Determination of Vehicle Emission Rates for Ammonia and Organic Molecular Markers Using a Chassis Dynamometer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Vehicle Selection
2.2. Test Mode
2.3. Chassis Dynamometer System and Exhaust Gas Analysis
3. Results
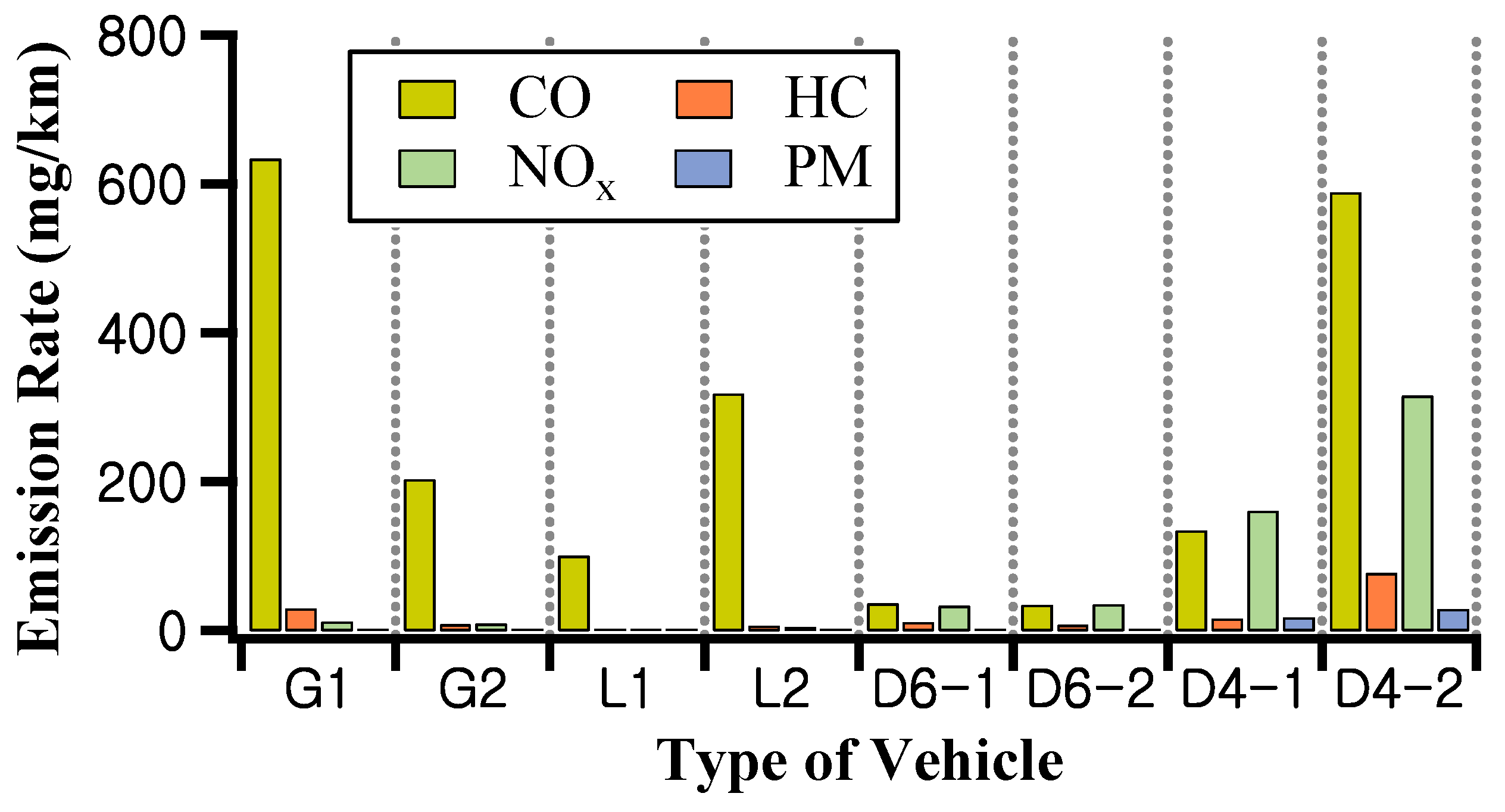
3.1. Emission Rate of CO, HC, NOx, and PM on the Regulatory Test Mode
3.2. Emission Rate of CO, HC, NOx, and PM on Diverse Test Mode Characteristics
3.3. Emission Rate of Ammonia
3.4. Emission Rate of Organic Molecular Markers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Vehicle | G1 | G2 | L1 | L2 |
|---|---|---|---|---|
| Fuel | Gasoline | Gasoline | LPG | LPG |
| Production year | 2015 | 2017 | 2017 | 2015 |
| Mileage | 76,000 | 26,000 | 4000 | 78,000 |
| Engine | Theta II | Alpha | NU | l4 |
| Fuel supply method | GDI (Turbocharger) | GDI | LPLi | LPGi |
| Displacement (cc) | 1999 | 1598 | 1999 | 1998 |
| Maximum output (HP) | 168 | 13 | 146 | 137 |
| Maximum torque (kg·m) | 20.5 | 15.7 | 19.5 | 18.7 |
| Fuel efficiency (km/L] | 10.8 | 13.7 | 10.3 | 8.3 |
| Reduction device | Three-way catalytic converter (Tier-2) | Three-way catalytic converter (Tier-2) | Three-way catalytic converter (Tier-3) | Three-way catalytic converter (Tier-2) |
| Vehicle | D6-1 | D6-2 | D4-1 | D4-2 |
|---|---|---|---|---|
| Fuel | Diesel | Diesel | Diesel | Diesel |
| Production year | 2018 | 2018 | 2007 | 2008 |
| Mileage | 4000 | 2500 | 89,000 | 190,000 |
| Engine | R VGT | UIII e-VGT | J3 | D-VGT |
| Fuel supply method | CRDI | CRDI | CRDI | CRDI |
| Displacement (cc) | 1995 | 1598 | 2902 | 1991 |
| Max. output (HP) | 186 | 136 | 174 | 151 |
| Max. torque (kg·m) | 41.0 | 32.6 | 36.0 | 34.0 |
| Fuel efficiency (km/L) | 13.8 | 16.3 | 10.0 | 12.6 |
| Reduction device | EGR + DOC + SCR + DPF (EURO-6) | EGR + LNT + SCR + DPF (EURO-6) | EGR + DOC (EURO-4) | EGR + DOC (EURO-4) |
References
- Meng, Z.; Chen, C.; Li, J.; Fang, J.; Tan, J.; Qin, Y.; Jiang, Y.; Qin, Z.; Bai, W.; Liang, K. Particle emission characteristics of DPF regeneration from DPF regeneration bench and diesel engine bench measurements. Fuel 2020, 262, 116589. [Google Scholar] [CrossRef]
- Wallington, T.J.; Sullivan, J.L.; Hurley, M.D. Emissions of CO2, CO, NOx, HC, PM, HFC-134a, N2O and CH4 from the global light duty vehicle fleet. Meteorol. Z. 2008, 17, 109–116. [Google Scholar] [CrossRef]
- Chow, J.C.; Watson, J.G.; Fujita, E.M.; Lu, Z.; Lawson, D.R.; Ashbaugh, L.L. Temporal and spatial variations of PM2.5 and PM10 aerosol in the southern California air quality study. Atmos. Environ. 1994, 28, 2061–2080. [Google Scholar] [CrossRef]
