Has the Toxicity of Therapeutic Deep Eutectic Systems Been Assessed?
Abstract
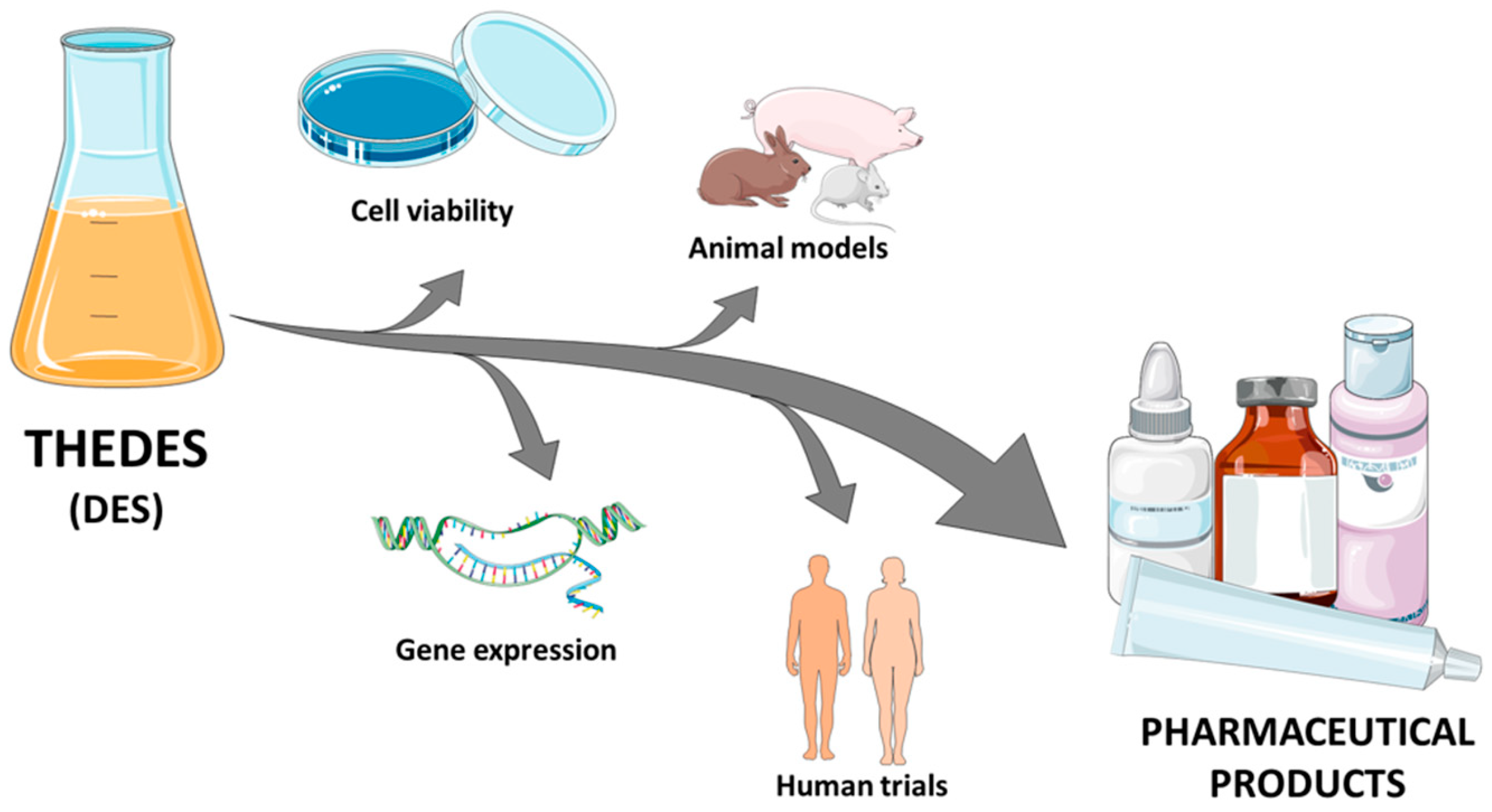
:Featured Application
Abstract
1. Introduction
2. Cell Lines
2.1. Cell Viability
2.2. Gene Expression
3. Animal Models
3.1. Injection Routes: Transdermal/Subcutaneous/Intravenous Administration
3.2. Oral Administration
3.3. Nasal Administration
4. Human Trials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| API | Active Pharmaceutical Ingredient |
| BCS | Biopharmaceutics Classification System |
| CAGE | Choline bicarbonate and Geranic acid |
| CAPD | Citric acid-1,2—propanediol 1:4 |
| ChCl | Choline Chloride |
| DES | Deep Eutectic Solvents |
| DHA | 2,4-Dihydroxybenzoic Acid |
| FA | Folic Acid |
| FMA | Fumaric Acid |
| FITIC-insulin | Fluorescein Isothiocyanate-Labeled Insulin |
| HBA | Hydrogen Bond Acceptor |
| HBD | Hydrogen Bond Donor |
| MSN | Silica Nanoparticle Matrix |
| NADES | Natural Deep Eutectic Solvents |
| PCL | Poly ε-Caprolactone |
| THEDES | Therapeutic Deep Eutectic Systems |
| THP | Trans-1,4,6-Trihydroxyphenanthrene |
References
- Paul, R.; Paul, S. Exploration on the drug solubility enhancement in aqueous medium with the help of endo -functionalized molecular tubes: A computational approach. Phys. Chem. Chem. Phys. 2021, 23, 18999–19010. [Google Scholar] [CrossRef] [PubMed]
- FDA. Formulating Drug Products for Optimized Absorption: Elucidating Amorphous Solid Dispersions. Available online: https://www.fda.gov/drugs/regulatory-science-action/formulating-drug-products-optimized-absorption-elucidating-amorphous-solid-dispersions (accessed on 5 May 2023).
