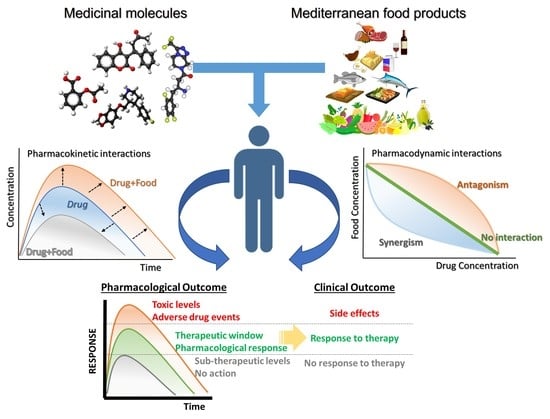
Drug-Food Interactions with a Focus on Mediterranean Diet
Abstract
:1. Introduction
2. Pharmacological Mechanisms of Drug-Food Interactions
2.1. Pharmacokinetic Drug-Food Interactions (PK-DFIs)
2.1.1. Absorption
2.1.2. Distribution
2.1.3. Metabolism
2.1.4. Elimination
2.2. Pharmacodynamic Drug-Food Interactions (PD-DFIs)
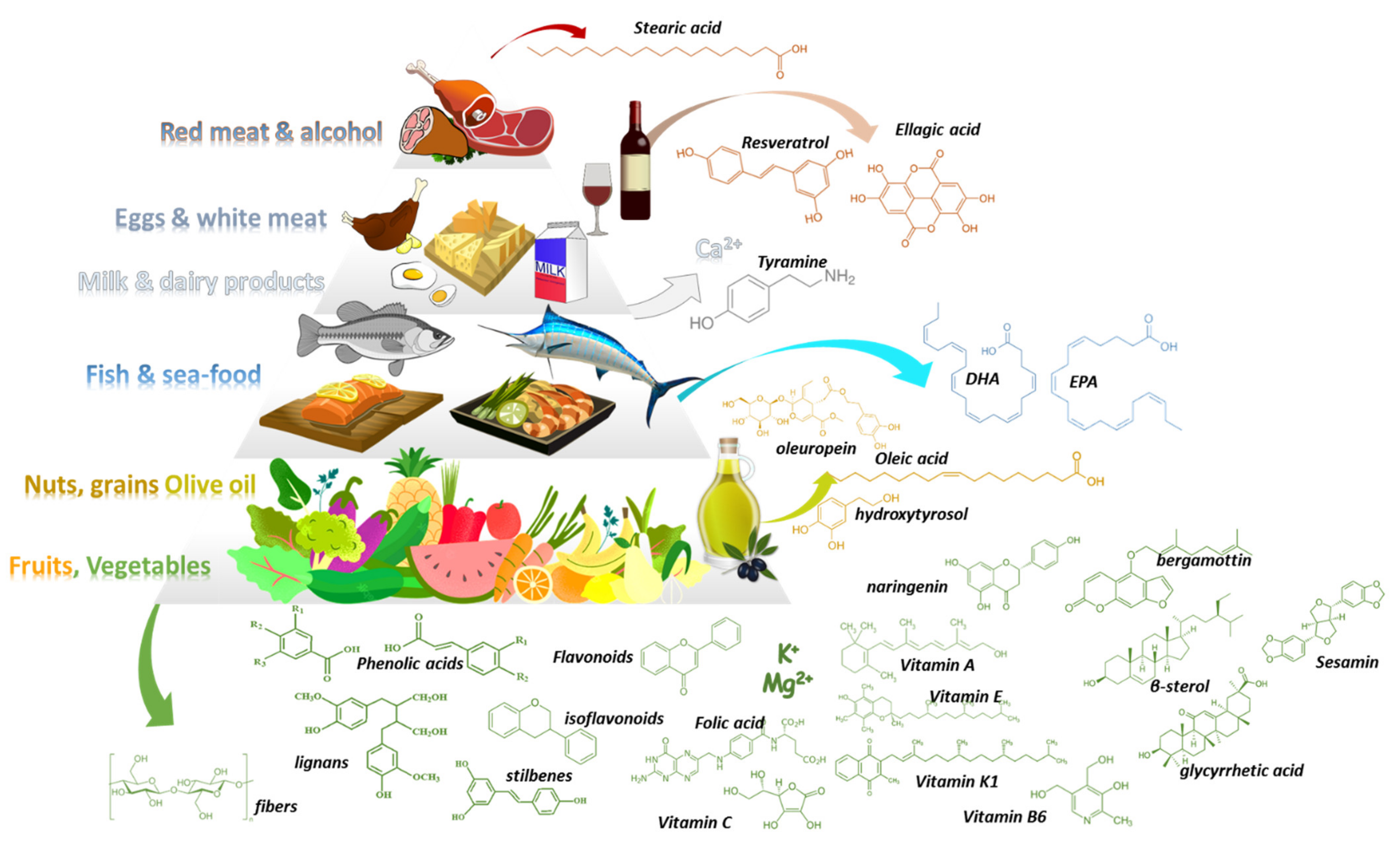
3. Mediterranean Food Products and Potential DFIs
3.1. Med-D Food Products
3.2. Drug-Med Diet Interactions
3.2.1. Vegetables, Herbals, Olive Oil, Cereals, and Nuts
3.2.2. Fruits and Fruit Juices
3.2.3. Fish and Sea Food
3.2.4. Milk Dairy Products, White and Red Meat
3.2.5. Wine and Other Beverages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martínez-González, M.Á.; Hershey, M.S.; Zazpe, I.; Trichopoulou, A. Transferability of the Mediterranean Diet to Non-Mediterranean Countries. What Is and What Is Not the Mediterranean Diet. Nutrients 2017, 9, 1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willett, W.C.; Sacks, F.; Trichopoulou, A.; Drescher, G.; Ferro-Luzzi, A.; Helsing, E.; Trichopoulos, D. Mediterranean Diet Pyramid: A Cultural Model for Healthy Eating. Am. J. Clin. Nutr. 1995, 61, 1402S–1406S. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean Diet Pyramid Today. Science and Cultural Updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- United Nations Educational, S. and C.O. The Mediterranean Diet. Intangible Heritage. Available online: https://www.unesco.org/archives/multimedia/document-1680-eng-2 (accessed on 8 August 2022).
- Wright, C.M. Biographical Notes on Ancel Keys and Salim Yusuf: Origins and Significance of the Seven Countries Study and the INTERHEART Study. J. Clin. Lipidol. 2011, 5, 434–440. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Martínez-González, M.A.; Tong, T.Y.N.; Forouhi, N.G.; Khandelwal, S.; Prabhakaran, D.; Mozaffarian, D.; de Lorgeril, M. Definitions and Potential Health Benefits of the Mediterranean Diet: Views from Experts around the World. BMC Med. 2014, 12, 112. [Google Scholar] [CrossRef] [Green Version]
- El Amrousy, D.; Elashry, H.; Salamah, A.; Maher, S.; Abd-Elsalam, S.M.; Hasan, S. Adherence to the Mediterranean Diet Improved Clinical Scores and Inflammatory Markers in Children with Active Inflammatory Bowel Disease: A Randomized Trial. J. Inflamm. Res. 2022, 15, 2075–2086. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean Diet and Survival in a Greek Population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef] [Green Version]
- Iaccarino Idelson, P.; Scalfi, L.; Valerio, G. Adherence to the Mediterranean Diet in Children and Adolescents: A Systematic Review. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 283–299. [Google Scholar] [CrossRef]
- García-Fernández, E.; Rico-Cabanas, L.; Estruch, R.; Estruch, R.; Estruch, R.; Bach-Faig, A. Mediterranean Diet and Cardiodiabesity: A Review. Nutrients 2014, 6, 3474–3500. [Google Scholar] [CrossRef]
- Dinu, M.; Pagliai, G.; Casini, A.; Sofi, F. Mediterranean Diet and Multiple Health Outcomes: An Umbrella Review of Meta-Analyses of Observational Studies and Randomised Trials. Eur. J. Clin. Nutr. 2017, 72, 30–43. [Google Scholar] [CrossRef]
- Du, H.; Cao, T.; Lu, X.; Zhang, T.; Luo, B.; Li, Z. Mediterranean Diet Patterns in Relation to Lung Cancer Risk: A Meta-Analysis. Front. Nutr. 2022, 9, 844382. [Google Scholar] [CrossRef]
- Bayán-Bravo, A.; Banegas, J.R.; Donat-Vargas, C.; Sandoval-Insausti, H.; Gorostidi, M.; Rodríguez-Artalejo, F.; Guallar-Castillón, P. The Mediterranean Diet Protects Renal Function in Older Adults: A Prospective Cohort Study. Nutrients 2022, 14, 432. [Google Scholar] [CrossRef]
- Forsyth, C.; Kouvari, M.; D’Cunha, N.M.; Georgousopoulou, E.N.; Panagiotakos, D.B.; Mellor, D.D.; Kellett, J.; Naumovski, N. The Effects of the Mediterranean Diet on Rheumatoid Arthritis Prevention and Treatment: A Systematic Review of Human Prospective Studies. Rheumatol. Int. 2018, 38, 737–747. [Google Scholar] [CrossRef]
- Morales-Ivorra, I.; Romera-Baures, M.; Roman-Viñas, B.; Serra-Majem, L. Osteoarthritis and the Mediterranean Diet: A Systematic Review. Nutrients 2018, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Rees, K.; Takeda, A.; Martin, N.; Ellis, L.; Wijesekara, D.; Vepa, A.; Das, A.; Hartley, L.; Stranges, S. Mediterranean-Style Diet for the Primary and Secondary Prevention of Cardiovascular Disease; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2019; Volume 2019. [Google Scholar]
- García-Casares, N.; Fuentes, P.G.; Barbancho, M.Á.; López-Gigosos, R.; García-Rodríguez, A.; Gutiérrez-Bedmar, M. Alzheimer’s Disease, Mild Cognitive Impairment and Mediterranean Diet. A Systematic Review and Dose-Response Meta-Analysis. J. Clin. Med. 2021, 10, 4642. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Chrysoula, L.; Leonida, I.; Kotzakioulafi, E.; Theodoridis, X.; Chourdakis, M. Impact of the Level of Adherence to the Mediterranean Diet on Blood Pressure: A Systematic Review and Meta-Analysis of Observational Studies. Clin. Nutr. 2021, 40, 5771–5780. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Chrysoula, L.; Kotzakioulafi, E.; Theodoridis, X.; Chourdakis, M. Impact of the Level of Adherence to Mediterranean Diet on the Parameters of Metabolic Syndrome: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2021, 13, 1514. [Google Scholar] [CrossRef]
- Finicelli, M.; Di Salle, A.; Galderisi, U.; Peluso, G. The Mediterranean Diet: An Update of the Clinical Trials. Nutrients 2022, 14, 2956. [Google Scholar] [CrossRef]
- Castro-Espin, C.; Agudo, A. The Role of Diet in Prognosis among Cancer Survivors: A Systematic Review and Meta-Analysis of Dietary Patterns and Diet Interventions. Nutrients 2022, 14, 348. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Pérez-Jiménez, A.; García-Salguero, L.; Mokhtari, K.; Reyes-Zurita, F.J.; Peragón-Sánchez, J.; Lupiáñez, J.A. Nutraceutical Role of Polyphenols and Triterpenes Present in the Extracts of Fruits and Leaves of Olea Europaea as Antioxidants, Anti-Infectives and Anticancer Agents on Healthy Growth. Molecules 2022, 27, 2341. [Google Scholar] [CrossRef]
- Caponio, G.R.; Lippolis, T.; Tutino, V.; Gigante, I.; De Nunzio, V.; Milella, R.A.; Gasparro, M.; Notarnicola, M. Nutraceuticals: Focus on Anti-Inflammatory, Anti-Cancer, Antioxidant Properties in Gastrointestinal Tract. Antioxidants 2022, 11, 1274. [Google Scholar] [CrossRef]
- Abenavoli, L.; Procopio, A.C.; Paravati, M.R.; Costa, G.; Milić, N.; Alcaro, S.; Luzza, F. Mediterranean Diet: The Beneficial Effects of Lycopene in Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2022, 11, 3477. [Google Scholar] [CrossRef]
