The Role of Nutritional Habits and Moderate Red Wine Consumption in PON1 Status in Healthy Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Evaluation of Macro- and Micronutrients
2.3. Sample Collection
2.4. PON1 Concentration
2.5. PON1 Activity
2.6. DNA Isolation and PON1 Genotyping
2.7. Wine Phytochemical Composition Analysis (Fingerprinting)
2.8. Statistical Analysis
3. Results
3.1. Study Subjects
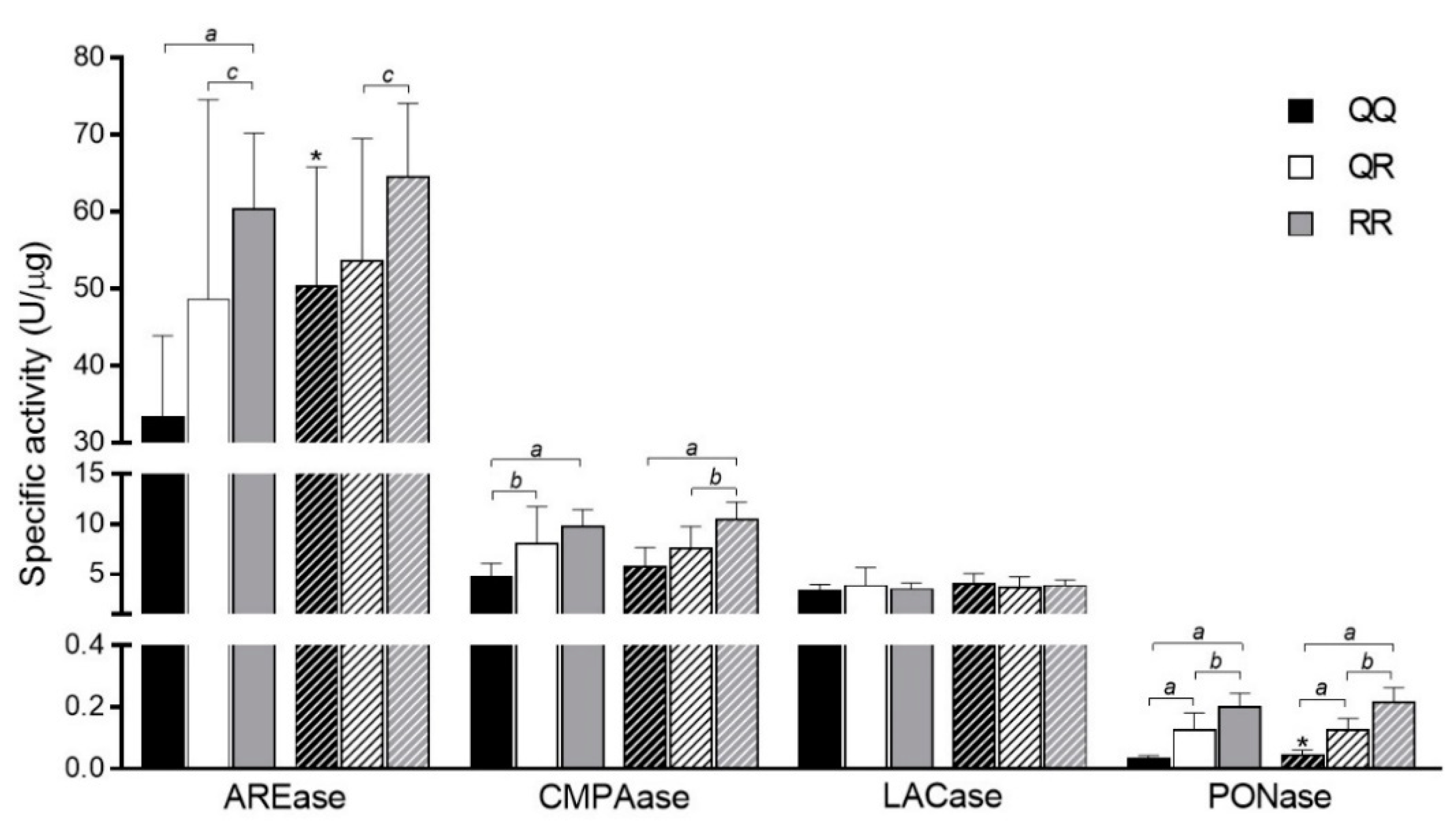
3.2. Allele Frequencies and PON1 Genotypes
3.3. Status of PON1 and Genotypes
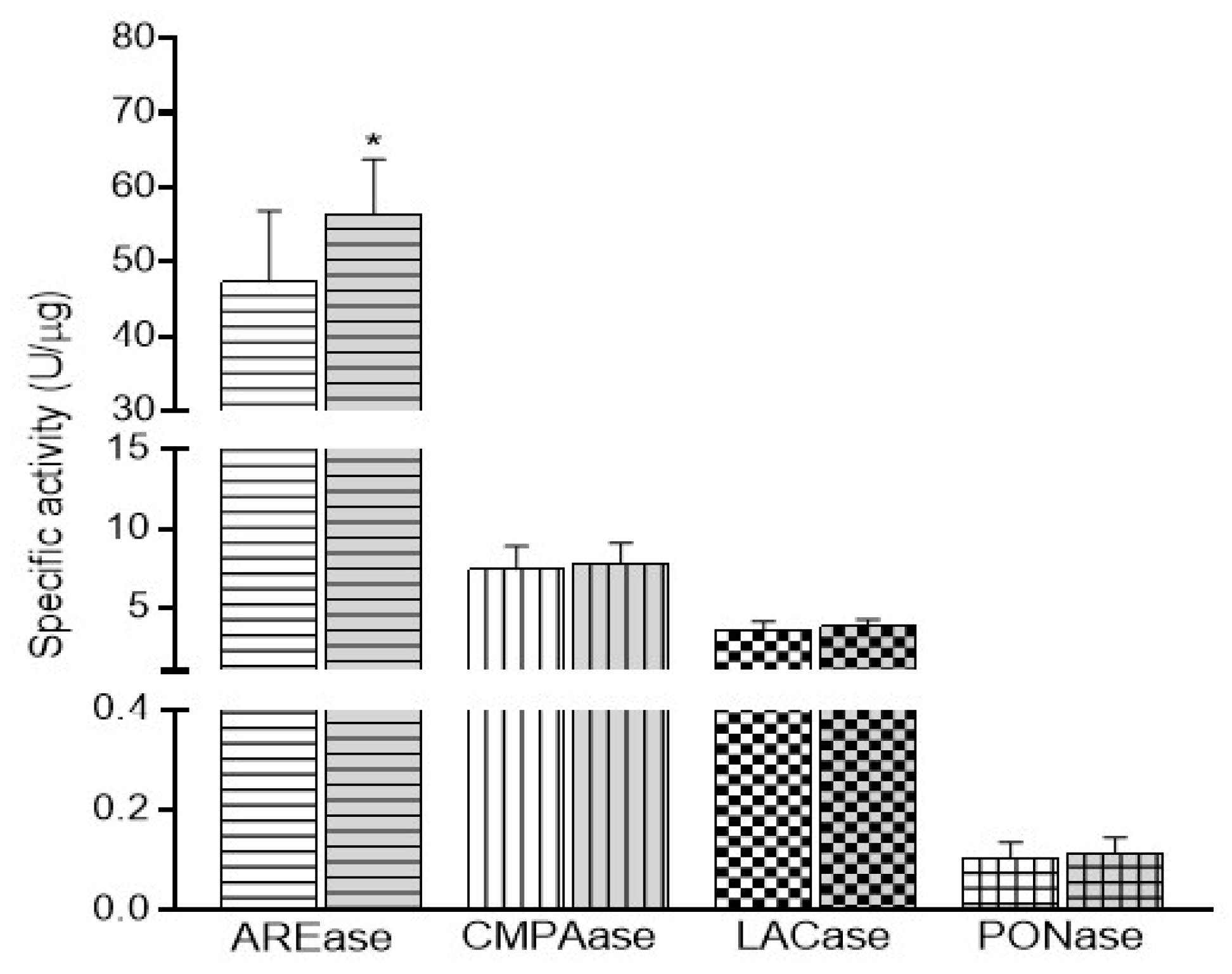
3.4. PON1 Activities and Nutrient Intake
3.5. Wine’s Phytochemical Composition
4. Discussion
5. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furlong, C.E.; Marsillach, J.; Jarvik, G.P.; Costa, L.G. Paraoxonases-1, -2 and -3: What are their functions? Chem. Biol. Interact. 2016, 259, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Fridman, O.; Fuchs, A.G.; Porcile, R.; Morales, A.V.; Gariglio, L.O. Paraoxonasa: Sus múltiples funciones y regulación farmacológica. Arch. Cardiol. Mex. 2011, 81, 251–260. [Google Scholar] [PubMed]
- Mackness, B.; Turkie, W.; Mackness, M. Paraoxonase-1 (PON1) promoter region polymorphisms, serum PON1 status and coronary heart disease. Arch. Med. Sci. 2013, 9, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Mackness, M.; Mackness, B. Human paraoxonase-1 (PON1): Gene structure and expression, promiscuous