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Rajpoot, K.; Tekade, M.; Sreeharsha, N.; Sharma, M.C.; Tekade, R.K. Recent advancements in solubilization of hydrophobic drugs. In The Future of Pharmaceutical Product Development and Research; Elsevier: Amsterdam, The Netherlands, 2020; pp. 109–144. ISBN 9780128144558. [Google Scholar]
- Kumar, S.; Bhargava, D.; Thakkar, A.; Arora, S. Drug carrier systems for solubility enhancement of BCS class II drugs: A critical review. Crit. Rev. Ther. Drug Carrier Syst. 2013, 30, 217–256. [Google Scholar] [CrossRef] [PubMed]
- Sekharan, T.R.; Chandira, R.M.; Tamilvanan, S.; Rajesh, S.C.; Venkateswarlu, B.S. Deep eutectic solvents as an alternate to other harmful solvents. Biointerface Res. Appl. Chem. 2022, 12, 847–860. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability Enhancement Techniques for Poorly Aqueous Soluble Drugs and Therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef]
- Savjani, K.T.; Gajjar, A.K.; Savjani, J.K. Drug Solubility: Importance and Enhancement Techniques. ISRN Pharm. 2012, 2012, 10. [Google Scholar] [CrossRef]
- Sikarra, D.; Shukla, V.; Kharia, A.A.; Chatterjee, D.P. Techniques for Solubility Enhancement of Poorly Soluble Drugs: An Overview. JMPAS 2012, 1, 1–22. [Google Scholar]
- Kanikkannan, N. Technologies to Improve the Solubility, Dissolution and Bioavailability of Poorly Soluble Drugs. J. Anal. Pharm. Res. 2018, 7, 1. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Tiwari, K.J.; Mahajan, V.R. Solubility Enhancement Techniques for Poorly Water-Soluble Drugs. Int. J. Pharm. Sci. Nanotechnol. 2017, 10, 3701–3708. [Google Scholar] [CrossRef]
- Abdkarimi, F.; Haghtalab, A. Solubility measurement and thermodynamic modeling of sertraline hydrochloride and clopidogrel bisulfate in deep eutectic solvent of choline chloride and malonic acid. J. Mol. Liq. 2021, 344, 117940. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chormale, J.H.; Bansal, A.K. Deep eutectic systems: An overview of fundamental aspects, current understanding and drug delivery applications. Int. J. Pharm. 2021, 610, 121203. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xue, Z.; Wang, J.; Jiang, J.; Zhao, X.; Mu, T. Investigation on the thermal stability of deep eutectic solvents. Acta Phys. Chim. Sin. 2018, 34, 904–911. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, T. Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem. Eng. 2021, 2, 174–186. [Google Scholar] [CrossRef]
- Fourmentin, S.; Gomes, M.C.; Lichtfouse, E. Deep Eutectic Solvents for Medicine, Gas Solubilization and Extraction of Natural Substances; Fourmentin, S., Costa Gomes, M., Lichtfouse, E., Eds.; Environmental Chemistry for a Sustainable World; Springer International Publishing: Cham, Switzerland, 2021; Volume 56, ISBN 978-3-030-53068-6. [Google Scholar]
- Nystedt, H.L.; Grønlien, K.G.; Tønnesen, H.H. Interactions of natural deep eutectic solvents (NADES) with artificial and natural membranes. J. Mol. Liq. 2021, 328, 115452. [Google Scholar] [CrossRef]
- Gala, U.; Chuong, M.C.; Varanasi, R.; Chauhan, H. Characterization and Comparison of Lidocaine-Tetracaine and Lidocaine-Camphor Eutectic Mixtures Based on Their Crystallization and Hydrogen-Bonding Abilities. AAPS PharmSciTech 2015, 16, 528–536. [Google Scholar] [CrossRef]
- Stott, P.W.; Williams, A.C.; Barry, B.W. Transdermal delivery from eutectic systems: Enhanced permeation of a model drug, ibuprofen. J. Control. Release 1998, 50, 297–308. [Google Scholar] [CrossRef]
- Abbott, A.P.; Ahmed, E.I.; Prasad, K.; Qader, I.B.; Ryder, K.S. Liquid pharmaceuticals formulation by eutectic formation. Fluid Phase Equilib. 2017, 448, 2–8. [Google Scholar] [CrossRef]
- Farooq, M.Q.; Abbasi, N.M.; Smith, E.A.; Petrich, J.W.; Anderson, J.L. Characterizing the Solvation Characteristics of Deep Eutectic Solvents Composed of Active Pharmaceutical Ingredients as a Hydrogen Bond Donor and/or Acceptor. ACS Sustain. Chem. Eng. 2022, 10, 3066–3078. [Google Scholar] [CrossRef]