- Scoditti, E.; Capurso, C.; Capurso, A.; Massaro, M. Vascular Effects of the Mediterranean Diet-Part II: Role of Omega-3 Fatty Acids and Olive Oil Polyphenols. Vascul. Pharmacol. 2014, 63, 127–134. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; De Caterina, R. Nutraceuticals and Prevention of Atherosclerosis: Focus on Omega-3 Polyunsaturated Fatty Acids and Mediterranean Diet Polyphenols. Cardiovasc. Ther. 2010, 28, e13–e19. [Google Scholar] [CrossRef] [Green Version]
- Augimeri, G.; Bonofiglio, D. The Mediterranean Diet as a Source of Natural Compounds: Does It Represent a Protective Choice against Cancer? Pharmaceuticals 2021, 14, 920. [Google Scholar] [CrossRef]
- Vivancos, M.; Moreno, J.J. Effect of Resveratrol, Tyrosol and Beta-Sitosterol on Oxidised Low-Density Lipoprotein-Stimulated Oxidative Stress, Arachidonic Acid Release and Prostaglandin E2 Synthesis by RAW 264.7 Macrophages. Br. J. Nutr. 2008, 99, 1199–1207. [Google Scholar] [CrossRef] [Green Version]
- Roman, G.C.; Jackson, R.E.; Gadhia, R.; Roman, A.N.; Reis, J. Mediterranean Diet: The Role of Long-Chain Omega-3 Fatty Acids in Fish; Polyphenols in Fruits, Vegetables, Cereals, Coffee, Tea, Cacao and Wine; Probiotics and Vitamins in Prevention of Stroke, Age-Related Cognitive Decline, and Alzheimer Disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Nadtochiy, S.M.; Redman, E.K. Mediterranean Diet and Cardioprotection: The Role of Nitrite, Polyunsaturated Fatty Acids, and Polyphenols. Nutrition 2011, 27, 733–744. [Google Scholar] [CrossRef] [Green Version]
- Nomikos, T.; Fragopoulou, E.; Antonopoulou, S.; Panagiotakos, D.B. Mediterranean Diet and Platelet-Activating Factor: A Systematic Review. Clin. Biochem. 2018, 60, 1–10. [Google Scholar] [CrossRef]
- Chatsisvili, A.; Sapounidis, I.; Pavlidou, G.; Zoumpouridou, E.; Karakousis, V.A.; Spanakis, M.; Teperikidis, L.; Niopas, I. Potential Drug-Drug Interactions in Prescriptions Dispensed in Community Pharmacies in Greece. Pharm. World Sci. 2010, 32, 187–193. [Google Scholar] [CrossRef]
- Kohler, G.I.; Bode-Boger, S.M.; Busse, R.; Hoopmann, M.; Welte, T.; Boger, R.H. Drug-Drug Interactions in Medical Patients: Effects of in-Hospital Treatment and Relation to Multiple Drug Use. Int. J. Clin. Pharmacol. Ther. 2000, 38, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Dechanont, S.; Maphanta, S.; Butthum, B.; Kongkaew, C. Hospital Admissions/Visits Associated with Drug-Drug Interactions: A Systematic Review and Meta-Analysis. Pharmacoepidemiol. Drug Saf. 2014, 23, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Spanakis, M.; Spanakis, E.G.; Kondylakis, H.; Sfakianakis, S.; Genitsaridi, I.; Sakkalis, V.; Tsiknakis, M.; Marias, K. Addressing drug-drug and drug-food interactions through personalized empowerment services for healthcare. In Proceedings of the 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBS 2016), Orlando, FL, USA, 16–20 August 2016. [Google Scholar]
- Vizirianakis, I.S.; Spanakis, M.; Termentzi, A.; Niopas, I.; Kokkalou, E. Clinical and Pharmacogenomic Assessment of Herb-Drug Interactions to Improve Drug Delivery and Pharmacovigilance. In Plants in Traditional and Modern Medicine: Chemistry and Activity; Kokkalou, E., Ed.; Transworld Research Network: Kerala, India, 2010; ISBN 978-81-7895-432-5. [Google Scholar]
- Spanakis, M.; Sfakianakis, S.; Sakkalis, V.; Spanakis, E.G. PharmActa: Empowering Patients to Avoid Clinical Significant Drug(-)Herb Interactions. Medicines 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, M.; Coimbra, M.A.; Costa, M.D.C.; Ramos, F. Food supplement vitamins, minerals, amino-acids, fatty acids, phenolic and alkaloid-based substances: An overview of their interaction with drugs. Crit. Rev. Food Sci. Nutr. 2021, 1–35. [Google Scholar] [CrossRef]
- Won, C.S.; Oberlies, N.H.; Paine, M.F. Mechanisms Underlying Food-Drug Interactions: Inhibition of Intestinal Metabolism and Transport. Pharmacol. Ther. 2012, 136, 186. [Google Scholar] [CrossRef] [Green Version]
- Frankel, E.H.; McCabe, B.J.; Wolfe, J.J. Handbook of Food-Drug Interactions; CRC Press: Boca Raton, FL, USA, 2003; ISBN 1135504571. [Google Scholar]
- Kirby, B.J.; Unadkat, J.D. Grapefruit Juice, a Glass Full of Drug Interactions? Clin. Pharmacol. Ther. 2007, 81, 631–633. [Google Scholar] [CrossRef]
- Brown, C.; Taniguchi, G.; Yip, K. The Monoamine Oxidase Inhibitor-Tyramine Interaction. J. Clin. Pharmacol. 1989, 29, 529–532. [Google Scholar] [CrossRef]
- Chan, L.N.; Anderson, G.D. Pharmacokinetic and Pharmacodynamic Drug Interactions with Ethanol (Alcohol). Clin. Pharmacokinet. 2014, 53, 1115–1136. [Google Scholar] [CrossRef]
- Amadi, C.N.; Mgbahurike, A.A. Selected Food/Herb-Drug Interactions: Mechanisms and Clinical Relevance. Am. J. Ther. 2018, 25, e423–e433. [Google Scholar] [CrossRef]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Serpe, L.; Canaparo, R.; Dell’Osso, B.; Galentino, R.; De Michele, S.; Dina, C.Z.; Porta, M.; et al. Food Bioactive Compounds and Their Interference in Drug Pharmacokinetic/Pharmacodynamic Profiles. Pharmaceutics 2018, 10, 277. [Google Scholar] [CrossRef]
- Spanakis, M.; Patelarou, A.; Patelarou, E.; Tzanakis, N. Drug Interactions for Patients with Respiratory Diseases Receiving COVID-19 Emerged Treatments. Int. J. Environ. Res. Public Health 2021, 18, 1711. [Google Scholar] [CrossRef]
- Spanakis, M.; Roubedaki, M.; Tzanakis, I.; Zografakis-Sfakianakis, M.; Patelarou, E.; Patelarou, A. Impact of Adverse Drug Reactions in Patients with End Stage Renal Disease in Greece. Int. J. Environ. Res. Public Health 2020, 17, 9101. [Google Scholar] [CrossRef]
- Spanakis, M.; Melissourgaki, M.; Lazopoulos, G.; Patelarou, A.E.; Patelarou, E. Prevalence and Clinical Significance of Drug–Drug and Drug–Dietary Supplement Interactions among Patients Admitted for Cardiothoracic Surgery in Greece. Pharmaceutics 2021, 13, 239. [Google Scholar] [CrossRef]
- Murad, M.H.; Asi, N.; Alsawas, M.; Alahdab, F. New Evidence Pyramid. Evid. Based Med. 2016, 21, 125–127. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Zhu, X.; Chen, Z.; Fan, C.H.; Kwan, H.S.; Wong, C.H.; Shek, K.Y.; Zuo, Z.; Lam, T.N. A Review of Food-Drug Interactions on Oral Drug Absorption. Drugs 2017, 77, 1833–1855. [Google Scholar] [CrossRef]
- Koziolek, M.; Alcaro, S.; Augustijns, P.; Basit, A.W.; Grimm, M.; Hens, B.; Hoad, C.L.; Jedamzik, P.; Madla, C.M.; Maliepaard, M.; et al. The Mechanisms of Pharmacokinetic Food-Drug Interactions—A Perspective from the UNGAP Group. Eur. J. Pharm. Sci. 2019, 134, 31–59. [Google Scholar] [CrossRef]
- Schmidt, L.E.; Dalhoff, K. Food-Drug Interactions. Drugs 2012, 62, 1481–1502. [Google Scholar] [CrossRef]
- Wu, C.Y.; Benet, L.Z. Predicting Drug Disposition via Application of BCS: Transport/Absorption/Elimination Interplay and Development of a Biopharmaceutics Drug Disposition Classification System. Pharm. Res. 2005, 22, 11–23. [Google Scholar] [CrossRef]
- Sharma, S.; Prasad, B. Meta-Analysis of Food Effect on Oral Absorption of Efflux Transporter Substrate Drugs: Does Delayed Gastric Emptying Influence Drug Transport Kinetics? Pharmaceutics 2021, 13, 1035. [Google Scholar] [CrossRef]