activities and multiple physiological roles. Gene 2015, 567, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Rainwater, D.L.; Rutherford, S.; Dyer, T.D.; Rainwater, E.D.; Cole, S.A.; VandeBerg, J.L.; Almasy, L.; Blangero, J.; MacCluer, J.W.; Mahaney, M.C. Determinants of variation in human serum paraoxonase activity. Heredity 2009, 102, 147–154. [Google Scholar] [CrossRef]
- Hassett, C.; Richter, R.J.; Humbert, R.; Chapline, C.; Crabb, J.W.; Omiecinski, C.J.; Furlong, C.E. Characterization of cDNA clones encoding rabbit and human serum paraoxonase: The mature protein retains its signal sequence. Biochemistry 1991, 30, 10141–10149. [Google Scholar] [CrossRef]
- Humbert, R.; Adler, D.A.; Disteche, C.M.; Hassett, C.; Omiecinski, C.J.; Furlong, C.E. The molecular basis of the human serum paraoxonase activity polymorphism. Nat. Genet. 1993, 3, 73–76. [Google Scholar] [CrossRef]
- Adkins, S.; Gan, K.N.; Mody, M.; La Du, B.N. Molecular basis for the polymorphic forms of human serum paraoxonase/arylesterase: Glutamine or arginine at position 191, for the respective A or B allozymes. Am. J. Hum. Genet. 1993, 52, 598–608. [Google Scholar]
- Garin, M.C.; James, R.W.; Dussoix, P.; Blanché, H.; Passa, P.; Froguel, P.; Ruiz, J. Paraoxonase polymorphism Met-Leu54 is associated with modified serum concentrations of the enzyme. A possible link between the paraoxonase gene and increased risk of cardiovascular disease in diabetes. J. Clin. Investig. 1997, 99, 62–66. [Google Scholar] [CrossRef]