- Tuntarawongsa, S.; Phaechamud, T. Menthol, borneol, camphor and WS-3 eutectic mixture. In Proceedings of the Advanced Materials Research; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2012; Volume 506, pp. 355–358. [Google Scholar]
- Morrison, H.G.; Sun, C.C.; Neervannan, S. Characterization of thermal behavior of deep eutectic solvents and their potential as drug solubilization vehicles. Int. J. Pharm. 2009, 378, 136–139. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Ferreira, A.S.D.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: Solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304. [Google Scholar] [CrossRef]
- Yin, T.; Wu, J.; Yuan, J.; Wang, X. Therapeutic deep eutectic solvent based on osthole and paeonol: Preparation, characterization, and permeation behavior. J. Mol. Liq. 2022, 346, 117133. [Google Scholar] [CrossRef]
- Lomba, L.; García, C.B.; Ribate, M.P.; Giner, B.; Zuriaga, E. Applications of deep eutectic solvents related to health, synthesis, and extraction of natural based chemicals. Appl. Sci. 2021, 11, 10156. [Google Scholar] [CrossRef]
- Silva, J.M.; Pereira, C.V.; Mano, F.; Silva, E.; Castro, V.I.B.; Sá-Nogueira, I.; Reis, R.L.; Paiva, A.; Matias, A.A.; Duarte, A.R.C. Therapeutic Role of Deep Eutectic Solvents Based on Menthol and Saturated Fatty Acids on Wound Healing. ACS Appl. Bio Mater. 2019, 2, 4346–4355. [Google Scholar] [CrossRef]
- Pedro, S.N.; Gomes, A.T.P.C.; Oskoei, P.; Oliveira, H.; Almeida, A.; Freire, M.G.; Silvestre, A.J.D.; Freire, C.S.R. Boosting antibiotics performance by new formulations with deep eutectic solvents. Int. J. Pharm. 2022, 616, 121566. [Google Scholar] [CrossRef]
- Aroso, I.M.; Silva, J.C.; Mano, F.; Ferreira, A.S.D.; Dionísio, M.; Sá-Nogueira, I.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Dissolution enhancement of active pharmaceutical ingredients by therapeutic deep eutectic systems. Eur. J. Pharm. Biopharm. 2016, 98, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Miguel Castro, M.; Santos, F.; Rita Jesus, A.; Paiva, A.; Oliveira, F.; Duarte, A.R.C. Selective terpene based therapeutic deep eutectic systems against colorectal cancer. Eur. J. Pharm. Biopharm. 2022, 175, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.V.; Silva, J.M.; Rodrigues, L.; Reis, R.L.; Paiva, A.; Duarte, A.R.C.; Matias, A. Unveil the Anticancer Potential of Limomene Based Therapeutic Deep Eutectic Solvents. Sci. Rep. 2019, 9, 14926. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Wang, L.; Han, W.; Gu, L.; Cui, X.; Wang, C. Novel Deep Eutectic Solvent–Hydrogel Systems for Synergistic Transdermal Delivery of Chinese Herb Medicine and Local Treatments for Rheumatoid Arthritis. Pharm. Res. 2022, 39, 2431–2446. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Duan, L.; Lin, Y.; Cui, X.; Yang, Y.; Wang, C. Deep Eutectic Systems as Novel Vehicles for Assisting Drug Transdermal Delivery. Pharmaceutics 2022, 14, 2265. [Google Scholar] [CrossRef]
- Emami, S.; Shayanfar, A. Deep eutectic solvents for pharmaceutical formulation and drug delivery applications. Pharm. Dev. Technol. 2020, 25, 779–796. [Google Scholar] [CrossRef]
- Aroso, I.M.; Craveiro, R.; Rocha, Â.; Dionísio, M.; Barreiros, S.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of controlled release systems for THEDES−Therapeutic deep eutectic solvents, using supercritical fluid technology. Int. J. Pharm. 2015, 492, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Sut, S.; Faggian, M.; Baldan, V.; Poloniato, G.; Castagliuolo, I.; Grabnar, I.; Perissutti, B.; Brun, P.; Maggi, F.; Voinovich, D.; et al. Natural Deep Eutectic Solvents (NADES) to enhance berberine absorption: An in vivo pharmacokinetic study. Molecules 2017, 22, 1921. [Google Scholar] [CrossRef] [PubMed]
