Evaluation of Microleakage of a New Bioactive Material for Restoration of Posterior Teeth: An In Vitro Radioactive Model
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maas, M.S.; Alania, Y.; Natale, L.C.; Rodrigues, M.C.; Watts, D.C.; Braga, R.R. Trends in Restorative Composites Research: What Is in the Future? Braz. Oral Res. 2017, 31, 23–36. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, N.; Weir, M.D.; Reynolds, M.A.; Bai, Y.; Xu, H.H.K. Bioactive Dental Composites and Bonding Agents Having Remineralizing and Antibacterial Characteristics. Dent. Clin. N. Am. 2017, 61, 669–687. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Abrantes, M.; Paula, A.; Casalta-Lopes, J.; Botelho, M.; Ferreira, M. Microleakage Study of a Restorative Material via Radioisotope Methods. Rev. Port. Estomatol. Med. Dentária Cir. Maxilofac. 2014, 55, 129–134. [Google Scholar] [CrossRef]
- Pawar, M.; Saleem Agwan, M.A.; Ghani, B.; Khatri, M.; Bopache, P.; Aziz, M.S. Evaluation of Class II Restoration Microleakage with Various Restorative Materials: A Comparative In vitro Study. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. 2), S1210–S1214. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.H.S.; Jamal, H.; Young, A.; Ashley, P. Scoping Review of Trials Evaluating Adhesive Strategies in Pediatric Dentistry: Where Do Simplified Strategies Lie? BMC Oral Health 2021, 21, 33. [Google Scholar] [CrossRef]
- Frankenberger, R.; Dudek, M.-C.; Winter, J.; Braun, A.; Krämer, N.; von Stein-Lausnitz, M.; Roggendorf, M.J. Amalgam Alternatives Critically Evaluated: Effect of Long-Term Thermomechanical Loading on Marginal Quality, Wear, and Fracture Behavior. J. Adhes. Dent. 2020, 22, 107–116. [Google Scholar] [CrossRef]
- Ayna, B.; Celenk, S.; Atas, O.; Tümen, E.C.; Uysal, E.; Toptanci, I.R. Microleakage of Glass Ionomer Based Restorative Materials in Primary Teeth: An In Vitro Study. Niger. J. Clin. Pract. 2018, 21, 1034–1037. [Google Scholar] [CrossRef]
- Pfefferkorn, F. Scientific Manual Surefil OneTM Self-Adhesive Composite Hybrid; Dentsply Sirona: Charlotte, NC, USA, 2019. [Google Scholar]
- Radhika, M.; Sajjan, G.S.; Kumaraswamy, B.; Mittal, N. Effect of Different Placement Techniques on Marginal Microleakage of Deep Class-II Cavities Restored with Two Composite Resin Formulations. J. Conserv. Dent. 2010, 13, 9. [Google Scholar] [CrossRef]
- Arora, R.; Kapur, R.; Sibal, N.; Juneja, S. Evaluation of Microleakage in Class II Cavities using Packable Composite Restorations with and without use of Liners. Int. J. Clin. Pediatr. Dent. 2012, 5, 178–184. [Google Scholar] [CrossRef]