- Jung, K.Y.; Choo, Y.K.; Kim, H.M.; Choi, B.K. Radish Extract Stimulates Motility of the Intestine via the Muscarinic Receptors. J. Pharm. Pharmacol. 2000, 52, 1031–1036. [Google Scholar] [CrossRef]
- Eussen, S.R.B.M.; Rompelberg, C.J.M.; Andersson, K.E.; Klungel, O.H.; Hellstrand, P.; Öste, R.; Van Kranen, H.; Garssen, J. Simultaneous Intake of Oat Bran and Atorvastatin Reduces Their Efficacy to Lower Lipid Levels and Atherosclerosis in LDLr-/- Mice. Pharmacol. Res. 2011, 64, 36–43. [Google Scholar] [CrossRef]
- Vaquero, M.P.; Muniz, F.J.S.; Redondo, S.J.; Oliván, P.P.; Higueras, F.J.; Bastida, S. Major Diet-Drug Interactions Affecting the Kinetic Characteristics and Hypolipidaemic Properties of Statins. Nutr. Hosp. 2010, 25, 193–206. [Google Scholar]
- Willemsen, A.E.C.A.B.; Lubberman, F.J.E.; Tol, J.; Gerritsen, W.R.; Van Herpen, C.M.L.; Van Erp, N.P. Effect of Food and Acid-Reducing Agents on the Absorption of Oral Targeted Therapies in Solid Tumors. Drug Discov. Today 2016, 21, 962–976. [Google Scholar] [CrossRef]
- Lewis, L.D.; Koch, K.M.; Reddy, N.J.; Cohen, R.B.; Lewis, N.L.; Whitehead, B.; Mackay, K.; Stead, A.; Beelen, A.P. Effects of Food on the Relative Bioavailability of Lapatinib in Cancer Patients. J. Clin. Oncol. 2009, 27, 1191–1196. [Google Scholar] [CrossRef]
- Kang, S.P.; Ratain, M.J. Inconsistent Labeling of Food Effect for Oral Agents across Therapeutic Areas: Differences between Oncology and Non-Oncology Products. Clin. Cancer Res. 2010, 16, 4446–4451. [Google Scholar] [CrossRef] [Green Version]
- Omachi, F.; Kaneko, M.; Iijima, R.; Watanabe, M.; Itagaki, F. Relationship between the Effects of Food on the Pharmacokinetics of Oral Antineoplastic Drugs and Their Physicochemical Properties. J. Pharm. Health Care Sci. 2019, 5, 26. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Perergina, A.A.; Rodriguez, J.M.; Moreno- Esparza, R. The Influence of Coffee with Milk and Tea with Milk on the Bioavailability of Tetracycline. Biopharm. Drug Dispos. 1997, 18, 459–463. [Google Scholar] [CrossRef]
- Estudante, M.; Morais, J.G.; Soveral, G.; Benet, L.Z. Intestinal Drug Transporters: An Overview. Adv. Drug Deliv. Rev. 2013, 65, 1340–1356. [Google Scholar] [CrossRef]
- Terada, T.; Hira, D. Intestinal and Hepatic Drug Transporters: Pharmacokinetic, Pathophysiological, and Pharmacogenetic Roles. J. Gastroenterol. 2015, 50, 508–519. [Google Scholar] [CrossRef] [Green Version]
- Lin, L.; Yee, S.W.; Kim, R.B.; Giacomini, K.M. SLC transporters as therapeutic targets: Emerging opportunities. Nat. Rev. Drug Discov. 2015, 14, 543–560. [Google Scholar] [CrossRef] [Green Version]
- Giacomini, K.M.; Huang, S.M.; Tweedie, D.J.; Benet, L.Z.; Brouwer, K.L.R.; Chu, X.; Dahlin, A.; Evers, R.; Fischer, V.; Hillgren, K.M.; et al. Membrane Transporters in Drug Development. Nat. Rev. Drug Discov. 2010, 9, 215–236. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, K. The Transporters of Intestinal Tract and Techniques Applied to Evaluate Interactions between Drugs and Transporters. Asian J. Pharm. Sci. 2013, 8, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Glaeser, H.; Bailey, D.G.; Dresser, G.K.; Gregor, J.C.; Schwarz, U.I.; McGrath, J.S.; Jolicoeur, E.; Lee, W.; Leake, B.F.; Tirona, R.G.; et al. Intestinal Drug Transporter Expression and the Impact of Grapefruit Juice in Humans. Clin. Pharmacol. Ther. 2007, 81, 362–370. [Google Scholar] [CrossRef]
- Bailey, D.G. Fruit Juice Inhibition of Uptake Transport: A New Type of Food-Drug Interaction. Br. J. Clin. Pharmacol. 2010, 70, 645–655. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Lim, L.Y.; Chowbay, B. Herbal Modulation of P-Glycoprotein. Drug Metab. Rev. 2004, 36, 57–104. [Google Scholar] [CrossRef]
- Nakanishi, T.; Tamai, I. Interaction of Drug or Food with Drug Transporters in Intestine and Liver. Curr. Drug Metab. 2015, 16, 753–764. [Google Scholar] [CrossRef]
- Deferme, S.; Augustijns, P. The Effect of Food Components on the Absorption of P-Gp Substrates: A Review. J. Pharm. Pharmacol. 2003, 55, 153–162. [Google Scholar] [CrossRef]
- Kim, T.H.; Shin, S.; Yoo, S.D.; Shin, B.S. Effects of Phytochemical P-Glycoprotein Modulators on the Pharmacokinetics and Tissue Distribution of Doxorubicin in Mice. Molecules 2018, 23, 349. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhou, P.; Asenso, J.; Yang, X.D.; Wang, C.; Wei, W. Advances in Plant-Based Inhibitors of P-Glycoprotein. J. Enzyme Inhib. Med. Chem. 2016, 31, 867–881. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, A.I.; Real, R.; Pérez, M.; Mendoza, G.; Prieto, J.G.; Merino, G. Modulation of the Activity of ABC Transporters (P-Glycoprotein, MRP2, BCRP) by Flavonoids and Drug Response. J. Pharm. Sci. 2010, 99, 598–617. [Google Scholar] [CrossRef]
- Katayama, K.; Masuyama, K.; Yoshioka, S.; Hasegawa, H.; Mitsuhashi, J.; Sugimoto, Y. Flavonoids Inhibit Breast Cancer Resistance Protein-Mediated Drug Resistance: Transporter Specificity and Structure-Activity Relationship. Cancer Chemother. Pharmacol. 2007, 60, 789–797. [Google Scholar] [CrossRef]
- Yao, H.T.; Hsu, Y.R.; Li, M.L. Beverage–Drug Interaction: Effects of Green Tea Beverage Consumption on Atorvastatin Metabolism and Membrane Transporters in the Small Intestine and Liver of Rats. Membranes 2020, 10, 233. [Google Scholar] [CrossRef]
- Onetto, A.J.; Shariff, S. Drug Distribution; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Caldwell, J.; Gardner, I.; Swales, N. An introduction to drug disposition: The basic principles of absorption, distribution, metabolism, and excretion. Toxicol. Pathol. 1995, 23, 102–114. [Google Scholar] [CrossRef]
- McElnay, J.C.; D’Arcy, P.F. Protein Binding Displacement Interactions and Their Clinical Importance. Drugs 1983, 25, 495–513. [Google Scholar] [CrossRef]
- Xiao, J.; Kai, G. A Review of Dietary Polyphenol-Plasma Protein Interactions: Characterization, Influence on the Bioactivity, and Structure-Affinity Relationship. Crit. Rev. Food Sci. Nutr. 2012, 52, 85–101. [Google Scholar] [CrossRef]
- López-Yerena, A.; Perez, M.; Vallverdú-Queralt, A.; Escribano-Ferrer, E. Insights into the Binding of Dietary Phenolic Compounds to Human Serum Albumin and Food-Drug Interactions. Pharmaceutics 2020, 12, 1123. [Google Scholar] [CrossRef]
- Rimac, H.; Dufour, C.; Debeljak, Ž.; Zorc, B.; Bojić, M. Warfarin and Flavonoids Do Not Share the Same Binding Region in Binding to the IIA Subdomain of Human Serum Albumin. Molecules 2017, 22, 1153. [Google Scholar] [CrossRef] [Green Version]
- Sim, S.C.; Ingelman-Sundberg, M. The Human Cytochrome P450 (CYP) Allele Nomenclature Website: A Peer-Reviewed Database of CYP Variants and Their Associated Effects. Hum. Genomics 2010, 4, 278–281. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.F.; Liu, J.P.; Chowbay, B. Polymorphism of Human Cytochrome P450 Enzymes and Its Clinical Impact. Drug Metab. Rev. 2009, 41, 89–295. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 Enzymes in Drug Metabolism: Regulation of Gene Expression, Enzyme Activities, and Impact of Genetic Variation. Pharmacol. Ther. 2013, 138, 103–141. [Google Scholar] [CrossRef]
