Effects of Porcine Whole-Blood Protein Hydrolysate on Exercise Function and Skeletal Muscle Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Amino Acid Content Determination
2.3. Animals and Treatment
2.4. Exercise Performance
2.5. Cell Culture and Myotube Differentiation
2.6. Western Blotting
2.7. Histological Analysis of Muscle
2.8. Statistical Analysis
3. Results
3.1. PWBPH Supplementation Increased Muscle Mass and Exercise Performance
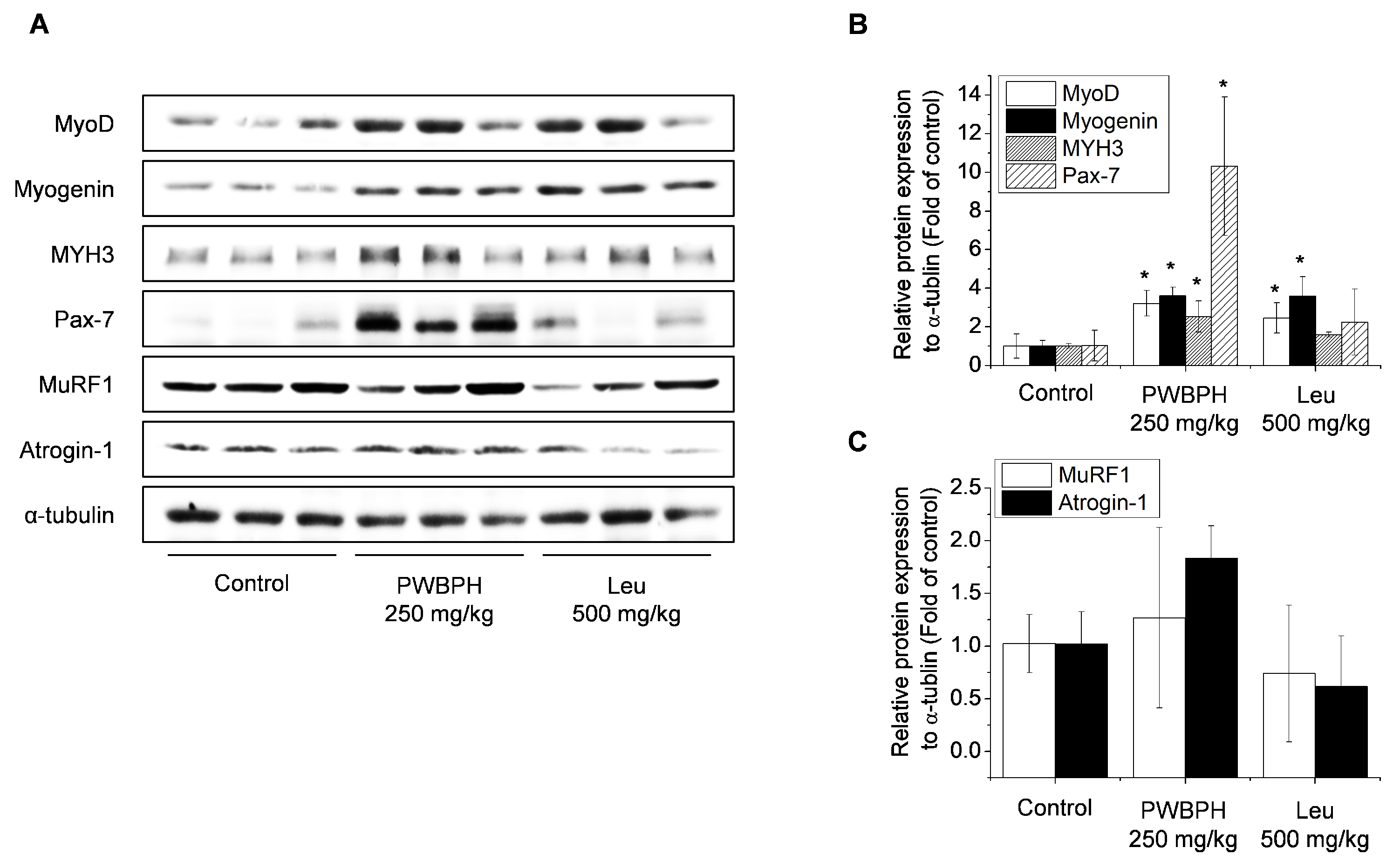
3.2. PWBPH Supplementation Enhanced Skeletal Muscle Differentiation In Vivo
3.3. PWBPH Supplementation Induced Differentiation and Hypertrophy of C2C12 Cells
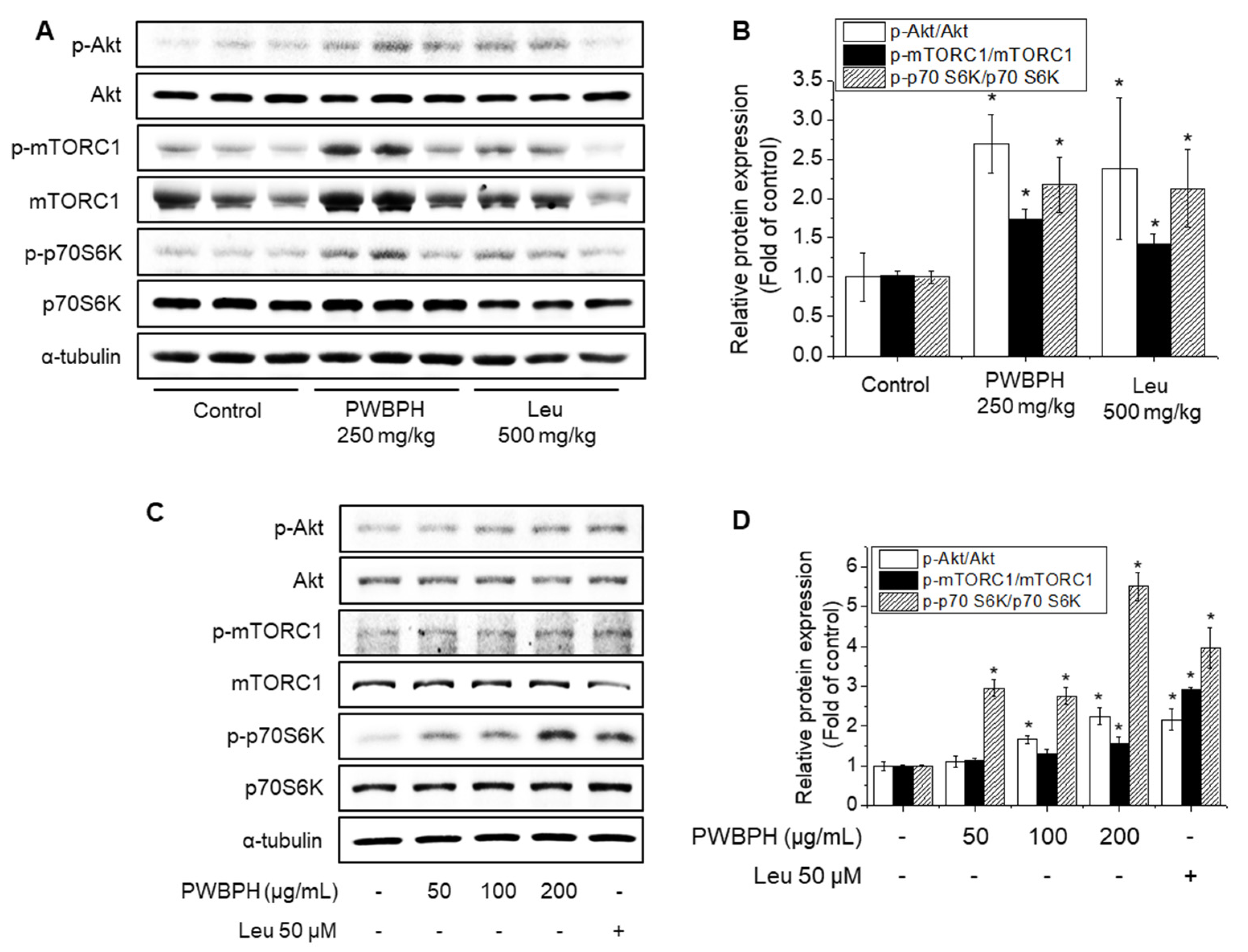
3.4. PWBPH Supplementation Induced Akt/mTOR Signaling Pathways In Vivo and In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duarte, R.T.; Carvalho Simões, M.C.; Sgarbieri, V.C. Bovine blood components: Fractionation, composition, and nutritive value. J. Agric. Food Chem. 1999, 47, 231–236. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, M.Y.; Jin, J.Y. Changes in physicochemical characteristics of porcine blood under various conditions of enzyme hydrolysis. Korean J. Food Preserv. 2014, 14, 6. [Google Scholar]
- De Vuono, M.; Penteado, C.; Lajolo, F.M.; dos Santos, N.P. Functional and nutritional properties of isolated bovine blood proteins. J. Sci. Food Agric. 1979, 30, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Salvador, P.; Saguer, E.; Parés, D.; Carretero, C.; Toldrà, M. Foaming and emulsifying properties of porcine red cell protein concentrate. Food Sci. Technol. Int. 2010, 16, 289–296. [Google Scholar] [CrossRef]
- Harper, A.E.; Miller, R.H.; Block, K.P. Branched-chain amino acid metabolism. Annu. Rev. Nutr. 1984, 4, 409–454. [Google Scholar] [CrossRef] [PubMed]
- Mantuano, P.; Bianchini, G.; Cappellari, O.; Boccanegra, B.; Conte, E.; Sanarica, F.; Mele, A.; Camerino, G.M.; Brandolini, L.; Allegretti, M.; et al. Ergogenic Effect of BCAAs and L-Alanine Supplementation: Proof-of-Concept Study in a Murine Model of Physiological Exercise. Nutrients 2020, 12, 2295. [Google Scholar] [CrossRef]