- François, P.; Remadi, A.; le Goff, S.; Abdel-Gawad, S.; Attal, J.P.; Dursun, E. Flexural Properties and Dentin Adhesion in Recently Developed Self-Adhesive Bulk-Fill Materials. J. Oral Sci. 2021, 63, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Mickenautsch, S. High-Viscosity Glass-Ionomer Cements for Direct Posterior Tooth Restorations in Permanent Teeth: The Evidence in Brief. J. Dent. 2016, 55, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Iaculli, F.; Salucci, A.; di Giorgio, G.; Luzzi, V.; Ierardo, G.; Polimeni, A.; Bossù, M. Bond Strength of Self-Adhesive Flowable Composites and Glass Ionomer Cements to Primary Teeth: A Systematic Review and Meta-Analysis of In Vitro Studies. Materials 2021, 14, 6694. [Google Scholar] [CrossRef] [PubMed]
- Francois, P.; Fouquet, V.; Attal, J.P.; Dursun, E. Commercially Available Fluoride-Releasing Restorative Materials: A Review and a Proposal for Classification. Materials 2020, 13, 2313. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Ahmed, M.H.; Okazaki, Y.; van Landuyt, K.L.; Huang, C.; van Meerbeek, B. Bonding Efficacy of a New Self-Adhesive Restorative onto Flat Dentin vs Class-I Cavity-Bottom Dentin. J. Adhes. Dent. 2020, 22, 65–77. [Google Scholar] [CrossRef] [PubMed]
- Vilkinis, V.; Hörsted-Bindslev, P.; Baelum, V. Two-Year Evaluation of Class II Resin-Modified Glass Ionomer Cement/Composite Open Sandwich and Composite Restorations. Clin. Oral Investig. 2000, 4, 133–139. [Google Scholar] [CrossRef]
- Banomyong, D.; Palamara, J.E.A.; Messer, H.H.; Burrow, M.F. Fluid Flow after Resin-Composite Restoration in Extracted Carious Teeth. Eur. J. Oral Sci. 2009, 117, 334–342. [Google Scholar] [CrossRef]
- Sidhu, S.K. Clinical Evaluations of Resin-Modified Glass-Ionomer Restorations. Dent. Mater. 2010, 26, 7–12. [Google Scholar] [CrossRef]
- Sidhu, S.; Nicholson, J. A Review of Glass-Ionomer Cements for Clinical Dentistry. J. Funct. Biomater. 2016, 7, 16. [Google Scholar] [CrossRef]
- Xie, D.; Brantley, W.A.; Culbertson, B.M.; Wang, G. Mechanical Properties and Microstructures of Glass-Ionomer Cements. Dent. Mater. 2000, 16, 129–138. [Google Scholar] [CrossRef]
- Yao, C.; Ahmed, M.H.; Zhang, F.; Mercelis, B.; van Landuyt, K.L.; Huang, C.; van Meerbeek, B. Structural/Chemical Characterization and Bond Strength of a New Self-Adhesive Bulk-Fill Restorative. J. Adhes. Dent. 2020, 22, 85–97. [Google Scholar] [CrossRef]
- Klee, J.E.; Renn, C.; Elsner, O. Development of Novel Polymer Technology for a New Class of Restorative Dental Materials. J. Adhes. Dent. 2020, 22, 35–45. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H.K. Time-Kill Behaviour against Eight Bacterial Species and Cytotoxicity of Antibacterial Monomers. J. Dent. 2013, 41, 881–891. [Google Scholar] [CrossRef]