- Ingelman-Sundberg, M.; Sim, S.C.; Gomez, A.; Rodriguez-Antona, C. Influence of Cytochrome P450 Polymorphisms on Drug Therapies: Pharmacogenetic, Pharmacoepigenetic and Clinical Aspects. Pharmacol. Ther. 2007, 116, 496–526. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.K.; Brockmoller, J.; Broly, F.; Eichelbaum, M.; Evans, W.E.; Gonzalez, F.J.; Huang, J.D.; Idle, J.R.; Ingelman-Sundberg, M.; Ishizaki, T.; et al. Nomenclature for Human CYP2D6 Alleles. Pharmacogenetics 1996, 6, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Huang, S.M. Scientific and Regulatory Perspectives on Metabolizing Enzyme-Transporter Interplay and Its Role in Drug Interactions: Challenges in Predicting Drug Interactions. Mol. Pharm. 2009, 6, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Borrelli, F.; Ernst, E.; Izzo, A.A. St John’s Wort: Prozac from the Plant Kingdom. Trends Pharmacol. Sci. 2001, 22, 292–297. [Google Scholar] [CrossRef]
- Chrubasik-Hausmann, S.; Vlachojannis, J.; McLachlan, A.J. Understanding Drug Interactions with St John’s Wort (Hypericum Perforatum L.): Impact of Hyperforin Content. J. Pharm. Pharmacol. 2019, 71, 129–138. [Google Scholar] [CrossRef] [Green Version]
- Madabushi, R.; Frank, B.; Drewelow, B.; Derendorf, H.; Butterweck, V. Hyperforin in St. John’s Wort Drug Interactions. Eur. J. Clin. Pharmacol. 2006, 62, 225–233. [Google Scholar] [CrossRef]
- Nicolussi, S.; Drewe, J.; Butterweck, V.; Meyer zu Schwabedissen, H.E. Clinical Relevance of St. John’s Wort Drug Interactions Revisited. Br. J. Pharmacol. 2020, 177, 1212–1226. [Google Scholar] [CrossRef]
- Soleymani, S.; Bahramsoltani, R.; Rahimi, R.; Abdollahi, M. Clinical Risks of St John’s Wort (Hypericum Perforatum) Co-Administration. Expert Opin. Drug Metab. Toxicol. 2017, 13, 1047–1062. [Google Scholar] [CrossRef]
- Van Der Weide, J.; Steijns, L.S.W.; Van Weelden, M.J.M. The Effect of Smoking and Cytochrome P450 CYP1A2 Genetic Polymorphism on Clozapine Clearance and Dose Requirement. Pharmacogenetics 2003, 13, 169–172. [Google Scholar] [CrossRef]
- Skupinska, K.; Misiewicz-Krzeminska, I.; Lubelska, K.; Kasprzycka-Guttman, T. The effect of isothiocyanates on CYP1A1 and CYP1A2 activities induced by polycyclic aromatic hydrocarbons in Mcf7 cells. Toxicol. In Vitro 2009, 23, 763–771. [Google Scholar] [CrossRef]
- Jiang, X.; Williams, K.M.; Liauw, W.S.; Ammit, A.J.; Roufogalis, B.D.; Duke, C.C.; Day, R.O.; McLachlan, A.J. Effect of Ginkgo and Ginger on the Pharmacokinetics and Pharmacodynamics of Warfarin in Healthy Subjects. Br. J. Clin. Pharmacol. 2005, 59, 425. [Google Scholar] [CrossRef]
- Von Moltke, L.L.; Weemhoff, J.L.; Bedir, E.; Khan, I.A.; Harmatz, J.S.; Goldman, P.; Greenblatt, D.J. Inhibition of Human Cytochromes P450 by Components of Ginkgo Biloba. J. Pharm. Pharmacol. 2004, 56, 1039–1044. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, L.; Duan, L.; Wu, J.J.; Hu, M.; Liu, Z.Q.; Wang, C. yan Potential of Herb-Drug/Herb Interactions between Substrates and Inhibitors of UGTs Derived from Herbal Medicines. Pharmacol. Res. 2019, 150, 104510. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Chen, J.; Hu, X.; Zhang, H.; Li, Z.; Lan, B.; Zhang, W.; Su, Y.; Zhang, C. Potential Interactions among Myricetin and Dietary Flavonols through the Inhibition of Human UDP-Glucuronosyltransferase in Vitro. Toxicol. Lett. 2022, 358, 40–47. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Z.; Wang, X.; Wang, S.; Wang, Z.; Liu, Y. Piceatannol exhibits potential food-drug interactions through the inhibition of human UDP-glucuronosyltransferase (UGT) in Vitro. Toxicol. In Vitro 2020, 67, 104890. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, H.; Hu, X.; Xu, M.; Su, Y.; Zhang, C.; Yue, Y.; Zhang, X.; Wang, X.; Cui, W.; et al. Phloretin exhibits potential food-drug interactions by inhibiting human UDP-glucuronosyltransferases in vitro. Toxicol. In Vitro 2022, 84, 105447. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F. Potential Renal Acid Load of Foods and Its Influence on Urine PH. J. Am. Diet. Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef]
- Freudenthaler, S.; Meineke, I.; Schreeb, K.H.; Boakye, E.; Gundert-Remy, U.; Gleiter, C.H. Influence of Urine PH and Urinary Flow on the Renal Excretion of Memantine. Br. J. Clin. Pharmacol. 1998, 46, 541. [Google Scholar] [CrossRef] [Green Version]
- Hertrampf, R.; Gundert-Remy, U.; Beckmann, J.; Hoppe, U.; Elsäßer, W.; Stein, H. Elimination of Flecainide as a Function of Urinary Flow Rate and PH. Eur. J. Clin. Pharmacol. 1991, 41, 61–63. [Google Scholar] [CrossRef]
- Couris, R.; Tataronis, G.; McCloskey, W.; Oertel, L.; Dallal, G.; Dwyer, J.; Blumberg, J.B. Dietary Vitamin K Variability Affects International Normalized Ratio (INR) Coagulation Indices. Int. J. Vitam. Nutr. Res. 2006, 76, 65–74. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, W.S.; Lee, J.H.; Lee, H.; Yang, D.H.; Chae, S.C. Relationship between Dietary Vitamin K Intake and the Stability of Anticoagulation Effect in Patients Taking Long-Term Warfarin. Thromb. Haemost. 2010, 104, 755–759. [Google Scholar] [CrossRef]
- Mahtani, K.R.; Heneghan, C.J.; Nunan, D.; Roberts, N.W. Vitamin K for improved anticoagulation control in patients receiving warfarin. Cochrane Database Syst. Rev. 2014, 5, CD009917. [Google Scholar] [CrossRef]
- Mohamed Pakkir Maideen, N.; Balasubramanian, R.; Muthusamy, S.; Nallasamy, V. An Overview of Clinically Imperative and Pharmacodynamically Significant Drug Interactions of Renin-Angiotensin-Aldosterone System (RAAS) Blockers. Curr. Cardiol. Rev. 2022, 18, e110522204611. [Google Scholar] [CrossRef]
- Batra, V.; Villgran, V. Hyperkalemia from Dietary Supplements. Cureus 2016, 8, e859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campos, M.G.; Machado, J.; Costa, M.L.; Lino, S.; Correia, F.; Maltez, F. Case Report: Severe Hematological, Muscle and Liver Toxicity Caused by Drugs and Artichoke Infusion Interaction in an Elderly Polymedicated Patient. Curr. Drug Saf. 2018, 13, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fragoso, L.; Martínez-Arismendi, J.L.; Orozco-Bustos, D.; Reyes-Esparza, J.; Torres, E.; Burchiel, S.W. Potential Risks Resulting from Fruit/Vegetable–Drug Interactions: Effects on Drug-Metabolizing Enzymes and Drug Transporters. J. Food Sci. 2011, 76, R112–R124. [Google Scholar] [CrossRef] [PubMed]