- Rönkkö, T.; Kuuluvainen, H.; Karjalanen, P.; Keskinen, J.; Hillamo, R.; Niemi, J.V.; Pirjola, L.; Timonen, H.; Saarikoski, S.; Saukko, E.; et al. Traffic is a major source of atmospheric nanocluster aerosol. Proc. Natl. Acad. Sci. USA 2017, 114, 7549–7554. [Google Scholar] [CrossRef]
- Feng, R.; Hu, X.; Li, G.; Sun, Z.; Deng, B. A comparative investigation between particle oxidation catalyst (POC) and diesel particulate filter (DPF) coupling aftertreatment system on emission reduction of a non-road diesel engine. Ecotoxicol. Environ. Saf. 2002, 238, 113576. [Google Scholar] [CrossRef]
- Pathak, S.K.; Sood, V.; Singh, Y.; Channiwala, S.A. Real world vehicle emissions: Their correlation with driving parameters. Transp. Res. Part D Transp. Environ. 2016, 44, 157–176. [Google Scholar] [CrossRef]
- Meng, Z.; Zeng, B.; Tan, J.; Chen, Z.; Ou, J. Study of gas and particulate emission characteristics during the fast regeneration period of DPF. Fuel 2022, 317, 123353. [Google Scholar] [CrossRef]
- Shibata, K.; Enya, K.; Ishikawa, N.; Sakamoto, K. EC/OC and PAHs Emissions from a Modern Diesel Engine with DPF Regeneration Fueled by 10% RME Biodiesel. Aerosol Air Qual. Res. 2019, 19, 1765–1774. [Google Scholar] [CrossRef]
- Cheung, K.L.; Polidori, A.; Ntziachristos, L.; Tzamkiozis, T.; Samaras, Z.; Cassee, F.R.; Gerlofs, M.; Sioutas, C. Chemical Characteristics and Oxidative Potential of Particulate Matter Emissions from Gasoline, Diesel, and Biodiesel Vehicles. Environ. Sci. Technol. 2009, 43, 6334–6340. [Google Scholar] [CrossRef]
- Tsai, J.H.; Chang, S.Y.; Chiang, H.L. Volatile organic compounds from the exhaust of light-duty diesel vehicles. Atmos. Environ. 2012, 61, 499–506. [Google Scholar] [CrossRef]
- Chiang, H.L.; Lai, Y.M.; Chang, S.Y. Pollutant constituents of exhaust emitted from light-duty diesel vehicles. Atmos. Environ. 2012, 47, 399–406. [Google Scholar] [CrossRef]
- Louis, C.; Liu, Y.; Tassel, P.; Perret, P.; Chaumond, A.; André, M. PAH, BTEX, carbonyl compound, black-carbon, NO2 and ultrafine particle dynamometer bench emissions for Euro 4 and Euro 5 diesel and gasoline passenger vehicles. Atmos. Environ. 2016, 141, 80–95. [Google Scholar] [CrossRef]
- Liu, Y.; Martinet, S.; Louis, C.; Pasquier, A.; Tassel, P.; Perret, P. Emission Characterization of In-Use Diesel and Gasoline Euro 4 to Euro 6 Passenger Vehicles Tested on Chassis Dynamometer Bench and Emission Model Assessment. Aerosol Air Qual. Res. 2017, 17, 2289–2299. [Google Scholar] [CrossRef]
- Yang, H.H.; Dhital, N.B.; Wang, L.C.; Hsieh, Y.S.; Lee, K.T.; Hsu, Y.T.; Huang, S.C. Chemical Characterization of Fine Particulate Matter in Gasoline and Diesel Vehicle Exhaust. Aerosol Air Qual. Res. 2019, 19, 1439–1449. [Google Scholar] [CrossRef]
- Šarkan, B.; Jaśkiewicz, M.; Kubiak, P.; Tarnapowicz, D.; Loman, M. Exhaust Emissions Measurement of a Vehicle with Retrofitted LPG System. Energies 2022, 15, 1184. [Google Scholar] [CrossRef]