- Imazato, S.; Tarumi, H.; Ebi, N.; Ebisu, S. Cytotoxic Effects of Composite Restorations Employing Self-Etching Primers or Experimental Antibacterial Primers. J. Dent. 2000, 28, 61–67. [Google Scholar] [CrossRef]
- Mazzi, U.; Schibli, R.; Pietzsch, H.J.; Künstler, J.U.; Spies, H. Technetium in Medicine. In Technetium-99m Pharmaceuticals: Preparation and Quality Control in Nuclear Medicine; Springer: Berlin/Heidelberg, Germany, 2007; pp. 7–58. [Google Scholar] [CrossRef]
- Pagella, P.; Stadlinger, B.; Mitsiadis, T.A. Isolation of Dental Pulp and Periodontal Cells from Human Teeth for Single-Cell RNA Sequencing. STAR Protoc. 2021, 2, 100953. [Google Scholar] [CrossRef]
- Wannous, M.; Abboud, S.A. Curing Depth and Degree of Conversion of Different Nano-Hybrid Composites. J. Stomatol. 2021, 74, 147–152. [Google Scholar] [CrossRef]
- Hameed, H.; Babu, B.P.; Sagir, V.M.M.; Chiriyath, K.J.; Mathias, J.; Shaji, A.P. Microleakage in Resin Composite Restoration Following Antimicrobial Pre-Treatments with 2% Chlorhexidine and Clearfil Protect Bond. J. Int. Oral Health 2015, 7, 71–76. [Google Scholar]
- BALLAL, N.V. Microleakage of Composite Resin Restorations. Aust. Dent. J. 2008, 53, 369. [Google Scholar] [CrossRef]
- Majety, K.K.; Pujar, M. In Vitro Evaluation of Microleakage of Class II Packable Composite Resin Restorations Using Flowable Composite and Resin Modified Glass Ionomers as Intermediate Layers. J. Conserv. Dent. 2011, 14, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Pereira, I.R.; Carvalho, C.; Paulo, S.; Martinho, J.P.; Coelho, A.S.; Paula, A.B.; Marto, C.M.; Carrilho, E.; Botelho, M.F.; Abrantes, A.M.; et al. Apical Sealing Ability of Two Calcium Silicate-Based Sealers Using a Radioactive Isotope Method: An In Vitro Apexification Model. Materials 2021, 14, 6456. [Google Scholar] [CrossRef] [PubMed]
- Jafari, F.; Rahimi, S.; Shahi, S.; Jafari, S. Endodontic Microleakage Studies: Correlation among Different Methods, Clinical Relevance, and Potential Laboratory Errors. Minerva Stomatol. 2017, 66, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Gogna, R.; Jagadis, S.; Shashikal, K. A Comparative in Vitro Study of Microleakage by a Radioactive Isotope and Compressive Strength of Three Nanofilled Composite Resin Restorations. J. Conserv. Dent. 2011, 14, 128. [Google Scholar] [CrossRef]