- Eagles, S.K.; Gross, A.S.; McLachlan, A.J. The Effects of Cruciferous Vegetable-Enriched Diets on Drug Metabolism: A Systematic Review and Meta-Analysis of Dietary Intervention Trials in Humans. Clin. Pharmacol. Ther. 2020, 108, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Lampe, J.W.; King, I.B.; Li, S.; Grate, M.T.; Barale, K.V.; Chen, C.; Feng, Z.; Potter, J.D. Brassica Vegetables Increase and Apiaceous Vegetables Decrease Cytochrome P450 1A2 Activity in Humans: Changes in Caffeine Metabolite Ratios in Response to Controlled Vegetable Diets. Carcinogenesis 2000, 21, 1157–1162. [Google Scholar] [CrossRef]
- Foster, B.C.; Foster, M.S.; Vandenhoek, S.; Krantis, A.; Budzinski, J.W.; Arnason, J.T.; Gallicano, K.D.; Choudri, S. An in Vitro Evaluation of Human Cytochrome P450 3A4 and P-Glycoprotein Inhibition by Garlic. J. Pharm. Pharm. Sci. 2001, 4, 176–184. [Google Scholar]
- Piscitelli, S.C.; Burstein, A.H.; Welden, N.; Gallicano, K.D.; Falloon, J. The Effect of Garlic Supplements on the Pharmacokinetics of Saquinavir. Clin. Infect. Dis. 2002, 34, 234–238. [Google Scholar] [CrossRef]
- Sunaga, K.; Ohkawa, K.; Nakamura, K.; Ohkubo, A.; Harada, S.; Tsuda, T. Mechanism-Based Inhibition of Recombinant Human Cytochrome P450 3A4 by Tomato Juice Extract. Biol. Pharm. Bull. 2012, 35, 329–334. [Google Scholar] [CrossRef] [Green Version]
- Ohkubo, A.; Chida, T.; Kikuchi, H.; Tsuda, T.; Sunaga, K. Effects of Tomato Juice on the Pharmacokinetics of CYP3A4-Substrate Drugs. Asian J. Pharm. Sci. 2017, 12, 464. [Google Scholar] [CrossRef]
- Tsujimoto, M.; Agawa, C.; Ueda, S.; Yamane, T.; Kitayama, H.; Terao, A.; Fukuda, T.; Minegaki, T.; Nishiguchi, K. Inhibitory Effects of Juices Prepared from Individual Vegetables on CYP3A4 Activity in Recombinant CYP3A4 and LS180 Cells. Biol. Pharm. Bull. 2017, 40, 1561–1565. [Google Scholar] [CrossRef]
- Kumar, S.; Bouic, P.J.; Rosenkranz, B. In Vitro Assessment of the Interaction Potential of Ocimum Basilicum (L.) Extracts on CYP2B6, 3A4, and Rifampicin Metabolism. Front. Pharmacol. 2020, 11, 517. [Google Scholar] [CrossRef]
- Samojlik, I.; Mijatović, V.; Petković, S.; Škrbić, B.; Božin, B. The Influence of Essential Oil of Aniseed (Pimpinella Anisum, L.) on Drug Effects on the Central Nervous System. Fitoterapia 2012, 83, 1466–1473. [Google Scholar] [CrossRef]
- Gupta, R.C.; Chang, D.; Nammi, S.; Bensoussan, A.; Bilinski, K.; Roufogalis, B.D. Interactions between Antidiabetic Drugs and Herbs: An Overview of Mechanisms of Action and Clinical Implications. Diabetol Metab Syndr 2017, 9, 59. [Google Scholar] [CrossRef]
- Hassanzadeh-Taheri, M.; Hassanpour-Fard, M.; Doostabadi, M.; Moodi, H.; Vazifeshenas-Darmiyan, K.; Hosseini, M. Co-Administration Effects of Aqueous Extract of Turnip Leaf and Metformin in Diabetic Rats. J. Tradit. Complement. Med. 2018, 8, 178. [Google Scholar] [CrossRef]
- Zeng, M.; Zhang, L.; Li, M.; Zhang, B.; Zhou, N.; Ke, Y.; Feng, W.; Zheng, X. Estrogenic Effects of the Extracts from the Chinese Yam (Dioscorea Opposite Thunb.) and Its Effective Compounds in Vitro and in Vivo. Molecules 2018, 23, 11. [Google Scholar] [CrossRef] [Green Version]
- Mohseni, M.S.M.; Golshani, B. Simultaneous Determination of Levodopa and Carbidopa from Fava Bean, Green Peas and Green Beans by High Performance Liquid Gas Chromatography. J. Clin. Diagn. Res. 2013, 7, 1004. [Google Scholar] [CrossRef]
- Fernandes, J.; Fialho, M.; Santos, R.; Peixoto-Plácido, C.; Madeira, T.; Sousa-Santos, N.; Virgolino, A.; Santos, O.; Vaz Carneiro, A. Is olive oil good for you? A systematic review and meta-analysis on anti-inflammatory benefits from regular dietary intake. Nutrition 2020, 69, 110559. [Google Scholar] [CrossRef]
- Papakonstantinou, V.D.; Lagopati, N.; Tsilibary, E.C.; Demopoulos, C.A.; Philippopoulos, A.I. A Review on Platelet Activating Factor Inhibitors: Could a New Class of Potent Metal-Based Anti-Inflammatory Drugs Induce Anticancer Properties? Bioinorg. Chem. Appl. 2017, 2017, 6947034. [Google Scholar] [CrossRef] [Green Version]
- Schwingshackl, L.; Krause, M.; Schmucker, C.; Hoffmann, G.; Rücker, G.; Meerpohl, J.J. Impact of Different Types of Olive Oil on Cardiovascular Risk Factors: A Systematic Review and Network Meta-Analysis. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1030–1039. [Google Scholar] [CrossRef]
- Albadawi, D.A.I.; Ravishankar, D.; Vallance, T.M.; Patel, K.; Osborn, H.M.I.; Vaiyapuri, S. Impacts of Commonly Used Edible Plants on the Modulation of Platelet Function. Int. J. Mol. Sci. 2022, 23, 605. [Google Scholar] [CrossRef]
- Jeong, H.U.; Kwon, S.S.; Kong, T.Y.; Kim, J.H.; Lee, H.S. Inhibitory Effects of Cedrol, β-Cedrene, and Thujopsene on Cytochrome P450 Enzyme Activities in Human Liver Microsomes. J. Toxicol. Environ. Health A 2014, 77, 1522–1532. [Google Scholar] [CrossRef]
- Bailey, D.G. Grapefruit-Medication Interactions. CMAJ 2013, 185, 507–508. [Google Scholar] [CrossRef] [Green Version]
- Hanley, M.J.; Cancalon, P.; Widmer, W.W.; Greenblatt, D.J. The Effect of Grapefruit Juice on Drug Disposition. Expert Opin. Drug Metab. Toxicol. 2011, 7, 267–286. [Google Scholar] [CrossRef]
- Seden, K.; Dickinson, L.; Khoo, S.; Back, D. Grapefruit-Drug Interactions. Drugs 2010, 70, 2373–2407. [Google Scholar] [CrossRef]
- Guo, L.Q.; Yamazoe, Y. Inhibition of Cytochrome P450 by Furanocoumarins in Grapefruit Juice and Herbal Medicines. Acta Pharmacol. Sin. 2004, 25, 129–136. [Google Scholar] [PubMed]
- Lown, K.S.; Bailey, D.G.; Fontana, R.J.; Janardan, S.K.; Adair, C.H.; Fortlage, L.A.; Brown, M.B.; Guo, W.; Watkins, P.B. Grapefruit Juice Increases Felodipine Oral Availability in Humans by Decreasing Intestinal CYP3A Protein Expression. J. Clin. Investig. 1997, 99, 2545–2553. [Google Scholar] [CrossRef] [Green Version]
- Bailey, D.G.; Dresser, G.; Arnold, J.M. Grapefruit-Medication Interactions: Forbidden Fruit or Avoidable Consequences? CMAJ 2013, 185, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, D.G. Better to Avoid Grapefruit with Certain Statins. Am. J. Med. 2016, 129, e301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, D.G.; Dresser, G.K.; Bend, J.R. Bergamottin, Lime Juice, and Red Wine as Inhibitors of Cytochrome P450 3A4 Activity: Comparison with Grapefruit Juice. Clin. Pharmacol. Ther. 2003, 73, 529–537. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, S.Y.; Fabriaga, E.; Zhang, P.H.; Zhou, Q. Food-Drug Interactions Precipitated by Fruit Juices Other than Grapefruit Juice: An Update Review. J. Food Drug Anal. 2018, 26, S61–S71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petric, Z.; Žuntar, I.; Putnik, P.; Kovačević, D.B. Food–Drug Interactions with Fruit Juices. Foods 2021, 10, 33. [Google Scholar] [CrossRef]
- An, G.; Mukker, J.K.; Derendorf, H.; Frye, R.F. Enzyme- and transporter-mediated beverage-drug interactions: An update on fruit juices and green tea. J. Clin. Pharmacol. 2015, 55, 1313–1331. [Google Scholar] [CrossRef]
- Dresser, G.K.; Bailey, D.G.; Leake, B.F.; Schwarz, U.I.; Dawson, P.A.; Freeman, D.J.; Kim, R.B. Fruit Juices Inhibit Organic Anion Transporting Polypeptide-Mediated Drug Uptake to Decrease the Oral Availability of Fexofenadine. Clin. Pharmacol. Ther. 2002, 71, 11–20. [Google Scholar] [CrossRef]