- Xu, Z.R.; Tan, Z.J.; Zhang, Q.; Gui, Q.F.; Yang, Y.M. The effectiveness of leucine on muscle protein synthesis, lean body mass and leg lean mass accretion in older people: A systematic review and meta-analysis. Br. J. Nutr. 2015, 113, 25–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, C.; He, T.; Zhang, W.; Zhang, G.; Ma, X. Branched Chain Amino Acids: Beyond Nutrition Metabolism. Int. J. Mol. Sci. 2018, 19, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bifari, F.; Nisoli, E. Branched-chain amino acids differently modulate catabolic and anabolic states in mammals: A pharmacological point of view. Br. J. Pharmacol. 2017, 174, 1366–1377. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.; Caille, O.; Ferreira, D.; Francaux, M. Pomegranate extract prevents skeletal muscle of mice against wasting induced by acute TNF-α injection. Mol. Nutr. Food Res. 2017, 61, 1600169. [Google Scholar] [CrossRef]
- Tajbakhsh, S. Skeletal muscle stem cells in developmental versus regenerative myogenesis. J. Intern. Med. 2009, 266, 372–389. [Google Scholar] [CrossRef]
- Yu, D.; Cai, Z.; Li, D.; Zhang, Y.; He, M.; Yang, Y.; Liu, D.; Xie, W.; Li, Y.; Xiao, W. Myogenic Differentiation of Stem Cells for Skeletal Muscle Regeneration. Stem Cells Int. 2021, 2021, 8884283. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Shibasaki, A.; Naka, A.; Saito, H.; Iida, K. Lactate Promotes Myoblast Differentiation and Myotube Hypertrophy via a Pathway Involving MyoD In Vitro and Enhances Muscle Regeneration In Vivo. Int. J. Mol. Sci. 2018, 19, 3649. [Google Scholar] [CrossRef] [Green Version]
- Lluís, F.; Perdiguero, E.; Nebreda, A.R.; Muñoz-Cánoves, P. Regulation of skeletal muscle gene expression by p38 MAP kinases. Trends Cell Biol. 2006, 16, 36–44. [Google Scholar] [CrossRef]
- Areta, J.L.; Hawley, J.A.; Ye, J.M.; Chan, M.H.; Coffey, V.G. Increasing leucine concentration stimulates mechanistic target of rapamycin signaling and cell growth in C2C12 skeletal muscle cells. Nutr. Res. 2014, 34, 1000–1007. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, X.; Huang, Z.; Chen, D.; Yu, B.; Chen, H.; Luo, J.; He, J.; Zheng, P.; Yu, J. Leucine promotes differentiation of porcine myoblasts through the protein kinase B (Akt)/Forkhead box O1 signalling pathway. Br. J. Nutr. 2018, 119, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Averous, J.; Gabillard, J.C.; Seiliez, I.; Dardevet, D. Leucine limitation regulates myf5 and myoD expression and inhibits myoblast differentiation. Exp. Cell Res. 2012, 318, 217–227. