Lipid Remodeling in the Mitochondria upon Ageing during the Long-Lasting Cultivation of Endomyces magnusii
Abstract
:1. Introduction
2. Results
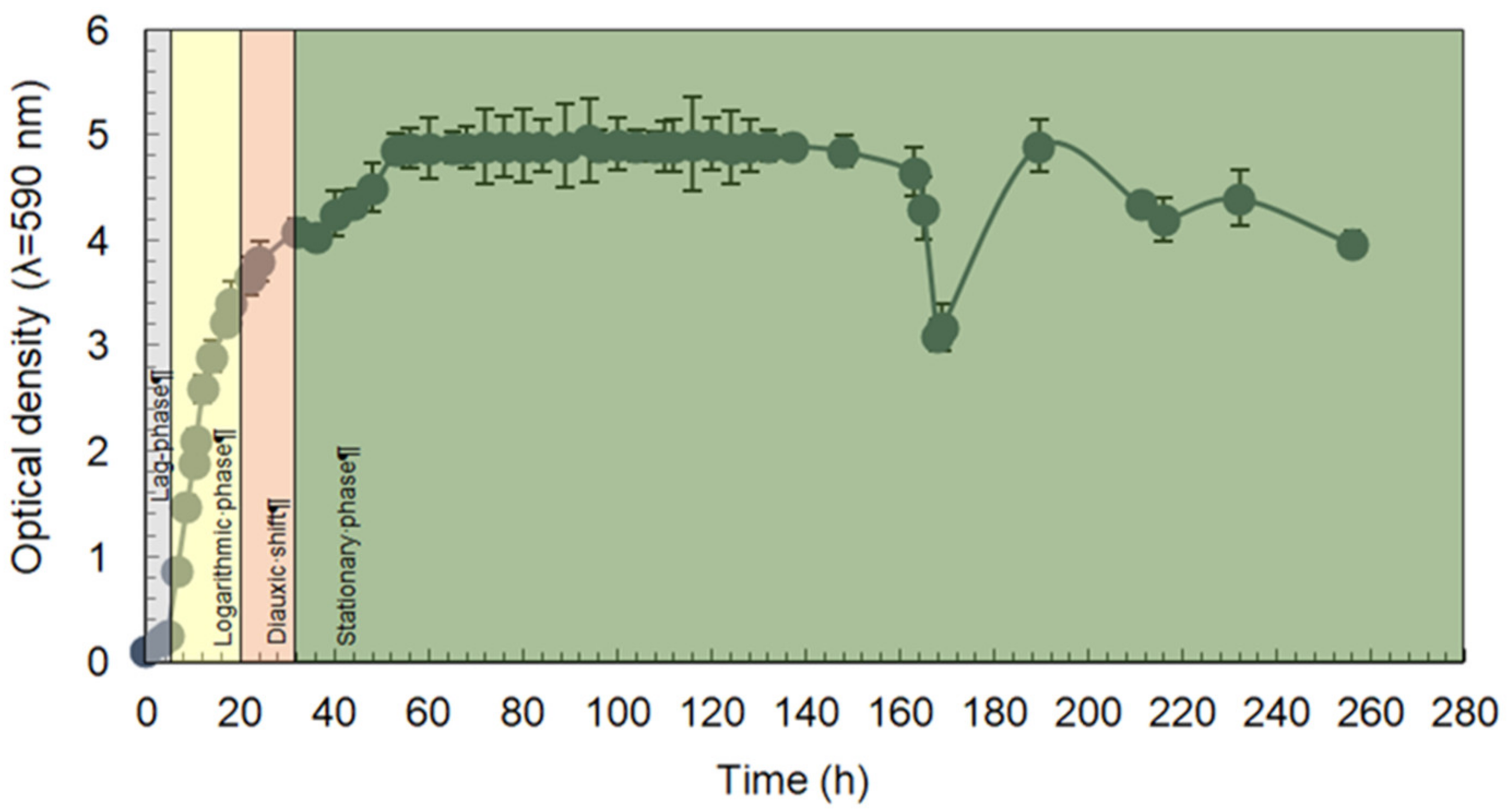
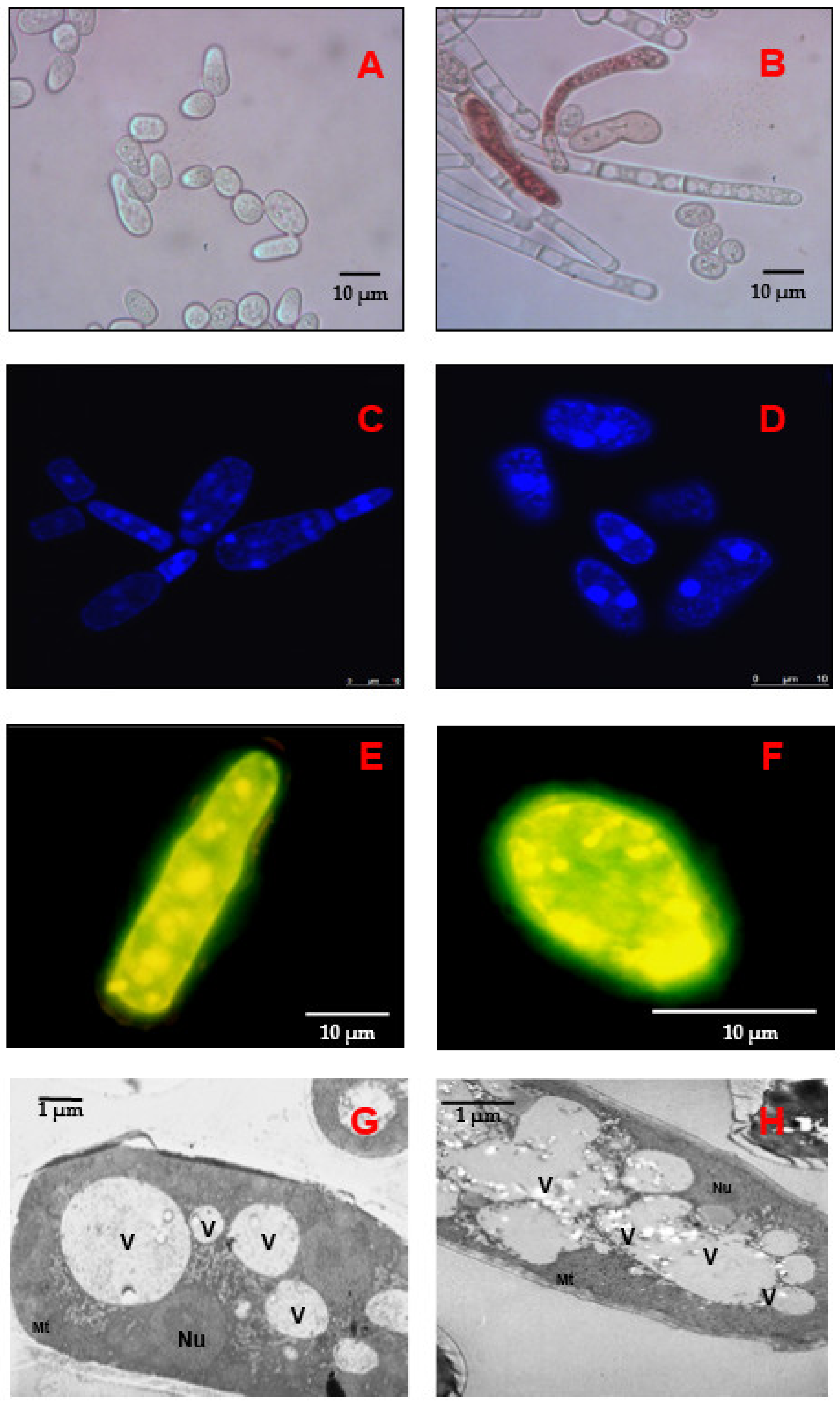
2.1. Long-Lasting Growth of the E. magnusii Yeast
2.2. Respiratory Activity of E. magnusii Yeast Mitochondria
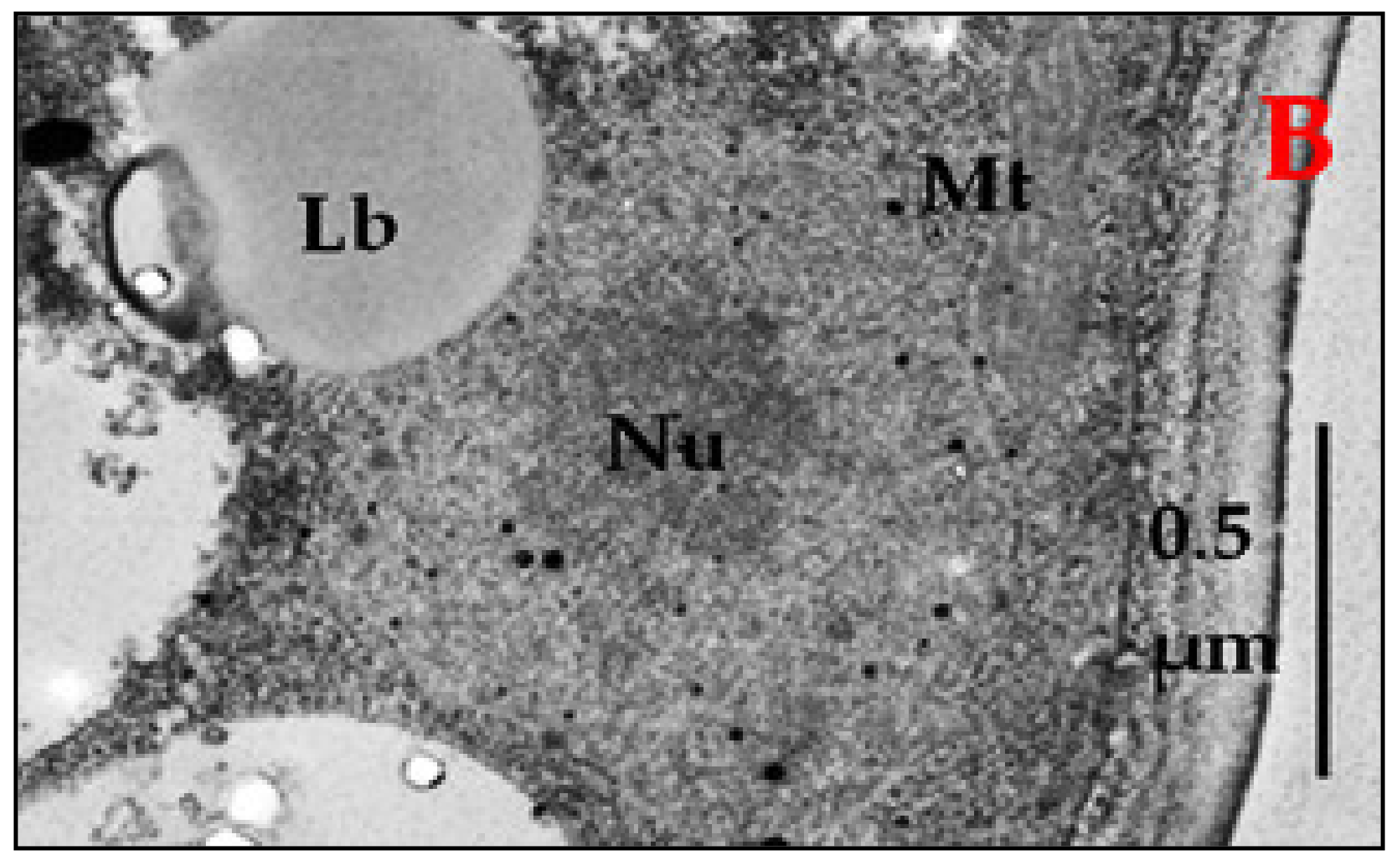
2.3. Storage Lipids Profile of E. magnusii Yeast in the Different Growth Phases
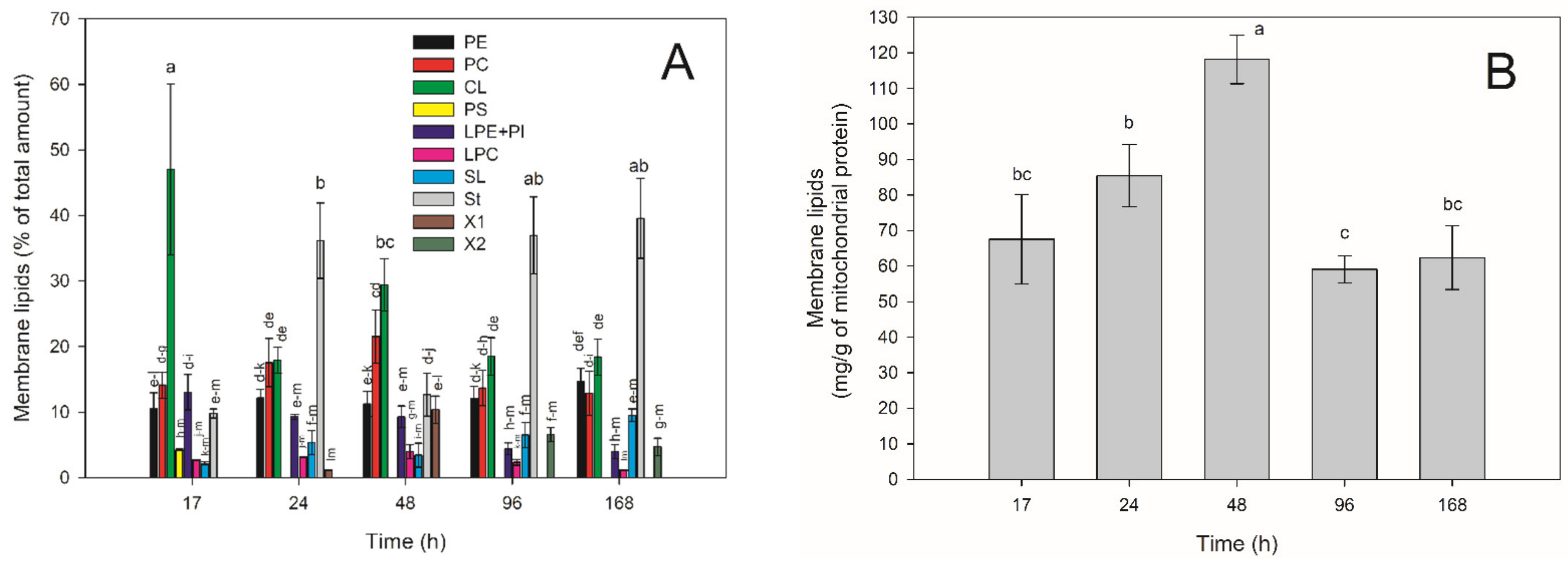
2.4. Membrane Lipids Profile of E. magnusii Mitochondria during Long-Lasting Cultivation
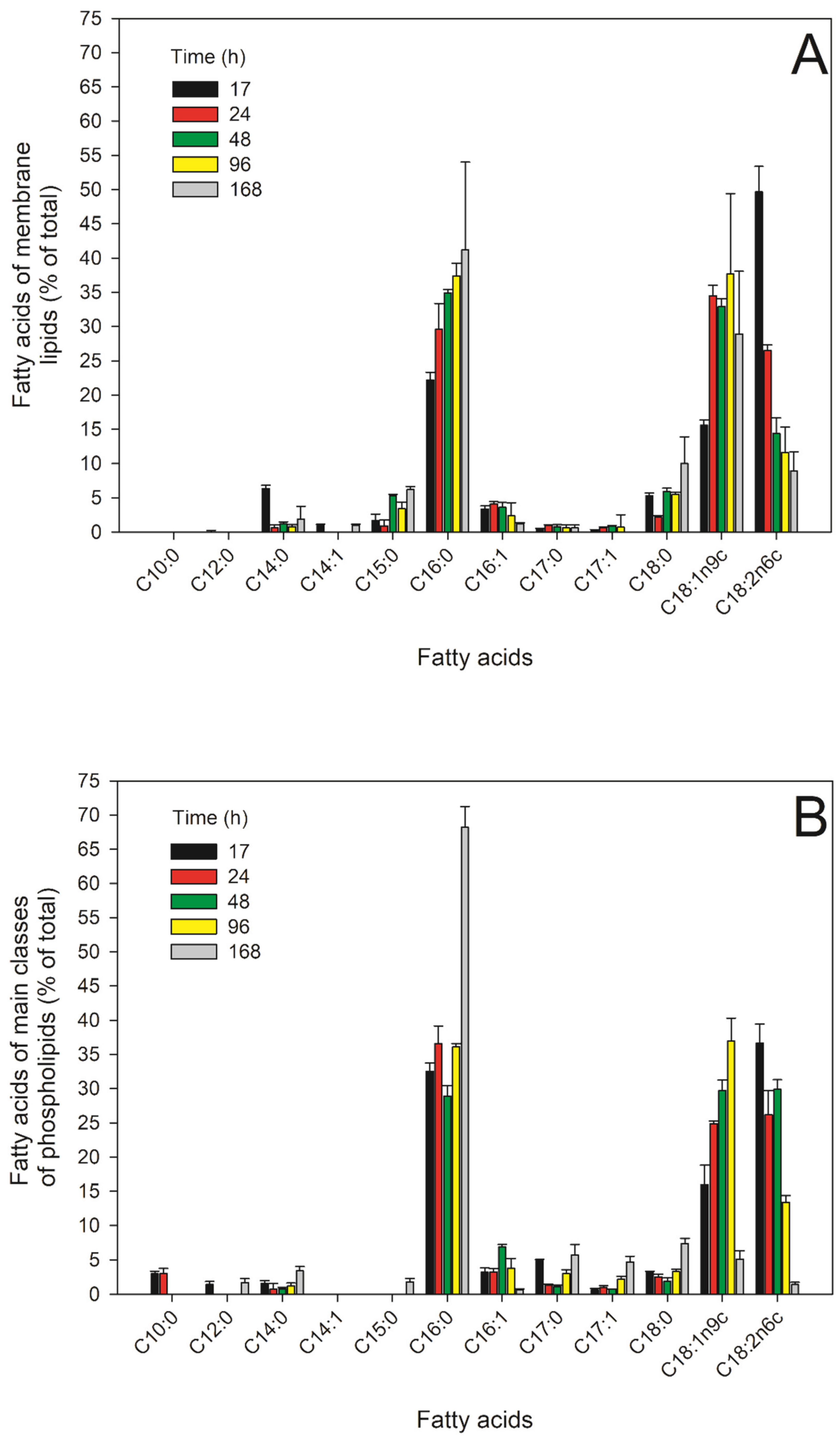
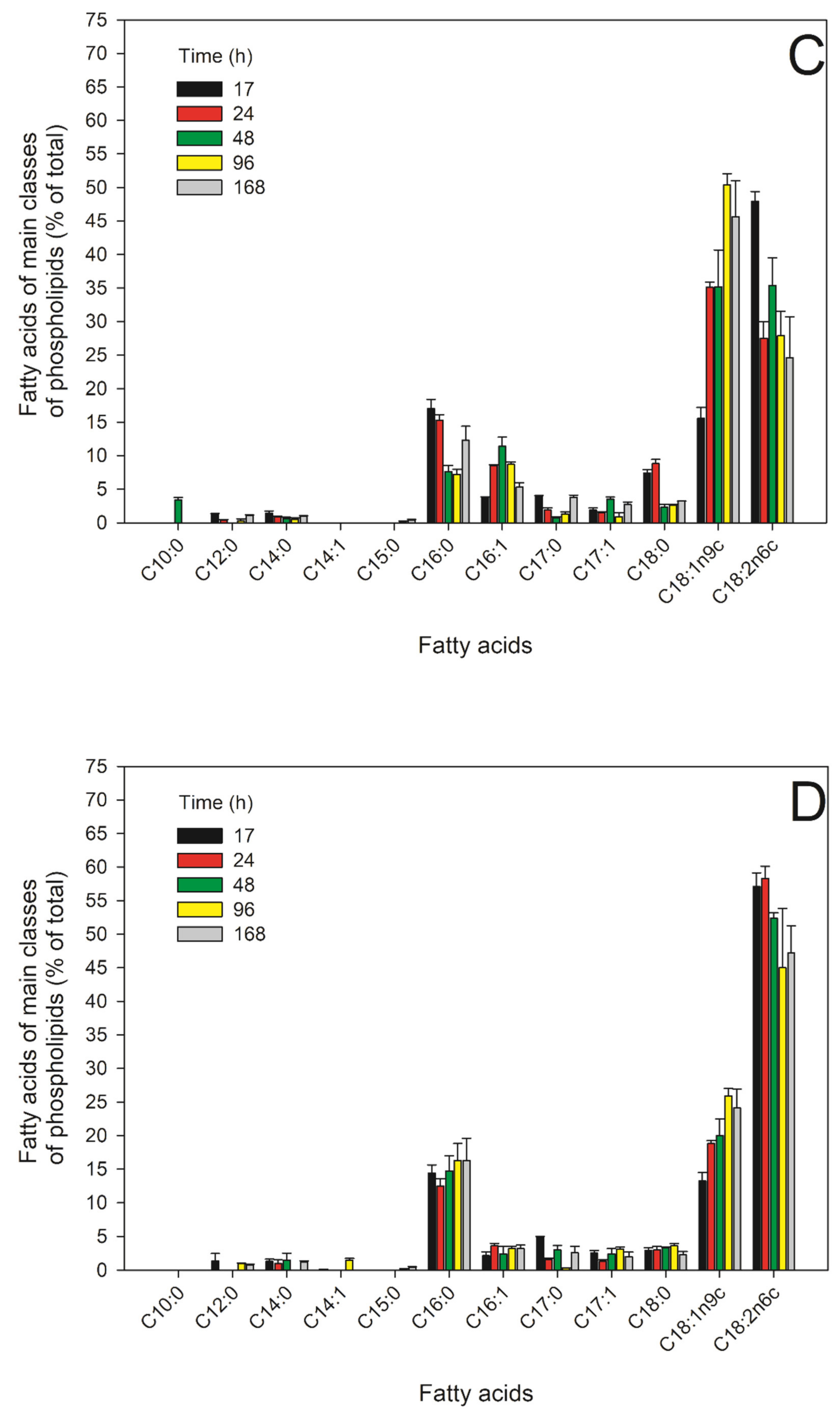
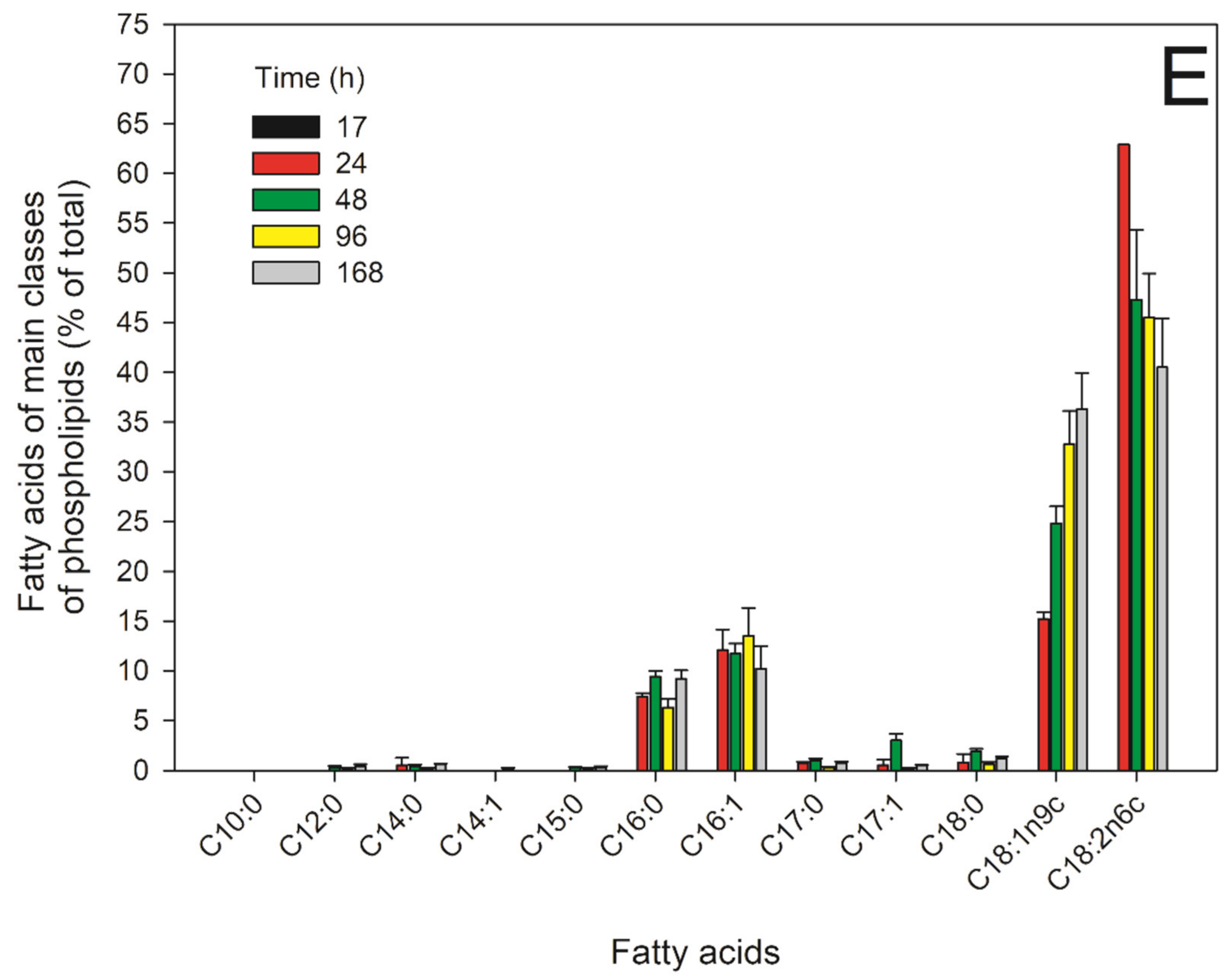
2.5. FAs of the Main PLs in the Different Growth Phases
3. Discussion
4. Materials and Methods
4.1. Yeast Strain and Culture Conditions
4.2. Potential-Dependent Staining
4.3. Staining with Neutral Red
4.4. Transmission Electron Microscopy (TEM)
4.5. Isolation of Mitochondria
4.6. Respiration Assessment
4.7. Preparation and Analysis of Lipids
4.8. Statistical analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Rogov, A.G.; Goleva, T.N.; Epremyan, K.K.; Kireev, I.I.; Zvyagilskaya, R.A. Propagation of Mitochondria-Derived Reactive Oxygen Species within the Dipodascus magnusii Cells. Antioxidants 2021, 10, 120. [Google Scholar] [CrossRef]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annesley, S.J.; Fisher, P.R. Mitochondria in Health and Disease. Cells 2019, 8, 680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harman, D. Aging: Overview. Ann. N. Y. Acad. Sci. 2001, 928, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Blum, A.; Liu, J.; Finkel, T. The role of mitochondria in aging. J. Clin. Investig. 2018, 128, 3662–3670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, M.; Kirkwood, T.B.L.; Bohr, V.A. Mitochondria in the signaling pathways that control longevity and health span. Ageing Res. Rev. 2019, 54, 100940. [Google Scholar] [CrossRef]