- Kamath, A.V.; Yao, M.; Zhang, Y.; Chong, S. Effect of Fruit Juices on the Oral Bioavailability of Fexofenadine in Rats. J. Pharm. Sci. 2005, 94, 233–239. [Google Scholar] [CrossRef]
- Grenier, J.; Fradette, C.; Morelli, G.; Merritt, G.J.; Vranderick, M.; Ducharme, M.P. Pomelo Juice, but Not Cranberry Juice, Affects the Pharmacokinetics of Cyclosporine in Humans. Clin. Pharmacol. Ther. 2006, 79, 255–262. [Google Scholar] [CrossRef]
- Gertz, B.J.; Holland, S.D.; Kline, W.F.; Matuszewski, B.K.; Freeman, A.; Quan, H.; Lasseter, K.C.; Mucklow, J.C.; Porras, A.G. Studies of the Oral Bioavailability of Alendronate. Clin. Pharmacol. Ther. 1995, 58, 288–298. [Google Scholar] [CrossRef]
- Tapaninen, T.; Neuvonen, P.J.; Niemi, M. Orange and Apple Juice Greatly Reduce the Plasma Concentrations of the OATP2B1 Substrate Aliskiren. Br. J. Clin. Pharmacol. 2011, 71, 718–726. [Google Scholar] [CrossRef]
- Malhotra, S.; Bailey, D.G.; Paine, M.F.; Watkins, P.B. Seville Orange Juice-Felodipine Interaction: Comparison with Dilute Grapefruit Juice and Involvement of Furocoumarins. Clin. Pharmacol. Ther. 2001, 69, 14–23. [Google Scholar] [CrossRef]
- Karmakar, S.; Biswas, S.; Bera, R.; Mondal, S.; Kundu, A.; Ali, M.A.; Sen, T. Beverage-Induced Enhanced Bioavailability of Carbamazepine and Its Consequent Effect on Antiepileptic Activity and Toxicity. J. Food Drug Anal. 2015, 23, 327–334. [Google Scholar] [CrossRef]
- Backman, J.T.; Mäenpää, J.; Belle, D.J.; Wrighton, S.A.; Kivistö, K.T.; Neuvonen, P.J. Lack of Correlation between in Vitro and m Vivo Studies on the Effects of Tangeretin and Tangerine Juice on Midazolam Hydroxylation. Clin. Pharmacol. Ther. 2000, 67, 382–390. [Google Scholar] [CrossRef]
- Morita, T.; Akiyoshi, T.; Sato, R.; Uekusa, Y.; Katayama, K.; Yajima, K.; Imaoka, A.; Sugimoto, Y.; Kiuchi, F.; Ohtani, H. Citrus Fruit-Derived Flavanone Glycoside Narirutin Is a Novel Potent Inhibitor of Organic Anion-Transporting Polypeptides. J. Agric. Food Chem. 2020, 68, 14182–14191. [Google Scholar] [CrossRef]
- Akamine, Y.; Miura, M.; Komori, H.; Saito, S.; Kusuhara, H.; Tamai, I.; Ieiri, I.; Uno, T.; Yasui-Furukori, N. Effects of One-Time Apple Juice Ingestion on the Pharmacokinetics of Fexofenadine Enantiomers. Eur. J. Clin. Pharmacol. 2014, 70, 1087–1095. [Google Scholar] [CrossRef]
- Jeon, H.; Jang, I.J.; Lee, S.; Ohashi, K.; Kotegawa, T.; Ieiri, I.; Cho, J.Y.; Yoon, S.H.; Shin, S.G.; Yu, K.S.; et al. Apple Juice Greatly Reduces Systemic Exposure to Atenolol. Br. J. Clin. Pharmacol. 2013, 75, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Morita, T.; Akiyoshi, T.; Tsuchitani, T.; Kataoka, H.; Araki, N.; Yajima, K.; Katayama, K.; Imaoka, A.; Ohtani, H. Inhibitory Effects of Cranberry Juice and Its Components on Intestinal OATP1A2 and OATP2B1: Identification of Avicularin as a Novel Inhibitor. J. Agric. Food Chem. 2022, 70, 3310–3320. [Google Scholar] [CrossRef]
- Srinivas, N.R. Cranberry Juice Ingestion and Clinical Drug-Drug Interaction Potentials; Review of Case Studies and Perspectives. J. Pharm. Pharm. Sci. 2013, 16, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Greenblatt, D.J.; Von Moltke, L.L.; Perloff, E.S.; Luo, Y.; Harmatz, J.S.; Zinny, M.A. Interaction of Flurbiprofen with Cranberry Juice, Grape Juice, Tea, and Fluconazole: In Vitro and Clinical Studies. Clin. Pharmacol. Ther. 2006, 79, 125–133. [Google Scholar] [CrossRef]
- Lilja, J.J.; Backman, J.T.; Neuvonen, P.J. Effects of Daily Ingestion of Cranberry Juice on the Pharmacokinetics of Warfarin, Tizanidine, and Midazolam—Probes of CYP2C9, CYP1A2, and CYP3A4. Clin. Pharmacol. Ther. 2007, 81, 833–839. [Google Scholar] [CrossRef]
- Srinivas, N.R. Is Pomegranate Juice a Potential Perpetrator of Clinical Drug-Drug Interactions? Review of the in Vitro, Preclinical and Clinical Evidence. Eur. J. Drug Metab. Pharmacokinet. 2013, 38, 223–229. [Google Scholar] [CrossRef]
- Yusuff, K.B.; Emeka, P.M.; Attimarad, M. Concurrent Administration of Date Palm Fruits with Lisinopril Increases Serum Potassium Level in Male Rabbits. Int. J. Pharmacol. 2018, 14, 93–98. [Google Scholar] [CrossRef]
- St-Jules, D.E.; Goldfarb, D.S.; Sevick, M.A. Nutrient Non-Equivalence: Does Restricting High-Potassium Plant Foods Help to Prevent Hyperkalemia in Hemodialysis Patients? J. Ren. Nutr. 2016, 26, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Jiménez-Molén, J.J.; Lindholm, B.; Cederholm, T.; Ärnlöv, J.; Risérus, U.; Sjögren, P.; Carrero, J.J. Mediterranean Diet, Kidney Function, and Mortality in Men with CKD. Clin. J. Am. Soc. Nephrol. 2013, 8, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Molina-Vega, M.; Gómez-Pérez, A.M.; Tinahones, F.J. Fish in the Mediterranean diet. In The Mediterranean Diet: An Evidence-Based Approach, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2020; pp. 275–284. [Google Scholar] [CrossRef]
- Méndez, L.; Dasilva, G.; Taltavull, N.; Romeu, M.; Medina, I. Marine Lipids on Cardiovascular Diseases and Other Chronic Diseases Induced by Diet: An Insight Provided by Proteomics and Lipidomics. Mar. Drugs 2017, 15, 258. [Google Scholar] [CrossRef] [Green Version]
- Vanschoonbeek, K.; Feijge, M.A.; Paquay, M.; Rosing, J.; Saris, W.; Kluft, C.; Giesen, P.L.; de Maat, M.P.; Heemskerk, J.W. Variable Hypocoagulant Effect of Fish Oil Intake in Humans: Modulation of Fibrinogen Level and Thrombin Generation. Arter. Thromb Vasc Biol 2004, 24, 1734–1740. [Google Scholar] [CrossRef]
- Buckley, M.S.; Goff, A.D.; Knapp, W.E. Fish Oil Interaction with Warfarin. Ann. Pharmacother. 2004, 38, 50–53. [Google Scholar] [CrossRef]
- Jalili, M.; Dehpour, A.R. Extremely Prolonged INR Associated with Warfarin in Combination with Both Trazodone and Omega-3 Fatty Acids. Arch. Med. Res. 2007, 38, 901–904. [Google Scholar] [CrossRef]
- Pryce, R.; Bernaitis, N.; Davey, A.K.; Badrick, T.; Anoopkumar-Dukie, S. The Use of Fish Oil with Warfarin Does Not Significantly Affect Either the International Normalised Ratio or Incidence of Adverse Events in Patients with Atrial Fibrillation and Deep Vein Thrombosis: A Retrospective Study. Nutrients 2016, 8, 578. [Google Scholar] [CrossRef] [Green Version]
- KANEKO, J.; TANIMUKAI, H. MONOAMINE OXIDASE INHIBITORS (MAO). Sogo. Rinsho. 1964, 13, 833–846. [Google Scholar] [CrossRef]
- Kibangou, I.B.; Bouhallab, S.; Henry, G.; Bureau, F.; Allouche, S.; Blais, A.; Guérin, P.; Arhan, P.; Bouglé, D.L. Milk Proteins and Iron Absorption: Contrasting Effects of Different Caseinophosphopeptides. Pediatr. Res. 2005, 58, 731–734. [Google Scholar] [CrossRef] [PubMed]