- Durand, E.; Lecomte, J.; Upasani, R.; Chabi, B.; Bayrasy, C.; Baréa, B.; Jublanc, E.; Clarke, M.J.; Moore, D.J.; Crowther, J.; et al. Evaluation of the ROS Inhibiting Activity and Mitochondrial Targeting of Phenolic Compounds in Fibroblast Cells Model System and Enhancement of Efficiency by Natural Deep Eutectic Solvent (NADES) Formulation. Pharm. Res. 2017, 34, 1134–1146. [Google Scholar] [CrossRef] [PubMed]
- Mano, F.; Martins, M.; Sá-Nogueira, I.; Barreiros, S.; Borges, J.P.; Reis, R.L.; Duarte, A.R.C.; Paiva, A. Production of Electrospun Fast-Dissolving Drug Delivery Systems with Therapeutic Eutectic Systems Encapsulated in Gelatin. AAPS PharmSciTech 2017, 18, 2579–2585. [Google Scholar] [CrossRef]
- Mokhtarpour, M.; Shekaari, H.; Shayanfar, A. Design and characterization of ascorbic acid based therapeutic deep eutectic solvent as a new ion-gel for delivery of sunitinib malate. J. Drug Deliv. Sci. Technol. 2020, 56, 101512. [Google Scholar] [CrossRef]
- Silva, J.M.; Reis, R.L.; Paiva, A.; Duarte, A.R.C. Design of Functional Therapeutic Deep Eutectic Solvents Based on Choline Chloride and Ascorbic Acid. ACS Sustain. Chem. Eng. 2018, 6, 10355–10363. [Google Scholar] [CrossRef]
- Lomba, L.; Ribate, M.P.; Zaragoza, E.; Concha, J.; Garralaga, M.P.; Errazquin, D.; García, C.B.; Giner, B. Deep eutectic solvents: Are they safe? Appl. Sci. 2021, 11, 10061. [Google Scholar] [CrossRef]
- Hayyan, M.; Looi, C.Y.; Hayyan, A.; Wong, W.F.; Hashim, M.A. In Vitro and in Vivo toxicity profiling of ammonium-based deep eutectic solvents. PLoS ONE 2015, 10, e0117934. [Google Scholar] [CrossRef]
- Radošević, K.; Cvjetko Bubalo, M.; Gaurina Srček, V.; Grgas, D.; Landeka Dragičević, T.; Redovniković, R.I. Evaluation of toxicity and biodegradability of choline chloride based deep eutectic solvents. Ecotoxicol. Environ. Saf. 2015, 112, 46–53. [Google Scholar] [CrossRef]
- Macário, I.P.E.; Oliveira, H.; Menezes, A.C.; Ventura, S.P.M.; Pereira, J.L.; Gonçalves, A.M.M.; Coutinho, J.A.P.; Gonçalves, F.J.M. Cytotoxicity profiling of deep eutectic solvents to human skin cells. Sci. Rep. 2019, 9, 3932. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents-Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Hayyan, M.; Mbous, Y.P.; Looi, C.Y.; Wong, W.F.; Hayyan, A.; Salleh, Z.; Mohd-Ali, O. Natural deep eutectic solvents: Cytotoxic profile. Springerplus 2016, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hayyan, M.; Hashim, M.A.; Al-Saadi, M.A.; Hayyan, A.; AlNashef, I.M.; Mirghani, M.E.S. Assessment of cytotoxicity and toxicity for phosphonium-based deep eutectic solvents. Chemosphere 2013, 93, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.Y.; Xu, P.; Yang, F.X.; Wu, H.; Zong, M.H.; Lou, W.Y. Biocompatible Deep Eutectic Solvents Based on Choline Chloride: Characterization and Application to the Extraction of Rutin from Sophora japonica. ACS Sustain. Chem. Eng. 2015, 3, 2746–2755. [Google Scholar] [CrossRef]
- Arafa, W.A.A. Deep eutectic solvent for an expeditious sono-synthesis of novel series of bis-quinazolin-4-one derivatives as potential anti-cancer agents. R. Soc. Open Sci. 2019, 6, 182046. [Google Scholar] [CrossRef]
- Mbous, Y.P.; Hayyan, M.; Wong, W.F.; Looi, C.Y.; Hashim, M.A. Unraveling the cytotoxicity and metabolic pathways of binary natural deep eutectic solvent systems. Sci. Rep. 2017, 7, 41257. [Google Scholar] [CrossRef]
- Shekaari, H.; Zafarani-Moattar, M.T.; Mokhtarpour, M.; Faraji, S. Exploring cytotoxicity of some choline-based deep eutectic solvents and their effect on the solubility of lamotrigine in aqueous media. J. Mol. Liq. 2019, 283, 834–842. [Google Scholar] [CrossRef]
- Van Osch, D.J.G.P.; Zubeir, L.F.; Van Den Bruinhorst, A.; Rocha, M.A.A.; Kroon, M.C. Hydrophobic deep eutectic solvents as water-immiscible extractants. Green Chem. 2015, 17, 4518–4521. [Google Scholar] [CrossRef]
- Khorsandi, M.; Shekaari, H.; Mokhtarpour, M.; Hamishehkar, H. Cytotoxicity of some choline-based deep eutectic solvents and their effect on solubility of coumarin drug. Eur. J. Pharm. Sci. 2021, 167, 106022. [Google Scholar] [CrossRef]