- Eleutherio, E.; Brasil, A.A.; França, M.B.; de Almeida, D.S.G.; Rona, G.B.; Magalhães, R.S.S. Oxidative stress and aging: Learning from yeast lessons. Fungal Biol. 2018, 122, 514–525. [Google Scholar] [CrossRef]
- Kaeberlein, M. Lessons on longevity from budding yeast. Nature 2010, 464, 513–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.V.; Vilaça, R.; Santos, C.N.; Costa, V.; Menezes, R. Exploring the power of yeast to model aging and age-related neurodegenerative disorders. Biogerontology 2017, 18, 3–34. [Google Scholar] [CrossRef]
- Herrero, E.; Ros, J.; Bel, G.; Cabiscol, E. Redox control and oxidative stress in yeast cells. Biochim. Biophys. Acta 2008, 1780, 1217–1235. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Withers, B.R.; Dickson, R.C. Sphingolipids and lifespan regulation. Biochim. Biophys. Acta 2014, 1841, 657–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jazwinski, S.M. Mitochondria to nucleus signaling and the role of ceramide in its integration into the suite of cell quality control processes during aging. Ageing Res. Rev. 2015, 23, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medkour, Y.; Dakik, P.; McAuley, M.; Mohammad, K.; Mitrofanova, D.; Titorenko, V.I. Mechanisms underlying the essential role of mitochondrial membrane lipids in yeast chronological aging. Oxid. Med. Cell. Longev. 2017, 2017, 2916985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handee, W.; Li, X.; Hall, K.W.; Deng, X.; Li, P.; Benning, C.; Williams, B.L.; Kuo, M.-H. An energy-independent pro-longevity function of triacylglycerol in yeast. PLoS Genet. 2016, 12, e1005878. [Google Scholar] [CrossRef] [Green Version]