- Han, S.B.; Kim, Y.T.; Lee, H.K.; Kang, J.H.; Jung, J.W.; Chung, Y.J. Characteristics Analysis of Exhaust Emission according to Fuels at CVS-75 Mode. J. Energy Eng. 2009, 18, 69–73. [Google Scholar]
- Park, G.; Mun, S.; Hong, H.; Chung, T.; Jung, S.; Kim, S.; Seo, S.; Kim, J.; Lee, J.; Kim, K.; et al. Characterization of Emission Factors Concerning Gasoline, LPG, and Diesel Vehicles via Transient Chassis-Dynamometer Tests. Appl. Sci. 2019, 9, 1573. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, B.; Deng, M.; Huang, S. An Overview of Chassis Dynamometer in the Testing of Vehicle Emission. MATEC Web Conf. 2018, 175, 02015. [Google Scholar] [CrossRef]
- Perrone, M.G.; Carbone, C.; Faedo, D.; Ferrero, L.; Maggioni, A.; Sangiorgi, G.; Bolzacchini, E. Exhaust emissions of polycyclic aromatic hydrocarbons, n-alkanes and phenols from vehicles coming within different European classes. Atmos. Environ. 2014, 82, 391–400. [Google Scholar] [CrossRef]
- Dunbar, J.C.; Lin, C.I.; Vergucht, I.; Wong, J.; Durant, J.L. Estimating the contributions of mobile sources of PAH to urban air using real-time PAH monitoring. Sci. Total Environ. 2001, 279, 1–19. [Google Scholar] [CrossRef]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R. Measurement of emissions from air pollution sources. 5. C1−C32 organic compounds from gasoline-powered motor vehicles. Environ. Sci. Technol. 2002, 36, 1169–1180. [Google Scholar] [CrossRef]
- Liu, P.; Ding, J.; Ji, Y.; Xu, H.; Liu, S.; Xiao, B.; Jin, H.; Zhong, X.; Guo, Z.; Wang, H.; et al. Satellite Support to Estimate Livestock Ammonia Emissions: A Case Study in Hebei, China. Atmosphere 2022, 13, 1552. [Google Scholar] [CrossRef]
- Hernández, D.L.; Vallano, D.M.; Zavaleta, E.S.; Tzankova, Z.; Pasari, J.R.; Weiss, S.; Selmants, P.C.; Morozumi, C. Nitrogen pollution is linked to US listed species declines. BioScience 2016, 66, 213–222. [Google Scholar] [CrossRef]
- Suarez-Bertoa, R.; Zardini, A.A.; Astorga, C. Ammonia exhaust emissions from spark ignition vehicles over the New European Driving Cycle. Atmos. Environ. 2014, 97, 43–53. [Google Scholar] [CrossRef]
- Livingston, C.; Rieger, P.; Winer, A. Ammonia emissions from a representative in-use fleet of light and medium-duty vehicles in the California South Coast Air Basin. Atmos. Environ. 2009, 43, 3326–3333. [Google Scholar] [CrossRef]
- Jo, S.; Cha, J.; Park, S. Exhaust emission characteristics of stoichiometric combustion applying to diesel particulate filter (DPF) and three-way catalytic converter (TWC). Energy 2022, 254, 124196. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Z.; Li, M.; Shao, S. Combustion and emissions of a Euro VI heavy-duty natural gas engine using EGR and TWC. J. Nat. Gas Sci. Eng. 2016, 28, 660–671. [Google Scholar] [CrossRef]