- Lomba, L.; Garralaga, M.P.; Werner, Á.; Giner, B.; Baptista, P.M.; Sánchez-Romero, N. Ibuprofen solubility and cytotoxic study of deep eutectic solvents formed by xylitol, choline chloride and water. J. Drug Deliv. Sci. Technol. 2023, 82, 104327. [Google Scholar] [CrossRef]
- Pedro, S.N.; Mendes, M.S.M.; Neves, B.M.; Almeida, I.F.; Costa, P.; Correia-Sá, I.; Vilela, C.; Freire, M.G.; Silvestre, A.J.D.; Freire, C.S.R. Deep Eutectic Solvent Formulations and Alginate-Based Hydrogels as a New Partnership for the Transdermal Administration of Anti-Inflammatory Drugs. Pharmaceutics 2022, 14, 827. [Google Scholar] [CrossRef] [PubMed]
- Olivares, B.; Martínez, F.A.; Ezquer, M.; Morales, B.J.; Fuentes, I.; Calvo, M.; Campodónico, P.R. Betaine-urea deep eutectic solvent improves imipenem antibiotic activity. J. Mol. Liq. 2022, 350, 118551. [Google Scholar] [CrossRef]
- Wu, J.; Yin, T. Insight into the physicochemical properties and bioactivities of therapeutic deep eutectic solvents based on matrine and fatty acids. J. Mol. Liq. 2022, 360, 119560. [Google Scholar] [CrossRef]
- Siddiqui, R.; Makhlouf, Z.; Akbar, N.; Khamis, M.; Ibrahim, T.; Khan, A.S.; Khan, N.A. Antiamoebic properties of salicylic acid-based deep eutectic solvents for the development of contact lens disinfecting solutions against Acanthamoeba. Mol. Biochem. Parasitol. 2022, 250, 111493. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.; Santos, F.; Duarte, A.R.C. Therapeutic deep eutectic systems towards the treatment of tuberculosis and colorectal cancer: Opportunities and challenges. Molecules 2021, 26, 7022. [Google Scholar] [CrossRef] [PubMed]
- Sarraguça, M.C.; Ribeiro, P.R.S.; Nunes, C.; Seabra, C.L. Solids Turn into Liquids—Liquid Eutectic Systems of Pharmaceutics to Improve Drug Solubility. Pharmaceuticals 2022, 15, 279. [Google Scholar] [CrossRef]
- Santos, F.; Leitão, M.I.P.S.; Duarte, A.R.C. Properties of therapeutic deep eutectic solvents of L-arginine and ethambutol for tuberculosis treatment. Molecules 2019, 24, 55. [Google Scholar] [CrossRef]
- Roda, A.; Santos, F.; Matias, A.A.; Paiva, A.; Duarte, A.R.C. Design and processing of drug delivery formulations of therapeutic deep eutectic systems for tuberculosis. J. Supercrit. Fluids 2020, 161, 104826. [Google Scholar] [CrossRef]
- De Oliveira, F.S.N.; Duarte, A.R.C. A Look on Target-Specificity of Eutectic Systems Based on Natural Bioactive Compounds, 1st ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2021; Volume 97, ISBN 9780128216910. [Google Scholar]
- Grozdanova, T.; Trusheva, B.; Alipieva, K.; Popova, M.; Dimitrova, L.; Najdenski, H.; Zaharieva, M.M.; Ilieva, Y.; Vasileva, B.; Miloshev, G.; et al. Extracts of medicinal plants with natural deep eutectic solvents: Enhanced antimicrobial activity and low genotoxicity. BMC Chem. 2020, 14, 1–9. [Google Scholar] [CrossRef]
- Mattioli, R.; Di Risola, D.; Federico, R.; Ciogli, A.; Gasparrini, F.; Villani, C.; Fontana, M.; Maggiore, A.; D’erme, M.; Mosca, L.; et al. Effect of Natural Deep Eutectic Solvents on trans-Resveratrol Photo-Chemical Induced Isomerization and 2,4,6-Trihydroxyphenanthrene Electro-Cyclic Formation. Molecules 2022, 27, 2348. [Google Scholar] [CrossRef]
- Shamseddin, A.; Crauste, C.; Durand, E.; Villeneuve, P.; Dubois, G.; Pavlickova, T.; Durand, T.; Vercauteren, J.; Veas, F. Resveratrol-Linoleate protects from exacerbated endothelial permeability via a drastic inhibition of the MMP-9 activity. Biosci. Rep. 2018, 38, BSR20171712. [Google Scholar] [CrossRef] [PubMed]