- Beach, A.; Richard, V.R.; Leonov, A.; Burstein, M.T.; Bourque, S.D.; Koupaki, O.; Juneau, M.; Feldman, R.; Iouk, T.; Titorenko, V.I. Mitochondrial membrane lipidome defines yeast longevity. Aging 2013, 5, 551–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burstein, M.T.; Titorenko, V.I. A mitochondrially targeted compound delays aging in yeast through a mechanism linking mitochondrial membrane lipid metabolism to mito-chondrial redox biology. Redox Biol. 2014, 2, 305–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonov, A.; Arlia-Ciommo, A.; Bourque, S.D.; Koupaki, O.; Kyryakov, P.; Dakik, P.; McAuley, M.; Medkour, Y.; Mohammad, K.; Di Maulo, T.; et al. Specific changes in mitochondrial lipidome alter mitochondrial proteome and increase the geroprotective efficiency of lithocholic acid in chronologically aging yeast. Oncotarget 2017, 8, 30672–30691. [Google Scholar] [CrossRef]
- Roberts, G.G.; Hudson, A.P. Rsf1p is required for an efficient metabolic shift from fermentative to glycerol-based respiratory growth in S. cerevisiae. Yeast 2009, 26, 95–110. [Google Scholar] [CrossRef] [Green Version]
- Deryabina, Y.; Isakova, E.; Sekova, V.; Antipov, A.; Saris, N.E. Inhibition of free radical scavenging enzymes affects mitochondrial membrane permeability transition during growth and aging of yeast cells. J. Bioenerg. Biomembr. 2014, 46, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Isakova, E.P.; Matushkina, I.N.; Popova, T.N.; Dergacheva, D.I.; Gessler, N.N.; Klein, O.I.; Semenikhina, A.V.; Deryabina, Y.I.; La Porta, N.; Saris, N.L. Metabolic remodeling during long-lasting cultivation of the Endomyces magnusii yeast on oxidative and fermentative substrates. Microorganisms 2020, 8, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner-Washburne, M.; Roy, S.; Davidson, G.S. Aging and the survival of quiescent and non-quiescent cells in yeast stationary-phase cultures. In Aging Research in Yeast; Subcellular Biochemistry; Breitenbach, M., Jazwinski, S., Laun, P., Eds.; Springer: Dordrecht, The Netherlands, 2011; Volume 57. [Google Scholar] [CrossRef]