- Liu, M.; Huang, X.; Song, Y.; Tang, J.; Cao, J.; Zhang, X.; Zhang, Q.; Wang, S.; Xu, T.; Kang, L.; et al. Ammonia emission control in China would mitigate haze pollution and nitrogen deposition, but worsen acid rain. Proc. Natl. Acad. Sci. USA 2019, 116, 7760–7765. [Google Scholar] [CrossRef]
- Pinder, R.W.; Adams, P.J.; Pandis, S.N. Ammonia emission controls as a cost-effective strategy for reducing atmospheric particulate matter in the eastern United States. Environ. Sci. Technol. 2007, 41, 380–386. [Google Scholar] [CrossRef]
- Wang, C.; Tan, J.; Harle, G.; Gong, H.; Xia, W.; Zheng, T.; Yang, D.; Ge, Y.; Zhao, Y. Ammonia Formation over Pd/ Rh Three-Way Catalysts during Lean-to-Rich Fluctuations: The Effect of the Catalyst Aging. Exhaust Temperature, Lambda, and Duration in Rich Conditions. Environ. Sci. Technol. 2019, 53, 12621–12628. [Google Scholar] [CrossRef]
- Liu, T.; Wang, X.; Wang, B.; Ding, X.; Deng, W.; Lu, S.; Zhang, Y. Emission rate of ammonia (NH3) from on-road vehicles in China: Tunnel tests in urban Guangzhou. Environ. Res. Lett. 2014, 9, 064027. [Google Scholar] [CrossRef]
- Wong, P.K.; Chadikolaei, M.A.; Chen, S.H.; Fadairo, A.A.; Ng, K.W.; Lee, S.M.Y.; Xu, J.C.; Lian, Z.D.; Li, L.; Wong, H.C.; et al. Physical, chemical, and cell toxicity properties of mature/aged particulate matter (PM) trapped in a diesel particulate filter (DPF) along with the results from freshly produced PM of a diesel engine. J. Hazard. Mater. 2022, 434, 128855. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Zou, Z.; Deng, C.; Huang, K.; Collett, J.L.; Lin, J.; Zhuang, G. The importance of vehicle emissions as a source of atmospheric ammonia in the megacity of Shanghai. Atmos. Chem. Phys. 2016, 16, 3577–3594. [Google Scholar] [CrossRef]
- Emmenegger, L.; Mohn, J.; Sigrist, M.; Marinov, D.; Steinemann, U.; Zumsteg, F.; Meier, M. Measurement of ammonia emissions using various techniques in a comparative tunnel study. Int. J. Environ. Pollut. 2004, 22, 326–341. [Google Scholar] [CrossRef]
- Tong, H.Y.; Hung, W.T.; Cheung, C.S. On-Road Motor Vehicle Emissions and Fuel Consumption in Urban Driving Conditions. J. Air Waste Manag. Assoc. 2000, 50, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Wang, Y.; Chong, J.; Lee, H.; Jang, J.; Song, H.; Kwak, N.; Borlaza, L.J.S.; Maeng, H.; Cosep, E.M.R.; et al. Simultaneous Measurements of Chemical Compositions of Fine Particles during Winter Haze Period in Urban Sites in China and Korea. Atmosphere 2020, 11, 292. [Google Scholar] [CrossRef]
