Study of Soot Deposits during Continuous Methane Pyrolysis in a Corundum Tube
Abstract
:1. Introduction
2. Experimental Part
2.1. Chemical Reagents
2.2. Experimental Plant
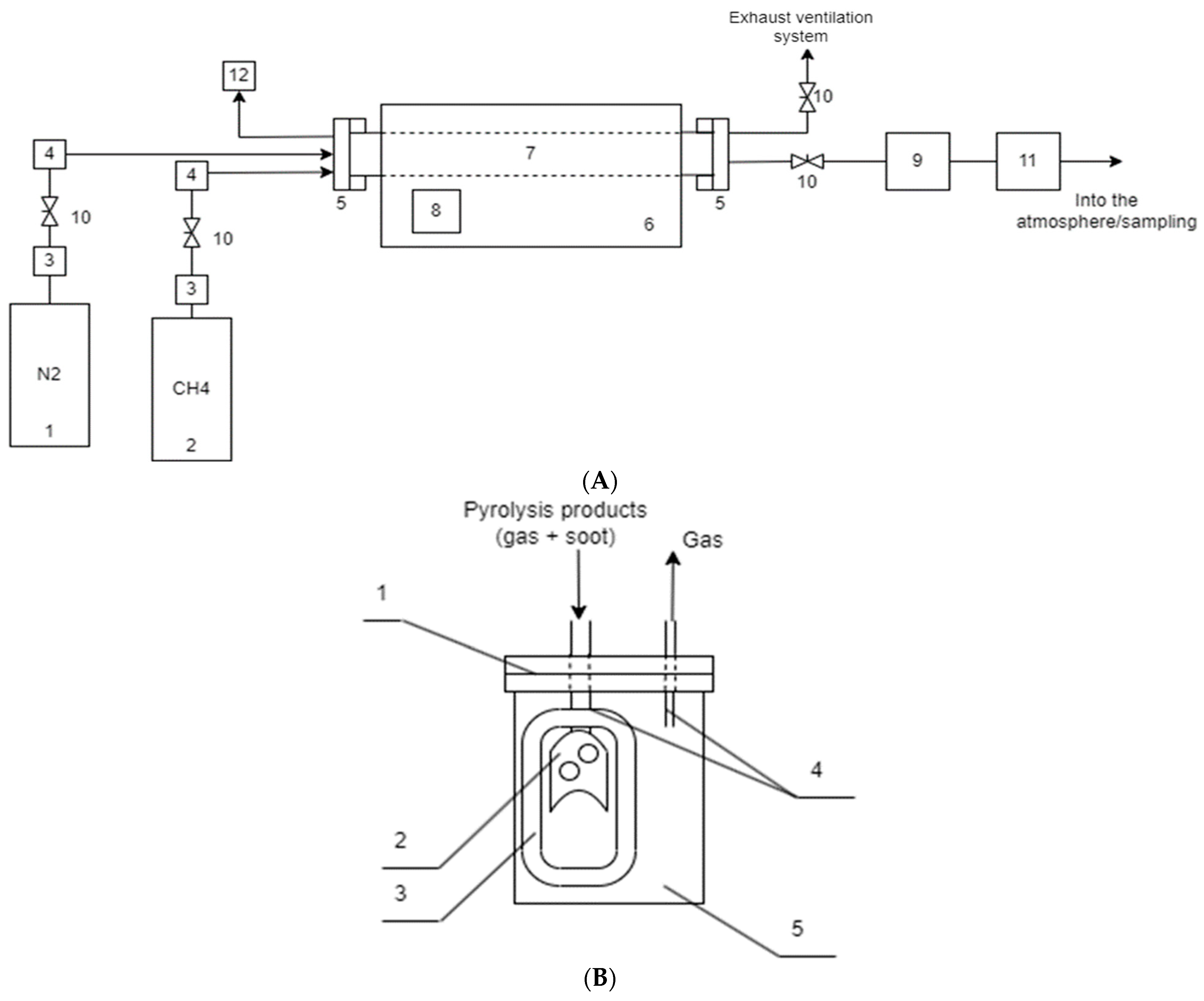
2.2.1. Schematic Diagram of the Experimental Plant
2.2.2. Methodology for the Experiments
2.3. Research Methods
2.3.1. Gas Chromatography
2.3.2. Microscopy
2.3.3. Low-Temperature Nitrogen Adsorption Method
2.3.4. Inductively Coupled Plasma Mass Spectrometry
3. Results and Discussion
3.1. Composition of Gaseous Product of Methane Pyrolysis
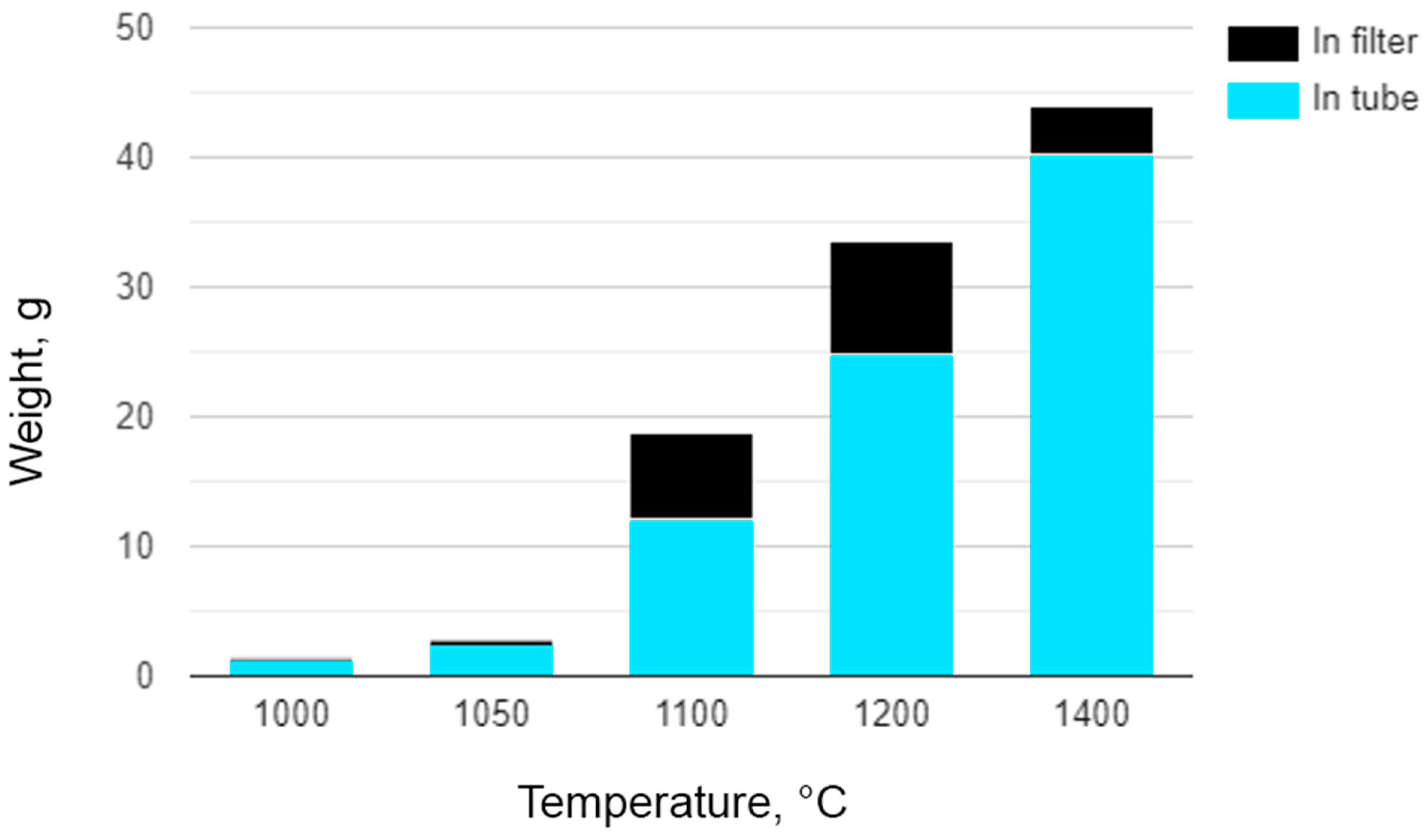
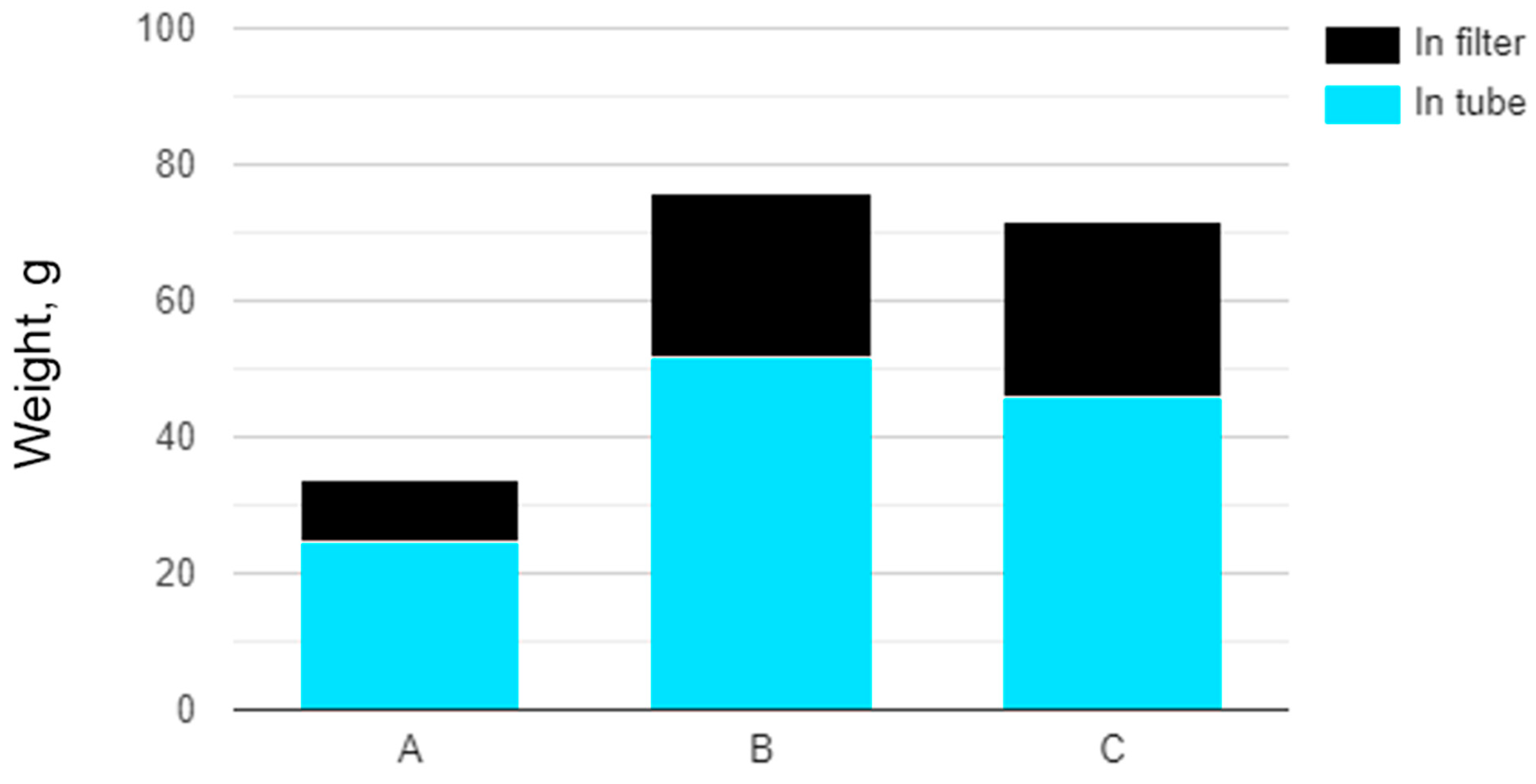
3.2. Yield of Soot
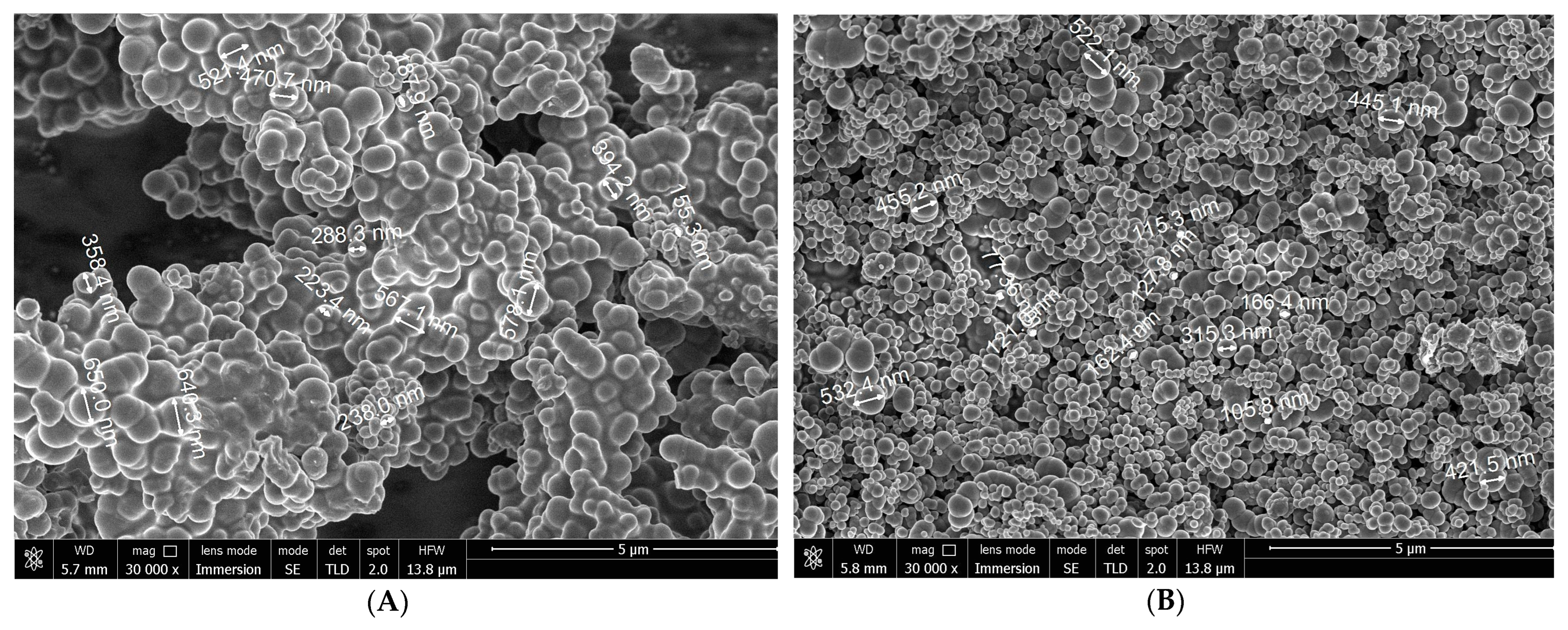
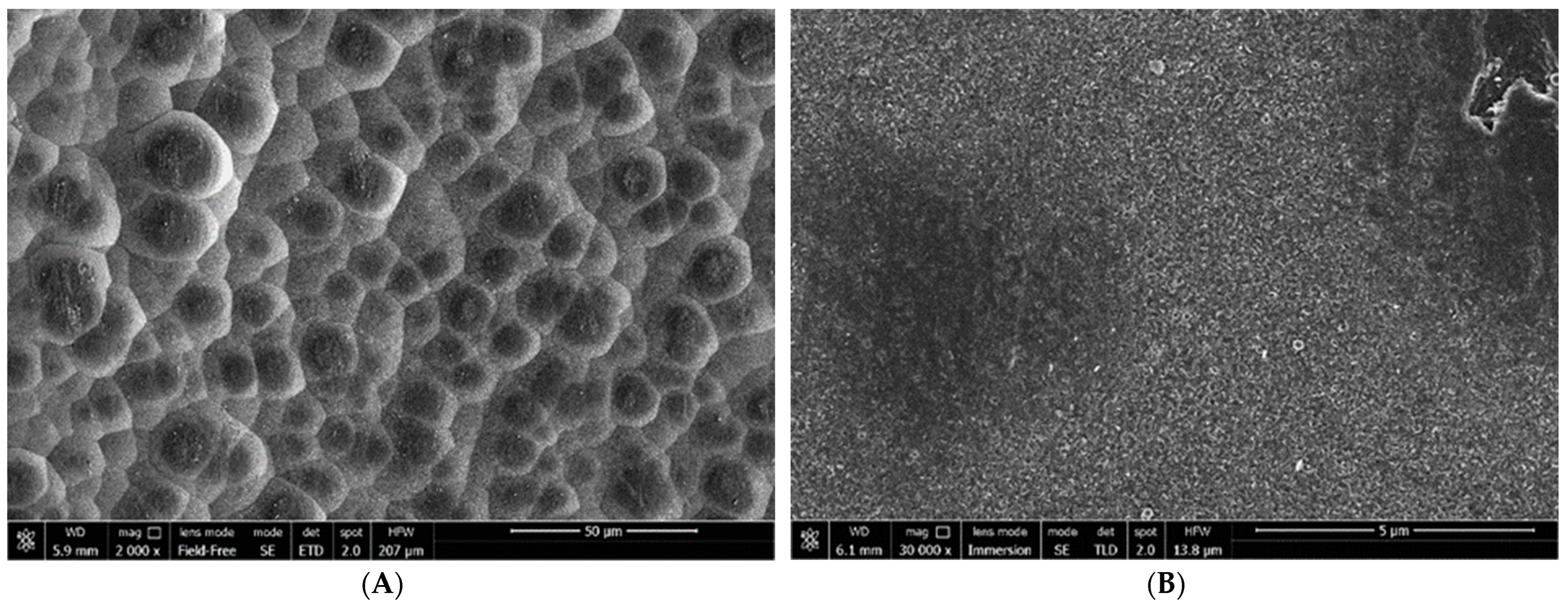
3.3. Microstructure of Soot Particles