- Rengo, C.; Goracci, C.; Ametrano, G.; Chieffi, N.; Spagnuolo, G.; Rengo, S.; Ferrari, M. Marginal Leakage of Class V Composite Restorations Assessed Using Microcomputed Tomography and Scanning Electron Microscope. Oper. Dent. 2015, 40, 440–448. [Google Scholar] [CrossRef]
- Camps, J.; Pashley, D. Reliability of the Dye Penetration Studies. J. Endod. 2003, 29, 592–594. [Google Scholar] [CrossRef]
- Afonso, T.; Lusitana, M.; de Andrade, S.; Pega, M.; Michelotto, A.L.D.C.; Margarida, A.; Abrantes, C.; Oliveiros, B.; Virgínia, E.; Carrilho, P.; et al. Effect of Calcium Hydroxide as Intracanal Medication on the Apical Sealing Ability of Mineral Trioxide Aggregate (MTA): An in Vitro Apexification Model Efeito Da Medicação Intra-Canal de Hidróxido de Cálcio Na Capacidade Seladora Do Mineral Trióxido Agregado (MTA): Um Modelo de Apicificação in Vitro. J. Health Sci. Inst. 2012, 30, 318–340. [Google Scholar]
- Ferreira, M.M. Quantitative Scintigraphic Analysis of the Apical Seal in Thermafil/Topseal and RealSeal 1/Realseal Filled Root Canals. World J. Stomatol. 2013, 2, 30. [Google Scholar] [CrossRef]
- Prasad, B.K.; Sudhakaran, S. Radioactive Isotope Evaluation of Coronal Leakage after Endodontic Treatment in Teeth Restored with Three Different Intracoronal Restorative Materials: An in Vitro Study. World J. Dent. 2011, 2, 35–38. [Google Scholar] [CrossRef]
- Schneider, L.F.J.; Cavalcante, L.M.; Silikas, N. Shrinkage Stresses Generated during Resin-Composite Applications: A Review. J. Dent. Biomech. 2010, 2010, 131630. [Google Scholar] [CrossRef]
- Kleverlaan, C.J.; Feilzer, A.J. Polymerization Shrinkage and Contraction Stress of Dental Resin Composites. Dent. Mater. 2005, 21, 1150–1157. [Google Scholar] [CrossRef]
- Alvarez-Gayosso, C.; Barceló-Santana, F.; Guerrero-Ibarra, J.; Sáez-Espínola, G.; Canseco-Martínez, M.A. Calculation of Contraction Rates Due to Shrinkage in Light-Cured Composites. Dent. Mater. 2004, 20, 228–235. [Google Scholar] [CrossRef]
- Lopes, L.G.; Franco, E.B.; Pereira, J.C.; Mondelli, R.F.L. Effect of Light-Curing Units and Activation Mode on Polymerization Shrinkage and Shrinkage Stress of Composite Resins. J. Appl. Oral Sci. 2008, 16, 35–42. [Google Scholar] [CrossRef]
- Giachetti, L.; Bertini, F.; Bambi, C.; Russo, D.S. A Rational Use of Dental Materials in Posterior Direct Resin Restorations in Order to Control Polymerization Shrinkage Stress. Minerva Stomatol. 2007, 56, 129–138. [Google Scholar]
- Wattanawongpitak, N.; Yoshikawa, T.; Burrow, M.F.; Tagami, J. The effect of thermal stress on bonding durability of resin composite adaptation to the cavity wall. Dent. Mater. J. 2007, 26, 445–450. [Google Scholar] [CrossRef][Green Version]