- Song, M.K.; Park, J.S.; Lim, Y.J.; Oh, S.H.; Lee, J.Y.; Lee, K.H.; Ro, C.U.; Bae, M.S. Long-range transport impacts from biomass burning and secondary pollutant sources based on receptor models during KORUS-AQ campaign. Atmos. Environ. 2022, 276, 119060. [Google Scholar] [CrossRef]
- Wang, C.; Xu, H.; Herreros, J.M.; Wang, J.; Cracknell, R. Impact of fuel and injection system on particle emissions from a GDI engine. Appl. Energy 2014, 132, 178–191. [Google Scholar] [CrossRef]
- Bonatesta, F.; Chiappetta, E.; La Rocca, A. Part-load particulate matter from a GDI engine and the connection with combustion characteristics. Appl. Energy 2014, 124, 366–376. [Google Scholar] [CrossRef]
- Durbin, T.D.; Wilson, R.D.; Norbeck, J.M.; Miller, J.W.; Huai, T.; Rhee, S.H. Estimates of the emission rates of ammonia from light-duty vehicles using standard chassis dynamometer test cycles. Atmos. Environ. 2002, 36, 1475–1482. [Google Scholar] [CrossRef]
- Selleri, T.; Melas, A.; Bonnel, P.; Suarez-Bertoa, R. NH3 and CO Emissions from Fifteen Euro 6d and Euro 6d-TEMP Gasoline-Fuelled Vehicles. Catalysts 2022, 12, 245. [Google Scholar] [CrossRef]
- Bae, W.B.; Kim, D.Y.; Hyun, S.W.; Hazlett, M.; Yoon, D.Y.; Jung, C.; Kim, C.H.; Kang, S.B. Emission of NH3 and N2O during NO reduction over commercial aged three-way catalyst (TWC): Role of individual reductants in simulated exhausts. Chem. Eng. J. Adv. 2022, 9, 100222. [Google Scholar] [CrossRef]
- Liu, Y.; Ge, Y.; Tan, J.; Wang, H.; Ding, Y. Research on ammonia emissions characteristics from light-duty gasoline vehicles. J. Environ. Sci. 2021, 106, 182–193. [Google Scholar] [CrossRef]
- Lyu, L.; Sun, W.; Feng, P.; Wang, H.; Hao, L.; Tan, J.; Wang, X.; Song, C.; Li, H.; Li, Z.; et al. NH3 and N2O emission durability of the heavy-duty diesel engine with DOC, DPF, SCR, and ASC through the accelerated aging method. Fuel 2023, 339, 126950. [Google Scholar] [CrossRef]
- Zhang, J.; Liggio, J.; Chan, T.W.; Huang, L.; Brook, J.R. Gasoline Direct Injection Engine Emissions of OC and EC: Laboratory Comparisons with Port Fuel Injection Engine. Aerosol Air Qual. Res. 2022, 22, 220032. [Google Scholar] [CrossRef]


| Fuel Type | Vehicle | PM (mg/km) | NOx (mg/km) | CO (mg/km) | CO2 (g/km) | Reference |
|---|---|---|---|---|---|---|
| Diesel | Euro4 | 27.10 | [9] | |||
| Euro5 DPF | 0.63 | [9] | ||||
| Light-duty | 1735 | 1395 | [10] | |||
| Light-duty | 1907 | 1138 | [10] | |||
| Light-duty | 98.00 | 1173 | 980 | 281.6 | [11] | |
| Light-duty | 85.00 | 1128 | 1685 | 283.9 | [11] | |
| Light-duty | 233.00 | 1001 | 1208 | 311.7 | [11] | |
| Light-duty | 243.00 | 3983 | 2650 | 442.4 | [11] | |
| Light-duty | 52.00 | 1642 | 671 | 377.2 | [11] | |
| Light-duty | 315.00 | 1480 | 1178 | 407.8 | [11] | |
| EURO5 DPF | 0.01–0.65 | 209–770 | [12] | |||
| EURO5 DPF | 0–0.32 | 158–900 | [12] | |||