- De Lemos, M.L.; Hamata, L.; Jennings, S.; Leduc, T. Interaction between Mercaptopurine and Milk. J. Oncol. Pharm. Pract. 2007, 13, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Sofianou-Katsoulis, A.; Khakoo, G.; Kaczmarski, R. Reduction in Bioavailability of 6-Mercaptopurine on Simultaneous Administration with Cow’s Milk. Pediatr. Hematol. Oncol. 2006, 23, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.C.C.; Vicente, A.F.R.B. Meat Nutritional Composition and Nutritive Role in the Human Diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V.; Santos-Buelga, C.; Feliciano, A.S. Wine Flavonoids in Health and Disease Prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of Red Wine Consumption to Human Health Protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renaud, S.; de Lorgeril, M. Wine, Alcohol, Platelets, and the French Paradox for Coronary Heart Disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Weathermon, R.; Crabb, D.W. Alcohol and Medication Interactions. Alcohol Res. Health 1999, 23, 40. [Google Scholar]
- Mergenhagen, K.A.; Wattengel, B.A.; Skelly, M.K.; Clark, C.M.; Russo, T.A. Fact versus Fiction: A Review of the Evidence behind Alcohol and Antibiotic Interactions. Antimicrob. Agents Chemother. 2020, 64, e02167-19. [Google Scholar] [CrossRef]
- Romeo, J.; Würnberg, J.; Nova, E.; Díaz, L.E.; Gómez-Martinez, S.; Marcos, A. Moderate Alcohol Consumption and the Immune System: A Review. Br. J. Nutr. 2007, 98, S111–S115. [Google Scholar] [CrossRef]
- Foster, J.H.; Powell, J.E.; Marshall, E.J.; Peters, T.J. Quality of Life in Alcohol-Dependent Subjects--a Review. Qual. Life Res. 1999, 8, 255–261. [Google Scholar] [CrossRef]
- Pauwels, E.K.J. The Protective Effect of the Mediterranean Diet: Focus on Cancer and Cardiovascular Risk. Med. Princ. Pract. 2011, 20, 103–111. [Google Scholar] [CrossRef]
- Tsigalou, C.; Konstantinidis, T.; Paraschaki, A.; Stavropoulou, E.; Voidarou, C.; Bezirtzoglou, E. Mediterranean diet as a tool to combat inflammation and chronic diseases. An overview. Biomedicines 2020, 8, 201. [Google Scholar] [CrossRef]
- Baute, V.; Sampath-Kumar, R.; Nelson, S.; Basil, B. Nutrition Education for the Health-care Provider Improves Patient Outcomes. Glob. Adv. Health Med. 2018, 7, 2164956118795995. [Google Scholar] [CrossRef]
- Spanakis, M.; Patelarou, A.E.; Patelarou, E. Nursing Personnel in the Era of Personalized Healthcare in Clinical Practice. J. Pers. Med. 2020, 10, 56. [Google Scholar] [CrossRef]
- Thorpe, M.G.; Milte, C.M.; Crawford, D.; McNaughton, S.A. Education and lifestyle predict change in dietary patterns and diet quality of adults 55 years and over. Nutr. J. 2019, 18, 67. [Google Scholar] [CrossRef] [Green Version]
- Tuttolomondo, A.; Simonetta, I.; Daidone, M.; Mogavero, A.; Ortello, A.; Pinto, A. Metabolic and Vascular Effect of the Mediterranean Diet. Int. J. Mol. Sci. 2019, 20, 4716. [Google Scholar] [CrossRef] [Green Version]
- Aljefree, N.M.; Almoraie, N.M.; Shatwan, I.M. Association of two types of dietary pattern scores with cardiovascular disease risk factors and serum 25 hydroxy vitamin D levels in Saudi Arabia. Food Nutr. Res. 2021, 65. [Google Scholar] [CrossRef]
- Herrera-Marcos, L.V.; Lou-Bonafonte, J.M.; Arnal, C.; Navarro, M.A.; Osada, J. Transcriptomics and the Mediterranean Diet: A Systematic Review. Nutrients 2017, 9, 472. [Google Scholar] [CrossRef] [Green Version]
- Badimon, L.; Vilahur, G.; Padro, T. Systems biology approaches to understand the effects of nutrition and promote health. Br. J. Clin. Pharmacol. 2017, 83, 38. [Google Scholar] [CrossRef] [Green Version]
- Yeh, C.T.; Yen, G.C. Effect of Vegetables on Human Phenolsulfotransferases in Relation to Their Antioxidant Activity and Total Phenolics. Free Radic. Res. 2005, 39, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Harasym, J.; Oledzki, R. Effect of Fruit and Vegetable Antioxidants on Total Antioxidant Capacity of Blood Plasma. Nutrition 2014, 30, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Ravera, A.; Carubelli, V.; Sciatti, E.; Bonadei, I.; Gorga, E.; Cani, D.; Vizzardi, E.; Metra, M.; Lombardi, C. Nutrition and Cardiovascular Disease: Finding the Perfect Recipe for Cardiovascular Health. Nutrients 2016, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Bjerrum, L.; Lopez-Valcarcel, B.G.; Petersen, G. Risk Factors for Potential Drug Interactions in General Practice. Eur. J. Gen. Pract. 2008, 14, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guttman, Y.; Kerem, Z. Computer-Aided (In Silico) Modeling of Cytochrome P450-Mediated Food-Drug Interactions (FDI). Int. J. Mol. Sci. 2022, 23, 8498. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Vadrev, S.M.; Magana-Mora, A.; Levman, J.; Soufan, O. A novel graph mining approach to predict and evaluate food-drug interactions. Sci. Rep. 2022, 12, 1061. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.Y.; Kim, H.U.; Lee, S.Y. Deep Learning Improves Prediction of Drug–Drug and Drug–Food Interactions. Proc. Natl. Acad. Sci. USA 2018, 115, E4304–E4311. [Google Scholar] [CrossRef] [Green Version]
- Car, J.; Tan, W.S.; Huang, Z.; Sloot, P.; Franklin, B.D. eHealth in the future of medications management: Personalisation, monitoring and adherence. BMC Med. 2017, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- Spanakis, E.G.; Santana, S.; Tsiknakis, M.; Marias, K.; Sakkalis, V.; Teixeira, A.; Janssen, J.H.; de Jong, H.; Tziraki, C. Technology-Based Innovations to Foster Personalized Healthy Lifestyles and Well-Being: A Targeted Review. J. Med. Internet Res. 2016, 18, e128. [Google Scholar] [CrossRef]
- Ware, P.; Bartlett, S.J.; Paré, G.; Symeonidis, I.; Tannenbaum, C.; Bartlett, G.; Poissant, L.; Ahmed, S. Using EHealth Technologies: Interests, Preferences, and Concerns of Older Adults. Interact. J. Med. Res. 2017, 6, e3. [Google Scholar] [CrossRef] [Green Version]
- Ossebaard, H.C.; Van Gemert-Pijnen, L. EHealth and Quality in Health Care: Implementation Time. Int. J. Qual. Health Care 2016, 28, 415–419. [Google Scholar] [CrossRef] [Green Version]
- Black, A.D.; Car, J.; Pagliari, C.; Anandan, C.; Cresswell, K.; Bokun, T.; McKinstry, B.; Procter, R.; Majeed, A.; Sheikh, A. The Impact of EHealth on the Quality and Safety of Health Care: A Systematic Overview. PLoS Med. 2011, 8, e1000387. [Google Scholar] [CrossRef] [Green Version]
- Maniadi, E.; Kondylakis, H.; Spanakis, E.G.; Spanakis, M.; Tsiknakis, M.; Marias, K.; Dong, F. Designing a digital patient avatar in the context of the MyHealthAvatar project initiative. In Proceedings of the 13th IEEE International Conference on BioInformatics and BioEngineering (BIBE 2013), Chania, Greece, 10–13 November 2013. [Google Scholar]
- Vasiloglou, M.F.; Lu, Y.; Stathopoulou, T.; Papathanail, I.; Faeh, D.; Ghosh, A.; Baumann, M.; Mougiakakou, S. Assessing Mediterranean Diet Adherence with the Smartphone: The Medipiatto Project. Nutrients 2020, 12, 3763. [Google Scholar] [CrossRef]
- McAleese, D.; Linardakis, M.; Papadaki, A. Quality and Presence of Behaviour Change Techniques in Mobile Apps for the Mediterranean Diet: A Content Analysis of Android Google Play and Apple App Store Apps. Nutrients 2022, 14, 1290. [Google Scholar] [CrossRef]
