, the effective imaging depth range would be doubled if the relative optical path delay was precisely tuned to make the two sensitivity curves cross at the −6 dB point from the sensitivity peaks.
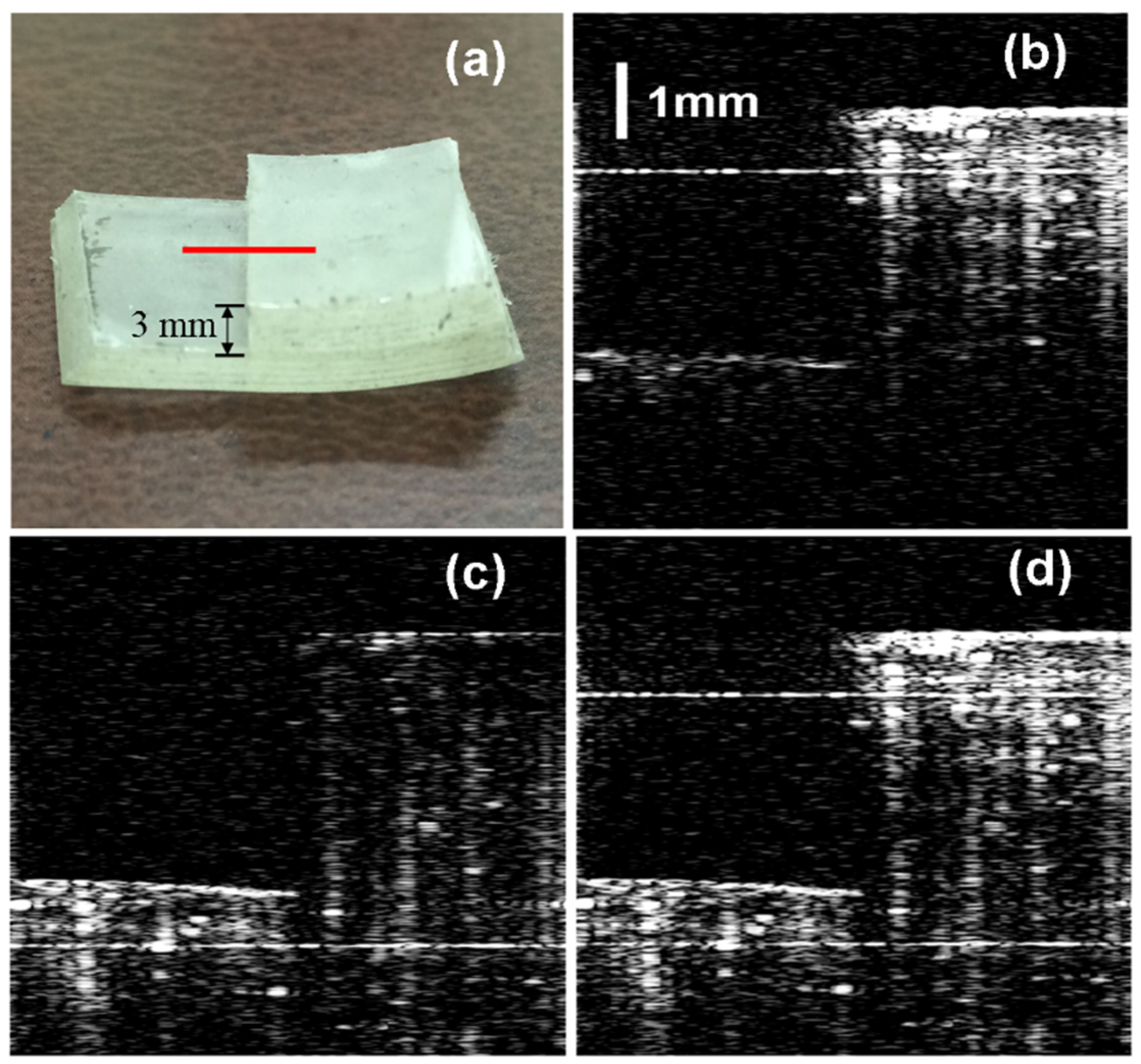
To demonstrate the ranging depth enhancement, we compare image results of the OCT images acquired by the proposed two-reference-arm spatial frequency domain multiplexing approach and the conventional single-reference-arm approach. Firstly, an artificial phantom is imaged by the system.
Figure 6a shows the photograph of the phantom taken with a digital camera. The phantom is made by sticking two blocks of sellotape to generate a step 3 mm high.