3.4. Impurity Composition of Carbon Black
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dadsetan, M.; Khan, M.F.; Salakhi, M.; Bobicki, E.R.; Thomson, M.J. CO2-free hydrogen production via microwave-driven methane pyrolysis. Int. J. Hydrogen Energy 2023, 48, 14565–14576. [Google Scholar] [CrossRef]
- Lott, P.; Mokashi, M.B.; Müller, H.; Heitlinger, D.J.; Lichtenberg, S.; Shirsath, A.B.; Janzer, C.; Tischer, S.; Maier, L.; Deutschmann, O. Hydrogen Production and Carbon Capture by Gas-Phase Methane Pyrolysis: A Feasibility Study. ChemSusChem 2022, 16, e202201720. [Google Scholar] [CrossRef]
- Tatum, J.; Punia, A.; Kostiuk, L.; Secanell, M.; Olfert, J. Dataset of methane pyrolysis products in a batch reactor as a function of time at high temperatures and pressures. Data Brief. 2023, 47, 108953. [Google Scholar] [CrossRef] [PubMed]
- International Energy Agency (IEA). Global Hydrogen Review 2022; IEA: Paris, France, 2022; Available online: https://www.iea.org/reports/global-hydrogen-review-2022 (accessed on 5 May 2023).
- Li, C.; Li, M.; Zheng, Y.; Fang, B.; Lin, J.; Ni, J.; Lin, B.; Jiang, L. Revealing hydrogen migration effect on ammonia synthesis activity over ceria-supported Ru catalysts. Appl. Catal. B Environ. 2023, 320, 121982. [Google Scholar] [CrossRef]
- Meesattham, S.; Kim-Lohsoontorn, P. Low-temperature alcohol-assisted methanol synthesis from CO2 and H2: The effect of alcohol type. Int. J. Hydrogen Energy 2022, 47, 22691–22703. [Google Scholar] [CrossRef]