| EURO4 DPF | 0.72–3.74 | 244–515 | [12] | |||
| EURO5 DPF | 0–0.18 | 311–747 | [12] | |||
| EURO5 1 | 212–763 | 14–572 | 89–172 | [13] | ||
| EURO5 2 | 162–910 | 17–185 | 87–169 | [13] | ||
| EURO4 l | 233–503 | 58–1625 | 108–226 | [13] | ||
| EURO5 3 | 315–746 | 3–871 | 92–167 | [13] | ||
| EURO6b | 201–1076 | 16–380 | 80–140 | [13] | ||
| SUV | 2 | 201 | 188 | [14] | ||
| Light truck | 13 | 371 | 115 | [14] | ||
| Heavy truck | 31 | 2560 | 209 | [14] | ||
| Heavy truck | 185 | 2180 | 1100 | [14] | ||
| Gasoline | EURO3 | 2.41 | [9] | |||
| EURO1 | 72.10 | [9] | ||||
| EURO4 | 0.04–18.38 | 10–125 | [12] | |||
| EURO5 | 2.67–29.44 | 5–214 | [12] | |||
| EURO4 | 11–122 | 21–954 | 113–205 | [13] | ||
| EURO5 | 128–128 | 32–32 | 233 | [13] | ||
| EURO6b | 3–15 | 32–27 | 91–174 | [13] | ||
| Sedan | 1.25 | 20 | 256 | [14] | ||
| Sedan | 1.10 | 84 | 714 | [14] | ||
| SUV | 3.28 | 32 | 2340 | [14] | ||
| Light truck | 0.64 | 39.1 | 1000 | [14] | ||
| LPG | EURO4 | 35 | 3000 | 233 | [15] | |
| EURO4 | 31 | 947 | 184 | [15] | ||
| EURO4 | 92 | 1949 | 230 | [15] |
| Parameters | Driving Mode | |||
|---|---|---|---|---|
| CVS-75 | NEDC | WLTC | US06 | |
| Driving time (second) | 1874 | 1180 | 1800 | 600 |
| Driving distance (km) | 17.84 | 11 | 23.26 | 12.9 |
| Average speed (km/h) | 34.1 | 33.6 | 46.3 | 77.9 |
| Maximum speed (km/h) | 91.2 | 120 | 131.6 | 128 |
| Vehicle | Test Mode | CO | HC | NOx | HC + NOx | PM |
|---|---|---|---|---|---|---|
| G1, G2 | CVS-75 | 1.31 | 0.034 | 0.044 | - | 0.004 |
| L1 | CVS-75 | 0.625 | - | 0.019 | - | 0.002 |
| L2 | CVS-75 | 1.31 | 0.034 | 0.044 | - | - |
| EURO6 | WLTC | 0.5 | - | 0.08 | 0.17 | 0.0045 |
| D4-1 | NEDC | 0.5 | - | 0.25 | 0.3 | 0.025 |
| D4-2 | NEDC | 0.74 | - | 0.39 | 0.46 | 0.06 |
| Compound | Mode | G1 | G2 | L1 | L2 | D6-1 | D6-2 | D4-1 | D4-2 |
|---|---|---|---|---|---|---|---|---|---|
| CO | CVS-75 | 634.7 | 204.0 | 101.4 | 500.5 | - | - | - | - |
| US06 | 2577.0 | 576.0 | 126.0 | 7660.4 | - | - | - | 130.0 | |
| NEDC | 1711.5 | 612.6 | - | - | - | - | 135.0 | 590.0 | |
| WLTC | 1770.5 | 491.7 | - | - | 37.0 | 35.0 | 0.0 | 125.0 | |
| HC | CVS-75 | 30.0 | 9.0 | 3.0 | 10.0 | - | - | - | - |
| US06 | 21.0 | 7.0 | 1.0 | 34.0 | 4.0 | 4.0 | 2.0 | 18.0 | |
| NEDC | 177.0 | 45.0 | - | - | - | - | 17.0 | 78.0 | |
| WLTC | 23.0 | 2.0 | - | - | 8.5 | 12.0 | 4.0 | 21.0 | |
| NOx | CVS-75 | 13.0 | 10.0 | 3.0 | 5.0 | - | - | - | - |
| US06 | 40.0 | 50.0 | 2.0 | 2.0 | 38.0 | 60.0 | 430.0 | 670.0 | |
| NEDC | 40.0 | 10.0 | - | - | - | - | 162.0 | 316.0 | |
| WLTC | 50.0 | 35.0 | - | - | 33.4 | 36.0 | 323.0 | 480.0 | |
| PM | CVS-75 | 1.2 | - | - | 0.2 | - | - | - | - |
| US06 | - | 2.0 | 0.4 | 0.9 | 0.4 | - | 21.4 | 33.0 | |
| NEDC | - | - | - | - | - | - | 18.3 | 29.8 | |