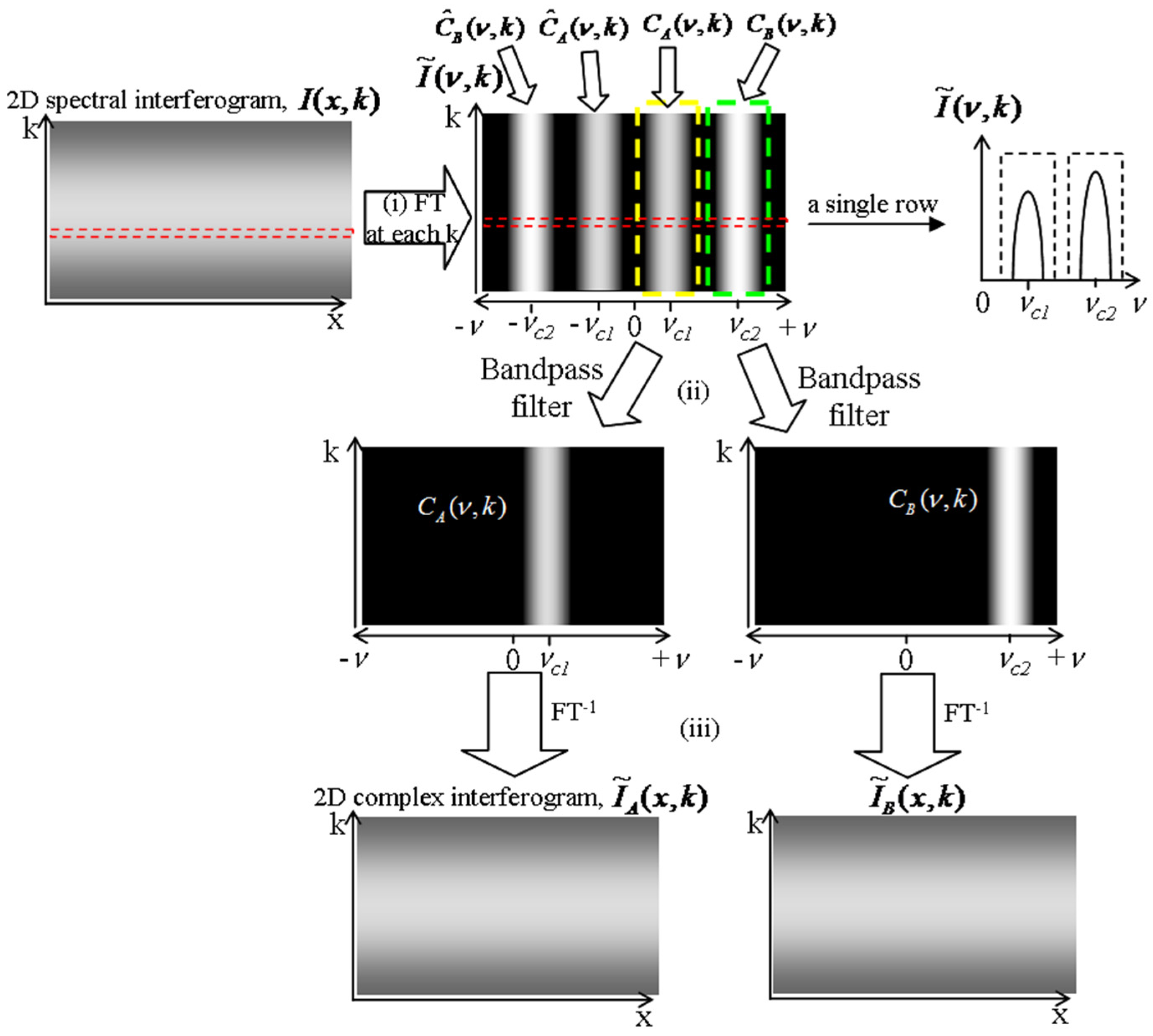
Figure 6b–d are the complex conjugate ambiguity free OCT images of the phantom reconstructed by the data processing algorithm described previously.
Figure 6b shows the image acquired with Reference Arm I open while blocking Reference Arm II. As we can see, the upper part of the step is clearly imaged, while the image of the lower part is hard to be discerned. The OPD between the upper part and Reference Arm I resides in the effective imaging range, while that of the lower part and Reference Arm I is larger than 6 mm, out of the effective imaging range.
Figure 6c shows the OCT image acquired with Reference Arm II open while blocking Reference Arm I. Likewise, the lower part of the step can be clearly imaged, while the upper part is not clear.
Figure 6d shows the reconstructed OCT image of the phantom acquired with both Reference Arms I and II open. The whole phantom including the upper and lower part of the step can be clearly seen. The imaging depth-enhanced OCT image of the artificial phantom demonstrates the feasibility of the proposed method for extending the OCT ranging depth.
The biological tissue of the swine adipose is also in vitro, imaged and compared with the results acquired by the proposed two-reference-arm spatial frequency domain multiplexing approach and the conventional single-reference-arm approach.
Figure 7a,b show the images recorded using the two Reference Arms I and II separately. In each case, the area of the tissue around the DC signal component has maximum sensitivity.
Figure 7c shows the OCT image of the tissue acquired with both of Reference Arms I and II open. As we can see, the upper and lower part of the biological sample is clearly appeared at the same time.
Figure 7d plots an A-scan profile from the same sample position indicated by the red arrows in
Figure 7a,b. It can be seen that the signal is about 13 dB stronger than that of the signal 3 mm away due to the sensitivity roll-off. The extended range OCT image of the biological tissue obtained with the system demonstrates the feasibility of the proposed method for extending the OCT ranging depth. Because the galvanometer scanners in the system are used for phase modulation of the lateral interference spectral signal, the phase noise does not affect the quality of the results. In future, the proposed method for extending the OCT ranging depth can be combined with the depth of the focus extension method to improve the quality of the extended range OCT image.