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Bell, R.A.; Al-Khalaf, M.; Megeney, L.A. The beneficial role of proteolysis in skeletal muscle growth and stress adaptation. Skelet Muscle 2016, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Keller, J.; Couturier, A.; Haferkamp, M.; Most, E.; Eder, K. Supplementation of carnitine leads to an activation of the IGF-1/PI3K/Akt signalling pathway and down regulates the E3 ligase MuRF1 in skeletal muscle of rats. Nutr. Metab. 2013, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Codina, M.; García de la Serrana, D.; Sánchez-Gurmaches, J.; Montserrat, N.; Chistyakova, O.; Navarro, I.; Gutiérrez, J. Metabolic and mitogenic effects of IGF-II in rainbow trout (Oncorhynchus mykiss) myocytes in culture and the role of IGF-II in the PI3K/Akt and MAPK signalling pathways. Gen. Comp. Endocrinol. 2008, 157, 116–124. [Google Scholar] [CrossRef]
- Egerman, M.A.; Glass, D.J. Signaling pathways controlling skeletal muscle mass. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.M.; Blenis, J. Molecular mechanisms of mTOR-mediated translational control. Nat. Rev. Mol. Cell Biol. 2009, 10, 307–318. [Google Scholar] [CrossRef]
- Cruz, B.; Oliveira, A.; Ventrucci, G.; Gomes-Marcondes, M.C.C. A leucine-rich diet modulates the mTOR cell signalling pathway in the gastrocnemius muscle under different Walker-256 tumour growth conditions. BMC Cancer 2019, 19, 349. [Google Scholar] [CrossRef]
- Drummond, M.J.; Rasmussen, B.B. Leucine-enriched nutrients and the regulation of mammalian target of rapamycin signalling and human skeletal muscle protein synthesis. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 222–226. [Google Scholar] [CrossRef]
- Gannon, N.P.; Schnuck, J.K.; Vaughan, R.A. BCAA Metabolism and Insulin Sensitivity—Dysregulated by Metabolic Status? Mol. Nutr. Food Res. 2018, 62, e1700756. [Google Scholar] [CrossRef]
- VanDusseldorp, T.A.; Escobar, K.A.; Johnson, K.E.; Stratton, M.T.; Moriarty, T.; Cole, N.; McCormick, J.J.; Kerksick, C.M.; Vaughan, R.A.; Dokladny, K.; et al. Effect of Branched-Chain Amino Acid Supplementation on Recovery Following Acute Eccentric Exercise. Nutrients 2018, 10, 1389. [Google Scholar] [CrossRef] [Green Version]
- Howatson, G.; Hoad, M.; Goodall, S.; Tallent, J.; Bell, P.G.; French, D.N. Exercise-induced muscle damage is reduced in resistance-trained males by branched chain amino acids: A randomized, double-blind, placebo controlled study. J. Int. Soc. Sports Nutr. 2012, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Wu, Z.; Dai, Z.; Wang, G.; Wu, G. Protein hydrolysates in animal nutrition: Industrial production, bioactive peptides, and functional significance. J. Anim. Sci. Biotechnol. 