| WLTC | 0.9 | - | - | - | 0.4 | 0.1 | 14.0 | 21.0 |
| Mode | G1 | G2 | L1 | L2 | D6-1 | D6--2 | D4-1 | D4-2 |
|---|---|---|---|---|---|---|---|---|
| CVS-75 | 61.8 | 8.7 | 4.4 | 17.8 | - | - | - | - |
| WLTC | 114.9 | 27.4 | - | - | 0.2 | 0.00 | 0.07 | 0.03 |
| US06 | 123.9 | 22.0 | 4.7 | 30.3 | 0.4 | 0.05 | 0.01 | 0.03 |
| NEDC | 106.0 | 15.0 | - | - | 0.4 | 0.09- | 0.04 | 0.12 |
| Vehicles | Mode | OC | EC | OC/EC |
|---|---|---|---|---|
| LPG | US06 | 0.04 | 0.01 | 4.0 |
| Gasoline (G1) | US06, CVS-75 | 0.10 | 0.03 | 0.3 |
| Gasoline (G2) | US06, CVS-75 | 0.02 | 0.02 | 1.2 |
| Diesel EURO4 | US06 | 1.44 | 10.38 | 0.1 |
| Diesel EURO6 | US06 | 0.01 | 0.01 | 0.9 |
| Diesel EURO6 during DPF regeneration | US06 | 0.03 | 0.04 | 0.6 |
| LPG | Gasoline | Gasoline | Diesel EURO4 | Diesel EURO6 | Diesel (DPFre) EURO6 | |
|---|---|---|---|---|---|---|
| Compounds | L2 + L1 | G1 | G2 | D4-1 + D4-2 | D6-1 + D6-2 | D6-1 + D6-2 |
| PAHs | 17.30 | 1482.09 | 90.44 | 62,997.68 | 0.43 | 49.28 |
| Hopanes | 1.83 | 10.44 | 4.60 | 854.52 | 0.53 | 5.31 |
| Cycloalkanes | 5.14 | 28.30 | 16.89 | 1208.50 | - | 47.68 |
| n-Alkanes | 211.13 | 717.99 | 355.88 | 85,290.67 | 171.17 | 1177.62 |
| Alkanoic acids | 1444.59 | 3836.63 | 1693.68 | 47,426.31 | 1913.94 | 2697.11 |
| Benzene carboxylic acids | 21.49 | 111.09 | 17.16 | 35,419.15 | 38.76 | 60.52 |
| Di-carboxylic acids | 193.07 | 1315.33 | 212.12 | 7430.16 | 72.65 | 228.02 |
| LPG | Gasoline | Gasoline | Diesel EURO4 | Diesel EURO6 | Diesel (DPFre) EURO6 | |
|---|---|---|---|---|---|---|
| Compounds | L2 + L1 | G1 | G2 | D4-1 + D4-2 | D6-1 + D6-2 | D6-1 + D6-2 |
| Phenanthrene | 0.52 | 234.99 | 8.19 | 14,826.95 | 0.27 | 41.42 |
| Anthracene | 0.21 | 63.60 | 2.01 | 3343.98 | - | 0.45 |
| Fluoranthene | 1.64 | 420.13 | 24.07 | 15,904.90 | - | 2.18 |
| Acephenanthrylene | 0.26 | 63.56 | 2.72 | 3341.99 | - | - |
| Pyrene | 4.68 | 576.89 | 20.02 | 18,199.49 | 0.16 | 2.85 |
| Benzo(ghi)fluoranthene | 2.45 | 30.21 | 5.18 | 1,588.51 | - | 0.44 |
| Cyclopenta(cd)pyrene | - | 2.83 | - | 148.72 | - | - |
| Benz(a)anthracene | 3.09 | 14.24 | 2.00 | 748.48 | - | 0.36 |
| Chrysene | 2.34 | 17.14 | 2.75 | 901.46 | - | 0.35 |
| Retene | 0.28 | 1.20 | 0.35 | 63.11 | - | - |
| Benzo(b)fluoranthene | 0.70 | 8.49 | 1.57 | 714.58 | - | - |
| Benzo(k)fluoranthene | 0.17 | 5.57 | 0.87 | 492.06 | - | - |
| Benzo(j)fluoranthene | 0.00 | 0.82 | 0.07 | 77.93 | - | - |
| Benzo(e)pyrene | 0.39 | 7.41 | 1.99 | 623.55 | - | - |
| Benzo(a)pyrene | 0.27 | 4.32 | 1.23 | 408.46 | - | - |
| Perylene | - | 0.88 | - | 46.29 | - | - |
| Indeno(123cd)pyrene | - | 8.11 | 3.00 | 426.25 | - | - |
| Benzo(ghi)perylene | 0.28 | 13.21 | 8.30 | 694.45 | - | 1.25 |
| Coronene | 0.00 | 8.49 | 5.77 | 446.52 | - | - |