- Polcic, P.; Sabová, L.; Kolarov, J. Fatty acids induced uncoupling of Saccharomyces cerevisiae mitochondria requires an intact ADP/ATP carrier. FEBS Lett. 1997, 412, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Medentsev, A.G.; Arinbasarova, A.Y.; Akimenko, V.K. Regulation and physiological role of cyanide-resistant oxidases in fungi and plants. Biochemistry 1999, 64, 1230–1243. [Google Scholar]
- Rogov, A.G.; Zvyagilskaya, R.A. Physiological role of alternative oxidase (from yeasts to plants). Biochemistry 2015, 80, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Bahr, J.T.; Bonner, W.D., Jr. Cyanide-insensitive respiration. II. Control of the alternate pathway. J. Biol. Chem. 1973, 248, 3446–3450. [Google Scholar] [CrossRef]
- Zelenshchikova, V.A.; Burbaev, D.S.; Zviagil’skaia, R.A. Reverse electron transfer in mitochondria of the yeast Endomyces magnusii grown on glycerol. Biokhimiia 1983, 48, 186–192. [Google Scholar]
- DeRisi, J.L.; Iyer, V.R.; Brown, P.O. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science 1997, 278, 680–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, G.G.; Hudson, A.P. Transcriptome profiling of Saccharomyces cerevisiae during a transition from fermentative to glycerol-based respiratory growth reveals extensive metabolic and structural remodeling. Mol. Genet. Genom. 2006, 276, 170–186. [Google Scholar] [CrossRef]
- Ohlmeier, S.; Kastaniotis, A.J.; Hiltunen, J.K.; Bergmann, U. The yeast mitochondrial proteome, a study of fermentative and respiratory growth. J. Biol. Chem. 2004, 279, 3956–3979. [Google Scholar] [CrossRef] [Green Version]