2017, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Bak, K.H.; Petersen, M.A.; Lametsch, R.; Hansen, E.T.; Ruiz-Carrascal, J. Development of Volatile Compounds during Hydrolysis of Porcine Hemoglobin with Papain. Molecules 2018, 23, 357. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.K.; Choi, J.S.; Yim, D.G. Hydrolysis Conditions of Porcine Blood Proteins and Antimicrobial Effects of Their Hydrolysates. Food Sci. Anim. Resour 2020, 40, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, R.R. Branched-chain amino acids and muscle protein synthesis in humans: Myth or reality? J. Int. Soc. Sports Nutr. 2017, 14, 30. [Google Scholar] [CrossRef] [Green Version]
- Dijk, F.J.; van Dijk, M.; Walrand, S.; van Loon, L.J.C.; van Norren, K.; Luiking, Y.C. Differential effects of leucine and leucine-enriched whey protein on skeletal muscle protein synthesis in aged mice. Clin. Nutr. ESPEN 2018, 24, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Santos, C.S.; Nascimento, F.E.L. Isolated branched-chain amino acid intake and muscle protein synthesis in humans: A biochemical review. Einstein 2019, 17, eRB4898. [Google Scholar] [CrossRef] [Green Version]
- Soundharrajan, I.; Kim, D.H.; Kuppusamy, P.; Choi, K.C. Modulation of osteogenic and myogenic differentiation by a phytoestrogen formononetin via p38MAPK-dependent JAK-STAT and Smad-1/5/8 signaling pathways in mouse myogenic progenitor cells. Sci. Rep. 2019, 9, 9307. [Google Scholar] [CrossRef] [Green Version]
- Rudnicki, M.A.; Braun, T.; Hinuma, S.; Jaenisch, R. Inactivation of MyoD in mice leads to up-regulation of the myogenic HLH gene Myf-5 and results in apparently normal muscle development. Cell 1992, 71, 383–390. [Google Scholar] [CrossRef]
- White, J.D.; Scaffidi, A.; Davies, M.; McGeachie, J.; Rudnicki, M.A.; Grounds, M.D. Myotube formation is delayed but not prevented in MyoD-deficient skeletal muscle: Studies in regenerating whole muscle grafts of adult mice. J. Histochem. Cytochem. 2000, 48, 1531–1544. [Google Scholar] [CrossRef] [Green Version]