- Spanakis, M.; Sfakianakis, S.; Spanakis, E.G.; Kallergis, G.; Sakkalis, V. PDCA: An EHealth Service for the Management of Drug Interactions with Complementary and Alternative Medicines. In Proceedings of the 2018 IEEE EMBS International Conference on Biomedical and Health Informatics (BHI), Las Vegas, NV, USA, 4–7 March 2018. [Google Scholar]
- Spanakis, M.; Sfakianakis, S.; Kallergis, G.; Spanakis, E.G.; Sakkalis, V. PharmActa: Personalized Pharmaceutical Care EHealth Platform for Patients and Pharmacists. J. Biomed. Inform. 2019, 100, 103336. [Google Scholar] [CrossRef]




| Food | Type of DFIs | Suggested Mechanism | Clinical Significance | Level of Evidence |
|---|---|---|---|---|
| Artichoke | PK | CYP mediated drug metabolism | Moderate | Moderate |
| Arugula | PD | Anticoagulants—Vitamin K | Moderate | Theoretical |
| Asparagus | PD | Anticoagulants—Vitamin K | Moderate | Theoretical |
| Beetroots | - | - | - | |
| Bell pepper | PD | Anticoagulants—Vitamin K | Moderate | Theoretical |
| Broccoli | PD | Anticoagulants—Vitamin K | Moderate | Theoretical |
| Brussel Sprouts | PK | CYP mediated drug metabolism | Moderate | Moderate |
| Cabbage | PK | CYP mediated drug metabolism | Moderate | Good |
| Carrots | PK | CYP mediated drug metabolism | Minor | Low |
| Cauliflower | PK | CYP mediated drug metabolism | Minor | Moderate |
| Celery | PD | Synergism sedatives | Minor | Good |
| Collard greens | PD | Synergism antidiabetic medications | Minor | Low |
| Cucumbers | - | - | - | - |
| Dandelion greens | PD | Lithium | Moderate | Low |
| Eggplant | - | - | - | - |
| Fennel | - | - | - | - |
| Fava beans | PD | synergism with anti-parkinson drugs (L-dopa content) | Moderate | Low |
| Garlic | PK | Moderate | Good | |
| Green beans | - | - | - | - |
| Kale | PD | Anticoagulants—Vitamin K | Moderate | Theoretical |
| Leeks | PD | Anticoagulants—Vitamin K | Minor | Theoretical |
| Mushrooms | - | - | - | - |
| Onions | PD | Anticoagulants—Vitamin K | Minor | Theoretical |
| Potatoes | - | - | - | - |
| Radish | PK | stimulate GI motility & transit time | Minor | Moderate |
| Scallions | PD | Anticoagulants—Vitamin K | Minor | Theoretical |
| Shallots | PD | Anticoagulants—Vitamin K | Minor | Theoretical |
| Spinach | PD | Anticoagulants—Vitamin K | Moderate | Theoretical |
| Squash | PD | stimulant laxative may alter Κ+ | Minor | Theoretical |
| Sweet potatoes | - | - | - | - |
| Swiss chard | PD | Anticoagulants—Vitamin K | Minor | Theoretical |
| Tomatoes | PK | CYP mediated drug metabolism | Minor | Moderate |
| Turnips | PD | Synergism antidiabetic medications | Minor | Moderate |
| Yams | PD | Synergism with estrogens | Moderate | Good |
| Zucchini | - | - | - | - |
| Anise | PK | CYP mediated metabolism | Moderate | Good |
| Basil | PD | Anticoagulants, n-3 FAs | Moderate | Low |
| Bayleaf | PD | Synergism with antidiabetics & sedatives | Minor | Low |
| Cinnamon | PD | Synergism antidiabetic & hepatoxicity | Minor | Low |
| Cloves | PD | potentiate the effects of drugs that affect hemostasis | Moderate | Moderate |
| Cumin | PD | Synergism antidiabetic medications | Moderate | Moderate |
| Fennel | - | - | - | - |
| Lavender | PD | Synergism with sedatives | Moderate | Low |
| Marjoram | PD | Potentiate effects of drugs for hemostasis | Moderate | Low |
| Mint | - | - | - | - |
| Oregano | PD | Potentiate effects of drugs for hemostasis | Moderate | Low |
| Parsley | - | - | - | - |
| Pepper | PK | CYP mediated drug metabolism | Minor | Moderate |
| Rosemary | - | - | - | - |
| Sage | PD | Synergism with sedatives | Moderate | Low |
| Savory | PD | Anticoagulants, n-3 FAs | Moderate | Low |
| Sumac | PD | Induce nephrotoxicity of drugs | Minor | Low |
| Tarragon | PD | Anticoagulants, n-3 FAs | Moderate | Low |
| Thyme | PD | Anticoagulants, n-3 FAs | Moderate | Low |
| Fruit | Type of DFIs | Suggested Mechanism | Clinical Significance | Level of Evidence |
|---|---|---|---|---|
| Apples | PK | OAT transporter inhibition | Moderate | Good |
| Apricots | - | - | - | - |
| Avocados | PD | Anticoagulants—Vitamin K | Moderate | Theoretical |
| Bananas | PD | Hyperkaliemia, high K+ | Moderate | Theoretical |
| Cherries | - | - | - | - |
| Clementines | PK | CYP mediated drug metabolism | Moderate | Moderate |
| Grapefruit | PK | P-gp transport & CYP mediated drug metabolism | Serious-avoid | High |
| Grapes | PK | UGT mediated drug metabolism | Moderate | Good |
| Lime | PK | |||
| Melons | PD | Synergism antidiabetic medications | Low | Theoretical |
| Oranges | PK | OAT transporter inhibition | Moderate | Good |
| Peaches | PD | Hyperkaliemia, high K+ | Moderate | Theoretical |
| Pears | - | - | - | - |
| Pomegranates | PK | CYP mediated drug metabolism | None | High |
| Pomelo | PK | P-gp transport & CYP mediated drug metabolism | Use with caution | Good |
| Strawberries | PK/PD | Metabolism & anticoagulation | Low | Theoretical |
| Tangerines | PK | CYP mediated drug metabolism | Moderate | Moderate |
| Food | Type of DFIs | Suggested Mechanism | Clinical Significance | Level of Evidence |
|---|---|---|---|---|
| Barley | PK | Modulation GI absorption | Moderate | Low |
| Buckwheat | - | - | - | - |
| Bulgur | - | - | - | - |
| Farro-flax seed | PD | Anticoagulants, n-3 FAs | Moderate | Low |
| Millet | - | - | - | - |
| Oats & fibers | PK | Modulation GI absorption | Moderate | Good |
| Polenta | - | - | - | - |
| Rice | - | - | - | - |
| Wheat Berries | PK | Modulation GI absorption | Moderate | Low |
| Breads | - | - | - | - |
| Couscous | - | - | - | - |
| Almonds | - | - | - | - |
| Cashews | PD | Synergism antidiabetic medications | Minor | Low |
| Chickpeas | ||||
| Fava beans | PD | Synergism with anti-Parkinson drugs (high L-dopa content) | Moderate | Low |
| Green peas | - | - | - | - |
| Hazelnuts | ||||
| Kidney beans | PK | Modulation GI absorption | Minor | Low |
| Lentils | PD | Hyperkaliemia, high K+ | Minor | Low |
| Peanuts | PD | Tyramine pressure effect (MAOIs) | Use with caution | Low |
| Pistachios | - | - | - | - |
| Sesame seeds (tachini) | PK | CYP mediated metabolism | None | Moderate |
| Split peas | PK | Modulation GI absorption | Minor | Theoretical |
| Walnuts | PK | Modulation GI absorption | Moderate | Theoretical |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spanakis, M.; Patelarou, E.; Patelarou, A. Drug-Food Interactions with a Focus on Mediterranean Diet. Appl. Sci. 2022, 12, 10207. https://doi.org/10.3390/app122010207
Spanakis M, Patelarou E, Patelarou A. Drug-Food Interactions with a Focus on Mediterranean Diet. Applied Sciences. 2022; 12(20):10207. https://doi.org/10.3390/app122010207
Chicago/Turabian StyleSpanakis, Marios, Evridiki Patelarou, and Athina Patelarou. 2022. "Drug-Food Interactions with a Focus on Mediterranean Diet" Applied Sciences 12, no. 20: 10207. https://doi.org/10.3390/app122010207