- Renvoisé, M.; Bonhomme, L.; Davanture, M.; Valot, B.; Zivy, M.; Lemaire, C. Quantitative variations of the mitochondrial proteome and phosphoproteome during fermentative and respiratory growth in Saccharomyces cerevisiae. J. Proteom. 2014, 106, 140–150. [Google Scholar] [CrossRef] [Green Version]
- Veiga, A.; Arrabaça, J.D.; Loureiro-Dias, M.C. Cyanide-resistant respiration, a very frequent metabolic pathway in yeasts. FEMS Yeast Res. 2003, 3, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Vanlerberghe, G.C. Alternative oxidase: A mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int. J. Mol. Sci. 2013, 14, 6805–6847. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Borovskii, G.; Panda, S.K. Alternative oxidase and plant stress tolerance. Plant Signal Behav. 2016, 11, e1256530. [Google Scholar] [CrossRef]
- Van Aken, O.; Giraud, E.; Clifton, R.; Whela, J. Alternative oxidase: A target and regulator of stress responses. Physiol. Plant 2009, 137, 354–361. [Google Scholar] [CrossRef]
- Lushchak, V.I. Adaptive response to oxidative stress: Bacteria, fungi, plants, and animals. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2011, 153, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Handee, W.; Kuo, M.H. The slim, the fat, and the obese: Guess who lives the longest? Curr. Genet. 2017, 63, 43–49. [Google Scholar] [CrossRef]
- Klug, L.; Daum, G. Yeast lipid metabolism at a glance. FEMS Yeast Res. 2014, 14, 369–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baile, M.G.; Lu, Y.W.; Claypool, S.M. The topology and regulation of cardiolipin biosynthesis and remodeling in yeast. Chem. Phys. Lipids 2014, 179, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Wenz, T.; Hielscher, R.; Hellwig, P.; Schagger, H.; Richers, S.; Hunte, C. Role of phospholipids in respiratory cytochrome bc (1) complex catalysis and supercomplex formation. Biochim. Biophys. Acta 2009, 1787, 609–616. [Google Scholar] [CrossRef] [Green Version]