- Knapp, J.R.; Davie, J.K.; Myer, A.; Meadows, E.; Olson, E.N.; Klein, W.H. Loss of myogenin in postnatal life leads to normal skeletal muscle but reduced body size. Development 2006, 133, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.H.; Gil, J.H.; Quan, H.; Viet, D.H.; Kim, C.K. Effect of 8-week leucine supplementation and resistance exercise training on muscle hypertrophy and satellite cell activation in rats. Physiol. Rep. 2018, 6, e13725. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, J.Y.; Jiang, Q.; Zhou, X.Q.; Feng, L.; Liu, Y.; Jiang, W.D.; Wu, P.; Zhou, J.; Zhao, J.; et al. Leucine Improved Growth Performance, Muscle Growth, and Muscle Protein Deposition Through AKT/TOR and AKT/FOXO3a Signaling Pathways in Hybrid Catfish. Cells 2020, 9, 327. [Google Scholar] [CrossRef] [Green Version]
- Atherton, P.J.; Smith, K. Muscle protein synthesis in response to nutrition and exercise. J. Physiol. 2012, 590, 1049–1057. [Google Scholar] [CrossRef] [Green Version]
- Guiraud, S.; Edwards, B.; Squire, S.E.; Moir, L.; Berg, A.; Babbs, A.; Ramadan, N.; Wood, M.J.; Davies, K.E. Embryonic myosin is a regeneration marker to monitor utrophin-based therapies for DMD. Hum. Mol. Genet. 2019, 28, 307–319. [Google Scholar] [CrossRef]


| List of Amino Acid | Contents (mg/100 g) | |
|---|---|---|
| Physiological amino acid | Threonine | 1197.01 |
| Cysteine | 70.79 | |
| Tyrosine | 1111.59 | |
| Arginine | - | |
| Alanine | 2056.28 | |
| Proline | 165.18 | |
| Lysine | 1464.38 | |
| Histidine | 984.23 | |
| Isoleucine | 611.30 | |
| Leucine | 6016.50 | |
| Methionine | 401.51 | |
| Phenylalanine | 2147.77 | |
| Tryptophan | 76.33 | |
| Valine | 1738.06 | |
| Glutamic Acid | 1850.13 | |
| Aspartic Acid | 1520.94 | |
| Serine | 1226.45 | |
| Glycine | 340.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.W.; Lee, G.H.; Kim, J.Y.; Kim, C.Y.; Choo, Y.M.; Cho, W.; Choi, J.H.; Han, E.H.; Hwang, Y.P.; Jeong, H.G. Effects of Porcine Whole-Blood Protein Hydrolysate on Exercise Function and Skeletal Muscle Differentiation. Appl. Sci. 2022, 12, 17. https://doi.org/10.3390/app12010017
Jin SW, Lee GH, Kim JY, Kim CY, Choo YM, Cho W, Choi JH, Han EH, Hwang YP, Jeong HG. Effects of Porcine Whole-Blood Protein Hydrolysate on Exercise Function and Skeletal Muscle Differentiation. Applied Sciences. 2022; 12(1):17. https://doi.org/10.3390/app12010017
Chicago/Turabian StyleJin, Sun Woo, Gi Ho Lee, Ji Yeon Kim, Chae Yeon Kim, Young Moo Choo, Whajung Cho, Jae Ho Choi, Eun Hee Han, Yong Pil Hwang, and Hye Gwang Jeong. 2022. "Effects of Porcine Whole-Blood Protein Hydrolysate on Exercise Function and Skeletal Muscle Differentiation" Applied Sciences 12, no. 1: 17. https://doi.org/10.3390/app12010017