- Papadopoulos, C.; Dionysopoulos, D.; Tolidis, K.; Kouros, P.; Koliniotou-Koumpia, E.; Tsitrou, E.A. Structural Integrity Evaluation of Large MOD Restorations Fabricated With a Bulk-Fill and a CAD/CAM Resin Composite Material. Oper. Dent. 2019, 44, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Maletin, A.; Markovic, D.; Neskovic, I.; Ramic, B.; Veljovic, T.; Ristic, I. Application of a Novel Modification of the Microbond Test for Evaluation of Adhesive Bond Strength Between Fiber Posts and Dual-Cure Dental Resin Cement. Med. Sci. Monit. 2019, 25, 3397–3405. [Google Scholar] [CrossRef]
- de Munck, J.; van Landuyt, K.; Coutinho, E.; Poitevin, A.; Peumans, M.; Lambrechts, P.; van Meerbeek, B. Micro-Tensile Bond Strength of Adhesives Bonded to Class-I Cavity-Bottom Dentin after Thermo-Cycling. Dent. Mater. 2005, 21, 999–1007. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Gallo, S.; Lelli, M.; Tarterini, F.; Giglia, F.; Scribante, A. SEM/EDS Evaluation of the Mineral Deposition on a Polymeric Composite Resin of a Toothpaste Containing Biomimetic Zn-Carbonate Hydroxyapatite (MicroRepair®) in Oral Environment: A Randomized Clinical Trial. Polymers 2021, 13, 2740. [Google Scholar] [CrossRef]
- Khanduri, N.; Kurup, D.; Mitra, M. Quantitative Evaluation of Remineralizing Potential of Three Agents on Artificially Demineralized Human Enamel Using Scanning Electron Microscopy Imaging and Energy-Dispersive Analytical X-Ray Element Analysis: An in Vitro Study. Dent. Res. J. 2020, 17, 366–372. [Google Scholar] [CrossRef]
- Ribeiras, I.; Vasconcelos, I.; Ramos, M.; Lopes, M.; Ginjeira, A. Estudo Comparativo Da Adaptação Marginal de 2 Cimentos Endodônticos. Rev. Port. Estomatol. Med. Dentária Cir. Maxilofac. 2015, 56, 173–181. [Google Scholar] [CrossRef]
- Vicente, A.; Ortiz, A.J.; Bravo, L.A. Microleakage beneath Brackets Bonded with Flowable Materials: Effect of Thermocycling. Eur. J. Orthod. 2009, 31, 390–396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roggendorf, M.J.; Krämer, N.; Appelt, A.; Naumann, M.; Frankenberger, R. Marginal Quality of Flowable 4-Mm Base vs. Conventionally Layered Resin Composite. J. Dent. 2011, 39, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Burke, F.J.T.; Crisp, R.J.; James, A.; MacKenzie, L.; Pal, A.; Sands, P.; Thompson, O.; Palin, W.M. Two Year Clinical Evaluation of a Low-Shrink Resin Composite Material in UK General Dental Practices. Dent. Mater. 2011, 27, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Ernst, C.P.; Brandenbusch, M.; Meyer, G.; Canbek, K.; Gottschalk, F.; Willershausen, B. Two-Year Clinical Performance of a Nanofiller vs a Fine-Particle Hybrid Resin Composite. Clin. Oral Investig. 2006, 10, 119–125. [Google Scholar] [CrossRef]