- Joshi, A.S.; Zhou, J.; Gohil, V.M.; Chen, S.; Greenberg, M.L. Cellular functions of cardiolipin in yeast. Biochim. Biophys. Acta 2009, 1793, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Joshi, A.S.; Thompson, M.N.; Fei, N.; Huttemann, M.; Greenberg, M.L. Cardiolipin and mitochondrial phosphatidylethanolamine have overlapping functions in mitochondrial fusion in Saccharomyces cerevisiae. J. Biol. Chem. 2012, 287, 17589–17597. [Google Scholar] [CrossRef] [Green Version]
- Mileykovskaya, E.; Dowhan, W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim. Biophys. Acta 2009, 1788, 2084–2091. [Google Scholar] [CrossRef] [Green Version]
- Rostovtseva, T.K.; Gurnev, P.A.; Chen, M.; Bezrukov, S.M. Membrane lipid composition regulates tubulin interaction with mitochondrial voltage-dependent anion channel. J. Biol. Chem. 2012, 287, 29589–29598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zinser, E.; Sperka-Gottlieb, C.D.; Fasch, E.V.; Kohlwein, S.D.; Paltauf, F.; Daum, G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J. Bacteriol. 1991, 173, 2026–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvath, S.E.; Wagner, A.; Steyrer, E.; Daum, G. Metabolic link between phosphatidylethanolamine and triacylglycerol metabolism in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 2011, 1811, 1030–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kieliszek, M.; Błażejak, S.; Bzducha-Wróbel, A.; Kot, A.M. Effect of selenium on lipid and amino acid metabolism in yeast cells. Biol. Trace Elem. Res. 2019, 187, 316–327. [Google Scholar] [CrossRef] [Green Version]