- Pye, S.; Welsby, D.; McDowall, W.; Reinauer, T.; Dessens, O.; Winning, M.; Calzadilla, A.; Bataille, C. Regional uptake of direct reduction iron production using hydrogen under climate policy. Energy Clim. Chang. 2022, 3, 100087. [Google Scholar] [CrossRef]
- Guan, D.; Chen, Z.; Chen, X.; Zhang, Y.; Qi, Q.; Shi, Q.; Zhao, S.; Xu, C.; Zhang, L. Molecular-level heavy petroleum hydrotreating modeling and comparison with high-resolution mass spectrometry. Fuel 2021, 297, 120792. [Google Scholar] [CrossRef]
- Sano, M.; Suzuki, M.; Homma, K.; Hayashida, K.; Tamura, T.; Matsuoka, T.; Katsumata, Y.; Onuki, S.; Sasaki, J. Promising novel therapy with hydrogen gas for emergency and critical care medicine. Acute Med. Surg. 2018, 5, 113–118. [Google Scholar] [CrossRef]
- Wang, W.-H.; Liu, X.; Bao, M. Chapter 14—Hydrogenation of fats and oils using supercritical carbon dioxide. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Inamuddin, A.M., Asiri, A., Suvardhan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 347–356. [Google Scholar]
- Wulf, C.; Zapp, P. Analyzing the future potential of defossilizing industrial specialty glass production with hydrogen by LCA. Procedia CIRP 2022, 105, 666–671. [Google Scholar] [CrossRef]
- Autrey, T.; Chen, P. Hydrogen energy carriers. J. Energy Chem. 2023, 77, 119–121. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- Mordor Intelligence. Carbon Black Market Size & Share Analysis—Growth Trends & Forecasts (2023–2028); Mordor Intelligence: Hyderabad, India, 2023; Available online: https://www.mordorintelligence.com/industry-reports/carbon-black-market (accessed on 5 May 2023).