- Amaral, F.L.B.; Colucci, V.; Palma-Dibb, R.G.; Corona, S.A.M. Assessment of In Vitro Methods Used to Promote Adhesive Interface Degradation: A Critical Review. J. Esthet. Restor. Dent. 2007, 19, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Yuasa, T.; Iijima, M.; Ito, S.; Muguruma, T.; Saito, T.; Mizoguchi, I. Effects of Long-Term Storage and Thermocycling on Bond Strength of Two Self-Etching Primer Adhesive Systems. Eur. J. Orthod. 2010, 32, 285–290. [Google Scholar] [CrossRef] [PubMed]

| Characterization | Chemical | Physical and Mechanical |
|---|---|---|
| Surefill One™ | Composition:
|
|
| Spectra™ ST HV | Composition:
|
|
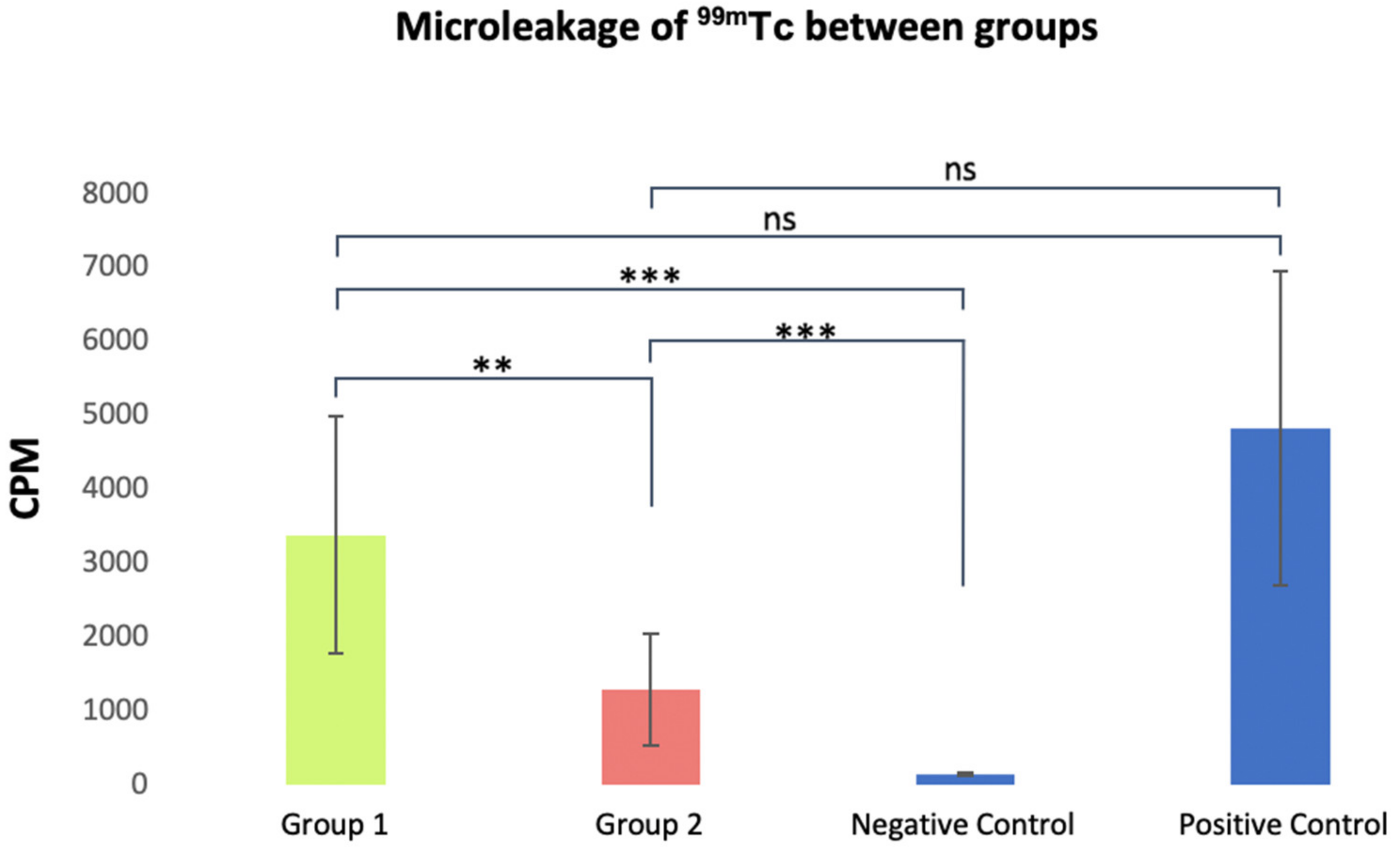
| Group | Average | Standard Deviation | Minimum Values | Maximum Values |
|---|---|---|---|---|
| Group 1 (n = 13) | 3371.1 | ±1548 | 1270 | 6717 |
| Group 2 (n = 13) | 1285.9 | ±724.1 | 293 | 2489 |
| Negative Control (n = 5) | 139.6 | ±12.3 | 120 | 156 |
| Positive Control (n = 5) | 4817.2 | ±1906.4 | 2369 | 8090 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neves, P.; Pires, S.; Marto, C.M.; Amaro, I.; Coelho, A.; Sousa, J.; Ferreira, M.M.; Botelho, M.F.; Carrilho, E.; Abrantes, A.M.; et al. Evaluation of Microleakage of a New Bioactive Material for Restoration of Posterior Teeth: An In Vitro Radioactive Model. Appl. Sci. 2022, 12, 11827. https://doi.org/10.3390/app122211827
Neves P, Pires S, Marto CM, Amaro I, Coelho A, Sousa J, Ferreira MM, Botelho MF, Carrilho E, Abrantes AM, et al. Evaluation of Microleakage of a New Bioactive Material for Restoration of Posterior Teeth: An In Vitro Radioactive Model. Applied Sciences. 2022; 12(22):11827. https://doi.org/10.3390/app122211827
Chicago/Turabian StyleNeves, Pedro, Salomé Pires, Carlos Miguel Marto, Inês Amaro, Ana Coelho, José Sousa, Manuel Marques Ferreira, Maria Filomena Botelho, Eunice Carrilho, Ana Margarida Abrantes, and et al. 2022. "Evaluation of Microleakage of a New Bioactive Material for Restoration of Posterior Teeth: An In Vitro Radioactive Model" Applied Sciences 12, no. 22: 11827. https://doi.org/10.3390/app122211827
APA StyleNeves, P., Pires, S., Marto, C. M., Amaro, I., Coelho, A., Sousa, J., Ferreira, M. M., Botelho, M. F., Carrilho, E., Abrantes, A. M., & Paula, A. B. (2022). Evaluation of Microleakage of a New Bioactive Material for Restoration of Posterior Teeth: An In Vitro Radioactive Model. Applied Sciences, 12(22), 11827. https://doi.org/10.3390/app122211827