- Szél, E.; Danis, J.; Sőrés, E.; Tóth, D.; Korponyai, C.; Degovics, D.; Prorok, J.; Acsai, K.; Dikstein, S.; Kemény, L.; et al. Protective effects of glycerol and xylitol in keratinocytes exposed to hyperosmotic stress. Clin. Cosmet. Investig. Dermatol. 2019, 12, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Wu, K.L.; Chang, S.F.; Chang, H.I.; Chen, C.N.; Chen, Y.Y. Fermented Ginger Extract in Natural Deep Eutectic Solvent Enhances Cytotoxicity by Inhibiting NF-κB Mediated CXC Chemokine Receptor 4 Expression in Oxaliplatin-Resistant Human Colorectal Cancer Cells. Antioxidants 2022, 11, 2057. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wan, R.; Huo, W.; Dong, H.; Chang, Z.; Xia, X. Cytotoxicity, genotoxicity, oxidative stress, and apoptosis in HepG2 cells induced by the imidazole ionic liquid 1-dodecyl-3-methylimidazolium chloride. Environ. Toxicol. 2020, 35, 665–672. [Google Scholar] [CrossRef] [PubMed]
- Lapeña, D.; Errazquin, D.; Lomba, L.; Lafuente, C.; Giner, B. Ecotoxicity and biodegradability of pure and aqueous mixtures of deep eutectic solvents: Glyceline, ethaline, and reline. Environ. Sci. Pollut. Res. 2021, 28, 8812–8821. [Google Scholar] [CrossRef] [PubMed]
- Vieira Sanches, M.; Freitas, R.; Oliva, M.; Mero, A.; De Marchi, L.; Cuccaro, A.; Fumagalli, G.; Mezzetta, A.; Colombo Dugoni, G.; Ferro, M.; et al. Are natural deep eutectic solvents always a sustainable option? A bioassay-based study. Environ. Sci. Pollut. Res. 2022, 30, 17268–17279. [Google Scholar] [CrossRef]
- Wen, Q.; Chen, J.X.; Tang, Y.L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef]
- Benlebna, M.; Ruesgas-Ramón, M.; Bonafos, B.; Fouret, G.; Casas, F.; Coudray, C.; Durand, E.; Cruz Figueroa-Espinoza, M.; Feillet-Coudray, C. Toxicity of Natural Deep Eutectic Solvent Betaine: Glycerol in Rats. J. Agric. Food Chem. 2018, 66, 6205–6212. [Google Scholar] [CrossRef]
- Nurunnabi, M.; Ibsen, K.N.; Tanner, E.E.L.; Mitragotri, S. Oral ionic liquid for the treatment of diet-induced obesity. Proc. Natl. Acad. Sci. USA 2019, 116, 25042–25047. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, T.; Lu, J.Z.; Ye, D.; Mu, S.; Tian, X.H.; Zhang, W.D.; Ma, B.L. Intra-Herb Interactions: Primary Metabolites in Coptidis Rhizoma Extract Improved the Pharmacokinetics of Oral Berberine Hydrochloride in Mice. Front. Pharmacol. 2021, 12, 1426. [Google Scholar] [CrossRef]
- Pradeepkumar, P.; Rajendran, N.K.; Alarfaj, A.A.; Munusamy, M.A.; Rajan, M. Deep Eutectic Solvent-Mediated FA-g-β-Alanine-co-PCL Drug Carrier for Sustainable and Site-Specific Drug Delivery. ACS Appl. Bio Mater. 2018, 1, 2094–2109. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Yang, J.; Zhao, Y.; Wan, H.; He, Y.; Zhang, L.; Wan, H.; Li, C. Greener extraction process and enhanced in vivo bioavailability of bioactive components from Carthamus tinctorius L. by natural deep eutectic solvents. Food Chem. 2021, 348, 129090. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhou, H.M.; Yue, S.J.; Feng, L.M.; Guo, D.Y.; Li, J.J.; Zhao, Q.; Huang, L.; Tang, Y.P. Oral Bioavailability-Enhancing and Anti-obesity Effects of Hydroxysafflor Yellow A in Natural Deep Eutectic Solvent. ACS Omega 2022, 7, 19225–19234. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, D.T.; Smaniotto, F.A.; Costa, I.F.; Baranzelli, J.; Muller, A.; Somacal, S.; Monteiro, C.S.A.; Vizzotto, M.; Rodrigues, E.; Barcia, M.T.; et al. Natural deep eutectic solvent (NADES): A strategy to improve the bioavailability of blueberry phenolic compounds in a ready-to-use extract. Food Chem. 2021, 364, 130370. [Google Scholar] [CrossRef] [PubMed]
- Semenov, A.L.; Tyndyk, M.L.; Von, J.D.; Ermakova, E.D.; Dorofeeva, A.A.; Tumanyan, I.A.; Radetskaya, E.A.; Yurova, M.N.; Zherebker, A.; Gorbunov, A.Y.; et al. Effects of Isoflavone-Rich NADES Extract of Pueraria lobata Roots and Astaxanthin-Rich Phaffia rhodozyma Extract on Prostate Carcinogenesis in Rats. Plants 2023, 12, 564. [Google Scholar] [CrossRef]
- Kim, J.; Shi, Y.; Kwon, C.J.; Gao, Y.; Mitragotri, S. A Deep Eutectic Solvent-Based Approach to Intravenous Formulation. Adv. Healthc. Mater. 2021, 10, 2100585. [Google Scholar] [CrossRef]
- Kim, J.; Gao, Y.; Zhao, Z.; Rodrigues, D.; Tanner, E.E.L.; Ibsen, K.; Sasmal, P.K.; Jaladi, R.; Alikunju, S.; Mitragotri, S. A deep eutectic-based, self-emulsifying subcutaneous depot system for apomorphine therapy in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2110450119. [Google Scholar] [CrossRef]