- Mathew, M.; Dominic, C.M.; Neenu, K.V.; Begum, P.S.; Dileep, P.; Kumar, T.A.; Sabu, A.A.; Nagane, D.; Parameswaranpillai, J.; Badawi, M. Carbon black and chitin nanofibers for green tyres: Preparation and property evaluation. Carbohydr. Polym. 2023, 310, 120700. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhai, Z.; Tang, H. Experimental investigations on thermo-stamping of carbon fiber reinforced polyamide 6 hat-shaped components with self-resistance electrical heating: Influence on microscopic and macroscopic properties from temperature related processing parameters. J. Manuf. Process. 2023, 85, 1133–1143. [Google Scholar] [CrossRef]
- Al-Lami, A.; Hilmer, P.; Sinapius, M. Eco-efficiency assessment of manufacturing carbon fiber reinforced polymers (CFRP) in aerospace industry. Aerosp. Sci. Technol. 2018, 79, 669–678. [Google Scholar] [CrossRef]
- Kareiva, A.; Beganskiene, A.; Senvaitiene, J.; Ramanaviciene, A.; Vaitkus, R.; Barkauskas, J.; Ramanavicius, A. Evaluation of carbon-based nanostructures suitable for the development of black pigments and glazes. Colloids Surf. A Physicochem. Eng. Asp. 2019, 580, 123718. [Google Scholar] [CrossRef]
- Gusev, A.L.; Jabbarov, T.G.; Mamedov, S.G.; Malikov, R.; Hajibalaev, N.M.; Abdullaeva, S.D.; Abbasov, N.M. Production of hydrogen and carbon in the petrochemical industry by cracking of hydrocarbons in the process of heat utilization in steel production. Int. J. Hydrogen Energy 2023, 48, 14954–14963. [Google Scholar] [CrossRef]
- Fincke, J.R.; Anderson, R.P.; Hyde, T.A.; Detering, B.A. Plasma Pyrolysis of Methane to Hydrogen and Carbon Black. Ind. Eng. Chem. Res. 2002, 41, 1425–1435. [Google Scholar] [CrossRef]
- Sigaeva, S.; Temerev, V.; Borisov, V.; Tsyrulnikov, P. Pyrolysis of Methane on a Fechral Resistive Catalyst under Addition of Hydrogen or Oxygen into Reaction Mixture. Kataliz V Promyshlennosti 2015, 15, 6–9. [Google Scholar]
- Sigaeva, S.; Temerev, V.; Kuznetsova, N.; Tsyrulnikov, P. Pyrolysis of Methane over Resistible Supported Catalysts MeOx/Carborundum, where MeOx are MgO, CaO, MgO/Al2O3, MgO/ZrO2, CaO/Al2O3, CaO/ZrO2. Kataliz V Promyshlennosti 2017, 17, 178–183. [Google Scholar] [CrossRef]
- Vlaskin, M.S.; Grigorenko, A.V.; Gromov, A.A.; Kumar, V.; Dudoladov, A.O.; Slavkina, O.V.; Darishchev, V.I. Methane pyrolysis on sponge iron powder for sustainable hydrogen production. Results Eng. 2022, 15, 100598. [Google Scholar] [CrossRef]
- Parfenov, V.; Nikitchenko, N.; Pimenov, A.; Kuz’min, A.; Kulikova, M.; Chupichev, O.; Maksimov, A.L. Methane Pyrolysis for Hydrogen Production: Specific Features of Using Molten Metals. Russ. J. Appl. Chem. 2020, 93, 625–632. [Google Scholar] [CrossRef]