- Sekova, V.Y.; Dergacheva, D.I.; Isakova, E.P.; Gessler, N.N.; Tereshina, V.M.; Deryabina, Y.I. Soluble sugar and lipid readjustments in the Yarrowia lipolytica yeast at various temperatures and pH. Metabolites 2019, 9, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glatz, A.; Pilbat, A.M.; Németh, G.L.; Vince-Kontár, K.; Jósvay, K.; Hunya, Á.; Udvardy, A.; Gombos, I.; Péter, M.; Balogh, G.; et al. Involvement of small heat shock proteins, trehalose, and lipids in the thermal stress management in Schizosaccharomyces pombe. Cell Stress Chaperones 2016, 21, 327–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deryabina, Y.I.; Zvyagilskaya, R.A. The Ca2+-Transport system of Yeast (Endomyces magnusii) mitochondria: Independent pathways for Ca2+ uptake and release. Biochemistry 2000, 65, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Bazhenova, E.N.; Saris, N.E.; Zvyagilskaya, R.A. Stimulation of the yeast mitochondrial calcium uniporter by hypotonicity and by ruthenium red. Biochim. Biophys. Acta 1998, 1371, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Nichols, B.W. Separation of the lipids of photosynthetic tissues: Improvements in analysis by thin-layer chromatography. Biochim. Biophys. Acta 1963, 70, 417–422. [Google Scholar] [CrossRef]
- Kates, M. Techniques of lipidology: Isolation, analysis and identifcation of lipids. In Laboratory Techniques in Biochemistry and Molecular Biology; Work, T.S., Work, E., Eds.; North-Holland: Amsterdam, The Netherlands, 1972; pp. 267–610. [Google Scholar]
- Benning, C.; Huang, Z.H.; Gage, D.A. Accumulation of a novel glycolipid and a betaine lipid in cells of Rhodobacter sphaeroides grown under phosphate limitation. Arch. Biochem. Biophys. 1995, 317, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Kates, M. Techniques of Lipidology: Isolation, Analysis, and Identification of Lipids, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 464. [Google Scholar] [CrossRef]
- Weete, J.D. Introduction to fungal lipids. In Fungal Lipid Biochemistry; Kritchevsky, D., Ed.; Springer: Berlin/Heidelberg, Germany, 1974. [Google Scholar]