| Dibenzo(ae)pyrene | 0.00 | 0.00 | 0.34 | - | - | - |
| LPG | Gasoline | Gasoline | Diesel EURO4 | Diesel EURO6 | Diesel (DPFre) EURO6 | |
|---|---|---|---|---|---|---|
| Compounds | L2 + L1 | G1 | G2 | D4-1 + D4-2 | D6-1 + D6-2 | D6-1 + D6-2 |
| 17A(H)-22,29,30-Trisnorhopane | 0.23 | 1.21 | 0.31 | 55.75 | - | 0.71 |
| 17A(H)-21B(H)-30-Norhopane | 0.53 | 4.57 | 1.32 | 235.82 | 0.15 | 1.81 |
| 17A(H)-21B(H)-Hopane | 0.51 | 2.20 | 0.92 | 140.73 | 0.38 | 1.07 |
| 22S-Homohopane | 0.19 | 1.55 | 0.42 | 84.46 | - | 0.72 |
| 22R-Homohopane | 0.16 | 0.92 | 0.36 | 63.98 | - | 0.51 |
| 22S-Bishomohopane | - | - | 0.35 | 49.56 | - | - |
| 22R-Bishomohopane | - | - | 0.37 | 37.19 | - | - |
| 22S-Trishomohopane | - | - | - | 30.80 | - | - |
| 22R-Trishomohopane | - | - | - | 18.78 | - | - |
| AAA-20S-C27-Cholestane | - | - | - | 29.84 | - | - |
| ABB-20R-C27-Cholestane | - | - | - | 22.22 | - | - |
| AAA-20R-27-Cholestane | - | - | - | 23.54 | - | - |
| ABB-20R-C28-Ergostane | - | - | 0.13 | 8.28 | - | - |
| ABB-20S-C28-Ergostane | - | - | 0.11 | 11.23 | - | - |
| ABB-20R-C29-Sitostane | 0.13 | - | 0.16 | 26.78 | - | 0.26 |
| ABB-20S-C29-Sitostane | 0.07 | - | 0.15 | 15.56 | - | 0.23 |
| LPG | Gasoline | Gasoline | Diesel EURO4 | Diesel EURO6 | Diesel (DPFre) EURO6 | |
|---|---|---|---|---|---|---|
| Compounds | L2 + L1 | G1 | G2 | D4-1 + D4-2 | D6-1 + D6-2 | D6-1 + D6-2 |
| Phthalic Acid | 12.50 | 50.57 | 10.20 | 1512.50 | 20.10 | 30.26 |
| Isophthalic Acid | 2.75 | 18.09 | 2.20 | 4959.33 | 5.50 | 10.02 |
| Terephthalic Acid | 4.10 | 36.62 | 3.38 | 2180.72 | 6.00 | 9.50 |
| 1,2,4-Benzene carboxylic Acid | - | - | - | 5936.00 | - | - |
| 1,2,3-Benzene carboxylic Acid | 1.17 | - | - | 16,689.74 | - | - |
| 1,3,5-Benzene carboxylic Acid | - | - | - | 486.09 | - | - |
| 1,2,4,5-Benzene carboxylic Acid | - | - | - | 2970.78 | - | - |
| Methylphthalic Acid | 0.96 | 5.82 | 1.38 | 683.98 | 7.17 | 10.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.-H.; Song, M.-K.; Oh, S.-H.; Choe, S.-Y.; Kim, M.-W.; Bae, M.-S. Determination of Vehicle Emission Rates for Ammonia and Organic Molecular Markers Using a Chassis Dynamometer. Appl. Sci. 2023, 13, 9366. https://doi.org/10.3390/app13169366
Yu G-H, Song M-K, Oh S-H, Choe S-Y, Kim M-W, Bae M-S. Determination of Vehicle Emission Rates for Ammonia and Organic Molecular Markers Using a Chassis Dynamometer. Applied Sciences. 2023; 13(16):9366. https://doi.org/10.3390/app13169366
Chicago/Turabian StyleYu, Geun-Hye, Myoung-Ki Song, Sea-Ho Oh, Seo-Yeong Choe, Min-Wook Kim, and Min-Suk Bae. 2023. "Determination of Vehicle Emission Rates for Ammonia and Organic Molecular Markers Using a Chassis Dynamometer" Applied Sciences 13, no. 16: 9366. https://doi.org/10.3390/app13169366