- Catalan, L.J.J.; Rezaei, E. Modelling the hydrodynamics and kinetics of methane decomposition in catalytic liquid metal bubble reactors for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 7547–7568. [Google Scholar] [CrossRef]
- Hu, X.; Hu, Y.; Xu, Q.; Wang, X.; Li, G.; Cheng, H.; Zou, X.; Lu, X. Molten salt-promoted Ni–Fe/Al2O3 catalyst for methane decomposition. Int. J. Hydrogen Energy 2020, 45, 4244–4253. [Google Scholar] [CrossRef]
- Abbas, H.F.; Ashik, U.P.M.; Mohammed, S.A.; Daud, W.M.A.W. Impact of reactor materials on methane decomposition for hydrogen production. Chem. Eng. Res. Des. 2021, 174, 127–136. [Google Scholar] [CrossRef]
- Younessi-Sinaki, M.; Matida, E.; Hamdullahpur, F. Kinetic model of homogeneous thermal decomposition of methane and ethane. Int. J. Hydrogen Energy 2009, 34, 3710–3716. [Google Scholar] [CrossRef]




| Component | Volumetric Content of the Component in the Gas Sample Depending on Methane Flow Rate and Process Temperature, vol.% | |||||
|---|---|---|---|---|---|---|
| 1000 °C | 1100 °C | 1200 °C | ||||
| 1 L/min | 5 L/min | 1 L/min | 5 L/min | 1 L/min | 5 L/min | |
| H2 | 28.64 | 1.10 | 81.41 | 52.06 | 92.74 | 72.09 |
| CH4 | 68.25 | 98.63 | 16.59 | 44.82 | 6.00 | 23.86 |
| O2 | 0.05 | 0.03 | 0.3 | 0.001 | 0.03 | 0.46 |
| N2 | 0.10 | 0.05 | 0.17 | 0.02 | 0.15 | 1.40 |
| C2H6 | 0.15 | 0.04 | 0.12 | 0.13 | 0.09 | 0.09 |
| C2H4 | 0.45 | 0.14 | 1.15 | 1.97 | 0.66 | 1.17 |
| C2H2 | 0.02 | 0.01 | 0 | 0.01 | 0 | <10−4 |
| C3H8 | 0.53 | 0.01 | 0.08 | 0.001 | <10−4 | <10−4 |
| C3H6 | 0.56 | <10−4 | 0.44 | 1.06 | 0.33 | 0.93 |
| T, °C | Methane Flow Rate, L/min | Operation Time, min | Mass of Soot, g | Soot Generation Rate, g/min | |||||
|---|---|---|---|---|---|---|---|---|---|
| Through Tube | Through Filter | In the Tube | In the Filter | Total | In the Tube | In the Filter | Total | ||
| 1000 | 1 | 74 | 60 | 1.28 | 0 | 1.28 | 0.017 | 0 | 0.017 |
| 1050 | 1 | 69 | 60 | 2.4 | 0.36 | 2.76 | 0.035 | 0.006 | 0.041 |
| 1100 | 1 | 90 | 78 | 12.12 | 6.65 | 18.77 | 0.135 | 0.085 | 0.22 |
| 1200 | 1 | 69 | 60 | 24.7 | 8.9 | 33.6 | 0.358 | 0.148 | 0.506 |
| 1400 | 1 | 69 | 60 | 40.3 | 3.6 | 43.9 | 0.584 | 0.06 | 0.644 |
| 1200 | 5 | 63 | 60 | 51.8 | 23.85 | 75.65 | 0.822 | 0.398 | 1.22 |
| 1200 | 5 | 63 | 60 | 45.91 | 25.86 | 71.77 | 0.729 | 0.431 | 1.16 |
| № n/a | The Element | Element Content in a Soot Sample, ppm | № n/a | The Element | Element Content in a Soot Sample, ppm | ||
|---|---|---|---|---|---|---|---|
| From the Tube | From the Filter | From the Tube | From the Filter | ||||