| Time, h | Respiration Rate, ng-Atom Consumed O per 1 mg of Protein | ||||
|---|---|---|---|---|---|
| V41 * | V3 ** | V42 * | +KCN *** Inhibition (% of Control) | +KCN + SHAM **** Inhibition (% of Control) | |
| 17 | 19.6 ± 0.63 f | 58.1 ± 0.08 b | 16.95 ± 1.23 f | 100 | 100 |
| 24 | 27.9 ± 1.68 f | 164.8 ± 1.82 c | 65.28 ± 4.67 e | 87.26 | 100 |
| 48 | 70.19 ± 1.68 e | 205.19 ± 1.82 b | 70.28 ± 4.67 e | 88.79 | 100 |
| 96 | 140.0 ± 14.14 cd | 311.18 ± 18.59 a | 139.26 ± 15.7 cd | 72.68 | 100 |
| 168 | 60.85 ± 4.6 e | 127.14 ± 18.13 d | 72.68 ± 14.18 e | 50.72 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isakova, E.P.; Gessler, N.N.; Dergacheva, D.I.; Tereshina, V.M.; Deryabina, Y.I.; Kieliszek, M. Lipid Remodeling in the Mitochondria upon Ageing during the Long-Lasting Cultivation of Endomyces magnusii. Appl. Sci. 2021, 11, 4069. https://doi.org/10.3390/app11094069
Isakova EP, Gessler NN, Dergacheva DI, Tereshina VM, Deryabina YI, Kieliszek M. Lipid Remodeling in the Mitochondria upon Ageing during the Long-Lasting Cultivation of Endomyces magnusii. Applied Sciences. 2021; 11(9):4069. https://doi.org/10.3390/app11094069
Chicago/Turabian StyleIsakova, Elena P., Natalya N. Gessler, Daria I. Dergacheva, Vera M. Tereshina, Yulia I. Deryabina, and Marek Kieliszek. 2021. "Lipid Remodeling in the Mitochondria upon Ageing during the Long-Lasting Cultivation of Endomyces magnusii" Applied Sciences 11, no. 9: 4069. https://doi.org/10.3390/app11094069
APA StyleIsakova, E. P., Gessler, N. N., Dergacheva, D. I., Tereshina, V. M., Deryabina, Y. I., & Kieliszek, M. (2021). Lipid Remodeling in the Mitochondria upon Ageing during the Long-Lasting Cultivation of Endomyces magnusii. Applied Sciences, 11(9), 4069. https://doi.org/10.3390/app11094069