- Banerjee, A.; Ibsen, K.; Iwao, Y.; Zakrewsky, M.; Mitragotri, S. Transdermal Protein Delivery Using Choline and Geranate (CAGE) Deep Eutectic Solvent. Adv. Healthc. Mater. 2017, 6, 1601411. [Google Scholar] [CrossRef]
- Tanner, E.E.L.; Ibsen, K.N.; Mitragotri, S. Transdermal insulin delivery using choline-based ionic liquids (CAGE). J. Control. Release 2018, 286, 137–144. [Google Scholar] [CrossRef]
- Curreri, A.M.; Kim, J.; Dunne, M.; Angsantikul, P.; Goetz, M.; Gao, Y.; Mitragotri, S. Deep Eutectic Solvents for Subcutaneous Delivery of Protein Therapeutics. Adv. Sci. 2023, 10, 2205389. [Google Scholar] [CrossRef]
- Khamoushian, S.; Madrakian, T.; Afkhami, A.; Ghoorchian, A.; Ghavami, S.; Tari, K.; Samarghandi, M.R. Transdermal Delivery of Insulin Using Combination of Iontophoresis and Deep Eutectic Solvents as Chemical Penetration Enhancers: In Vitro and in Vivo Evaluations. J. Pharm. Sci. 2023, in press. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Adwan, S.; Khanfer, M.; Idkaidek, N.; Al-Remawi, M. A Novel Eutectic-Based Transdermal Delivery System for Risperidone. AAPS PharmSciTech 2021, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cui, H.; Cao, Y.; Lin, Y.; Yang, Y.; Gao, M.; Zhang, W.; Wang, C. Deep eutectic solvents—Hydrogels for the topical management of rheumatoid arthritis. J. Control. Release 2023, 354, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Lai, Z.; Zhu, L.; Ding, X.; Tong, X.; Wang, Z.; Bi, Q.; Tan, N. Novel amorphous solid dispersion based on natural deep eutectic solvent for enhancing delivery of anti-tumor RA-XII by oral administration in rats. Eur. J. Pharm. Sci. 2021, 166, 105931. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.D.; Raval, M.K.; Sheth, N.R. Formation of Diacerein − fumaric acid eutectic as a multi-component system for the functionality enhancement. J. Drug Deliv. Sci. Technol. 2020, 58, 101562. [Google Scholar] [CrossRef]
- Patel, R.D.; Raval, M.K.; Pethani, T.M.; Sheth, N.R. Influence of eutectic mixture as a multi-component system in the improvement of physicomechanical and pharmacokinetic properties of diacerein. Adv. Powder Technol. 2020, 31, 1441–1456. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Q.; Liu, M.; Zhang, L. The effect of deep eutectic solvent on the pharmacokinetics of salvianolic acid B in rats and its acute toxicity test. J. Chromatogr. B 2017, 1063, 60–66. [Google Scholar] [CrossRef]
- Banerjee, A.; Ibsen, K.; Brown, T.; Chen, R.; Agatemor, C.; Mitragotri, S. Ionic liquids for oral insulin delivery. Proc. Natl. Acad. Sci. USA 2018, 115, 7296–7301. [Google Scholar] [CrossRef]
- Vaidya, A.; Mitragotri, S. Ionic liquid-mediated delivery of insulin to buccal mucosa. J. Control. Release 2020, 327, 26–34. [Google Scholar] [CrossRef]
- Li, Y.; Wu, X.; Zhu, Q.; Chen, Z.; Lu, Y.; Qi, J.; Wu, W. Improving the hypoglycemic effect of insulin via the nasal administration of deep eutectic solvents. Int. J. Pharm. 2019, 569, 118584. [Google Scholar] [CrossRef]
- Godin, B.; Touitou, E. Transdermal skin delivery: Predictions for humans from in vivo, ex vivo and animal models. Adv. Drug Deliv. Rev. 2007, 59, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Mandal, A.; Dhawan, S.; Shevachman, M.; Mitragotri, S.; Joshi, N. Clinical translation of choline and geranic acid deep eutectic solvent. Bioeng. Transl. Med. 2021, 6, e10191. [Google Scholar] [CrossRef] [PubMed]
- Zakrewsky, M.; Banerjee, A.; Apte, S.; Kern, T.L.; Jones, M.R.; Sesto, R.E.D.; Koppisch, A.T.; Fox, D.T.; Mitragotri, S. Choline and Geranate Deep Eutectic Solvent as a Broad-Spectrum Antiseptic Agent for Preventive and Therapeutic Applications. Adv. Healthc. Mater. 2016, 5, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- API|cgb-400. Available online: https://www.cdek.liu.edu/api/119951/#trials (accessed on 24 March 2023).