| 1 | Na | 5.0 ± 0.2 | 6.7 ± 0.5 | 27 | Tl | <0.002 | <0.04 |
| 2 | Mg | 89 ± 2 | 10 ± 4 | 28 | Bi | <0.07 | 0.0088 ± 0.0002 |
| 3 | Al | 94 ± 4 | 81 ± 6 | 29 | Th | 0.043 ± 0.006 | <0.002 |
| 4 | K | <2 | <14 | 30 | U | 0.063 ± 0.004 | <0.0003 |
| 5 | Ca | 332 ± 16 | 250 ± 30 | 31 | Ru | <0.00004 | <0.0007 |
| 6 | Cr | 1.3 ± 0.4 | 16 ± 3 | 32 | Rh | <0.00004 | <0.0003 |
| 7 | Mn | 6.1 ± 0.5 | 1.3 ± 0.2 | 33 | Pd | 0.0008 ± 0.0002 | <0.004 |
| 8 | Fe | 12 ± 4 | 63 ± 9 | 34 | Ir | <0.0001 | <0.005 |
| 9 | Ni | 0.87 ± 0.01 | 11 ± 1 | 35 | Pt | 0.0065 ± 0.0005 | <0.001 |
| 10 | Zn | 2.1 ± 0.4 | 4.3 ± 0.3 | 36 | Sc | 0.21 ± 0.02 | <0.002 |
| 11 | Ba | 1.8 ± 0.1 | 1.1 ± 0.3 | 37 | Y | 0.63 ± 0.04 | 0.0034 ± 0.0009 |
| 12 | Pb | <0.5 | 9 ± 1 | 38 | In | 0.0004 ± 0.0001 | <0.0003 |
| 13 | Li | 0.04 ± 0.01 | 0.18 ± 0.03 | 39 | La | 0.07 ± 0.01 | 0.0020 ± 0.0002 |
| 14 | Be | 0.0052 ± 0.0004 | <0.007 | 40 | Ce | 0.13 ± 0.01 | 0.0020 ± 0.0007 |
| 15 | V | 0.15 ± 0.03 | 0.06 ± 0.02 | 41 | Pr | 0.015 ± 0.001 | 0.0011 ± 0.0001 |
| 16 | Co | 0.025 ± 0.001 | 0.10 ± 0.01 | 42 | Nd | 0.058 ± 0.005 | 0.25 ± 0.01 |
| 17 | Cu | 2.7 ± 0.3 | 0.51 ± 0.02 | 43 | Sm | 0.013 ± 0.001 | <0.0004 |
| 18 | Ga | 0.020 ± 0.003 | 0.029 ± 0.005 | 44 | Eu | 0.0029 ± 0.0002 | <0.0001 |
| 19 | As | <0.004 | <0.02 | 45 | Gd | 0.016 ± 0.001 | 0.0003 ± 0.0001 |
| 20 | Se | <0.03 | <0.2 | 46 | Tb | 0.0035 ± 0.0001 | 0.00014 ± 0.00004 |
| 21 | Rb | 0.016 ± 0.005 | <0.002 | 47 | Dy | 0.030 ± 0.001 | <0.0003 |
| 22 | Sr | 1.25 ± 0.06 | 0.09 ± 0.03 | 48 | Ho | 0.010 ± 0.001 | <0.00008 |
| 23 | Cd | 0.0020 ± 0.0003 | <0.002 | 49 | Er | 0.041 ± 0.004 | 0.0005 ± 0.0001 |
| 24 | Te | <0.003 | <0.01 | 50 | Tm | 0.008 ± 0.001 | <0.00007 |
| 25 | Cs | 0.0011 ± 0.0004 | <0.0005 | 51 | Yb | 0.097 ± 0.006 | 0.00022 ± 0.00001 |
| 26 | Re | 0.0010 ± 0.0001 | <0.0006 | 52 | Lu | 0.013 ± 0.001 | <0.00009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galtsov-Tsientsiala, M.S.; Dudoladov, A.O.; Grigorenko, A.V.; Vlaskin, M.S. Study of Soot Deposits during Continuous Methane Pyrolysis in a Corundum Tube. Appl. Sci. 2023, 13, 10817. https://doi.org/10.3390/app131910817
Galtsov-Tsientsiala MS, Dudoladov AO, Grigorenko AV, Vlaskin MS. Study of Soot Deposits during Continuous Methane Pyrolysis in a Corundum Tube. Applied Sciences. 2023; 13(19):10817. https://doi.org/10.3390/app131910817
Chicago/Turabian StyleGaltsov-Tsientsiala, Matvey S., Aleksandr O. Dudoladov, Anatoly V. Grigorenko, and Mikhail S. Vlaskin. 2023. "Study of Soot Deposits during Continuous Methane Pyrolysis in a Corundum Tube" Applied Sciences 13, no. 19: 10817. https://doi.org/10.3390/app131910817