| Potential Advantages | Applications |
|---|---|
| Higher API solubility | Enhancement of therapeutic action of API |
| Higher API permeability | Faster drug delivery |
| Improved API absorption | Controlled drug release |
| Improved API bioavailability |
| Therapeutic Area | Components | Cell Lines | Cytotoxicity Assay | Reference |
|---|---|---|---|---|
| Anticoagulation | ChCl: urea/ethylene glycol/glycerol + coumarin | Melanoma skin cell line | MTT cell viability assay | [53] |
| Anti-inflammation | ChCl: ascorbic acid + dexamethasone | Immortalized mouse lung fibroblasts cell line (L929) | MTS cell viability assay | [40] |
| Anti-inflammation | Arginine: glycerol + ibuprofen | Murine raw 264.7 macrophages | Resazurin cell viability assay | [55] |
| Antimicrobial | ChCl + mandelic acid | Immortalized mouse lung fibroblasts cell line (L929) | MTS cell viability assay | [38] |
| Antimicrobial | Menthol + stearic acid/myristic acid/lauric acid | Immortalized human epidermal keratinocytes (HaCaT) | MTS cell viability assay | [27] |
| Antimicrobial | Betaine: urea + imipenem | Human primary dermal fibroblasts | CellTiter-Blue® Cell Viability Assay (resazurin) | [56] |
| Antimicrobial | Proline: urea: malonic acid/citric acid: xylitol + ciprofloxacin | Immortalized human epidermal keratinocytes cell line (HaCaT) | MTT cell viability assay | [28] |
| Antimicrobial | Trioctylphosphine/trihexylamine/trioctylamine + malonic acid/salicylic acid | Henrietta Lacks cervical cancer cell line (HeLa) | LDH cytotoxicity assay | [58] |
| Cancer | Menthol/capric acid/ibuprofen + limonene | Human colon adenocarcinoma cell lines (Caco-2 and HT29) | MTS cell viability assay (and antiproliferative assay) | [31] |
| Cancer | Safranal/menthol/linalool + ibuprofen/ketoprofen/flurbiprofen | Human colon adenocarcinoma cell lines (Caco-2 and HT29) | MTS cell viability assay (and antiproliferative assay) | [30] |
| Diabetes mellitus | Tromethamide: water + clorpropamide/tolbutamide | Immortalized mouse lung fibroblasts cell line (L929) and human colon adenocarcinoma cell line (Caco-2) | MTT cell viability assay | [60] |
| Tuberculosis | Citric acid + L-arginine/ethambutol | Human colon adenocarcinoma cell line (Caco-2) | MTS cell viability assay | [61] |
| Tuberculosis | Citric acid + L-arginine | Immortalized mouse lung fibroblasts cell line (L929) | MTS cell viability assay | [62] |
| Route of Administration | Components | Animal Model | Reference |
|---|---|---|---|
| Injection: intravenous (in vivo) | Choline bicarbonate: oleic acid (CODES) + verteporfin | Balb/c female mice | [81] |
| Injection: subcutaneous (in vivo) + skin from dorsal side (ex vivo) | CAGE + Apomorphine | Wistar rats and pigs | [82] |
| Injection: transdermal (in vivo) + porcine skin (ex vivo) | CAGE + insulin | Male Wistar rats and Porcine skin | [83] |
| Injection: transdermal skin (ex vivo) | CAGE + insulin | Porcine skin | [84] |
| Injection: subcutaneous (in vivo) | Choline glycolate, acetylcholine glycolate, choline lactate, acetyl-choline lactate, choline propionate, acetylcholine propionate, choline hexenoate, acetylcholine hexenoate, CAGE, and acetylcholine geranate (aCAGE) + insulin. | Balb/c female mice | [85] |
| Injection: transdermal iontophoresis (in vivo) | ChCl/urea, ChCl/glycerol, and ChCl/ethylene glycol + insuline | Diabetic rats | [86] |
| Injection: skin topical (in vivo) | Capric acid: Risperidone | Male rodents | [87] |
| Injection: intraperitoneal (in vivo) | 3-(4-(4- (bis(2chloroethyl) amino)phenyl)butanoyloxy)-N,N,N-trimethyl propane-1-aminium chloride (CABAL), 1,4-butanediol + doxorubicin | Adult female Sprague Dawley rats | [76] |
| Injection: topical and subcutaneous (in vivo) | Arginine (Arg)-citric acid (CA) + Methotrexate | Male Wistar rats | [88] |
| Oral (in vivo) | Betaine, Mandelic acid (Bet-Man NADES) + RA-XII | Healthy male Sprague Dawley rats | [89] |
| Oral (in vivo) | Fumaric acid + Diacerein 2,4-dihydroxybenzoic acid + Diacerein | Healthy Sprague Dawley rats | [90,91] |
| Oral (in vivo) | Mixtures of sugars (glucose, xylitol, sorbitol), amino acids (glutamic acid, proline), organic acids (citric, malic, oxalic, and tartaric acid), and other nitrogen-containing compounds (urea, ChCl, acetylcarnitine and carnitine) + Berberine | Balb/c female mice | [36] |
| Oral (in vivo) | Malic acid + Berberine | Grade II ICR mice (male and female) | [75] |
| Oral (in vivo) | ChCl/glycerol + salvianolic acid B | Mice (male and female) and Sprague Dawley male rats | [92] |
| Oral (in vivo) | CAGE + insulin | Adult nondiabetic male Wistar rats | [93] |
| Oral (in vivo) + porcine buccal tissue (ex vivo) | CAGE + chitosan + insulin | Nondiabetic adult male Wistar rats and Yorkshire pigs | [94] |
| Nasal (in vivo) | ChCl and malic acid (CM-DES) + insulin | Male Sprague Dawley rats | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García, C.B.; Concha, J.; Culleré, L.; Lomba, L.; Sangüesa, E.; Ribate, M.P. Has the Toxicity of Therapeutic Deep Eutectic Systems Been Assessed? Appl. Sci. 2023, 13, 5980. https://doi.org/10.3390/app13105980
García CB, Concha J, Culleré L, Lomba L, Sangüesa E, Ribate MP. Has the Toxicity of Therapeutic Deep Eutectic Systems Been Assessed? Applied Sciences. 2023; 13(10):5980. https://doi.org/10.3390/app13105980
Chicago/Turabian StyleGarcía, Cristina B., Julia Concha, Laura Culleré, Laura Lomba, Estela Sangüesa, and Mª Pilar Ribate. 2023. "Has the Toxicity of Therapeutic Deep Eutectic Systems Been Assessed?" Applied Sciences 13, no. 10: 5980. https://doi.org/10.3390/app13105980