- Rojas-García, A.E.; Solís-Heredia, M.J.; Piña-Guzmán, B.; Vega, L.; López-Carrillo, L.; Quintanilla-Vega, B. Genetic polymorphisms and activity of PON1 in a Mexican population. Toxicol. Appl. Pharmacol. 2005, 205, 282–289. [Google Scholar] [CrossRef]
- Turgut Cosan, D.; Colak, E.; Saydam, F.; Yazici, H.U.; Degirmenci, I.; Birdane, A.; Colak, E.; Gunes, H.V. Association of paraoxonase 1 (PON1) gene polymorphisms and concentration with essential hypertension. Clin. Exp. Hypertens. 2016, 38, 602–607. [Google Scholar] [CrossRef]
- Davies, H.G.; Richter, R.J.; Keifer, M.; Broomfield, C.A.; Sowalla, J.; Furlong, C.E. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat. Genet. 1996, 14, 334–336. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Gabás-Rivera, C.; Navarro, M.A.; Osada, J. PON1 and Mediterranean Diet. Nutrients 2015, 7, 4068–4092. [Google Scholar] [CrossRef]
- Lou-Bonafonte, J.M.; Gabás-Rivera, C.; Navarro, M.A.; Osada, J. The Search for Dietary Supplements to Elevate or Activate Circulating Paraoxonases. Int. J. Mol. Sci. 2017, 18, 416. [Google Scholar] [CrossRef]
- Otocka-Kmiecik, A.; Lewandowski, M.; Szkudlarek, U.; Nowak, D.; Orlowska-Majdak, M. Aerobic training modulates the effects of exercise-induced oxidative stress on PON1 activity: A preliminary study. Sci. World J. 2014, 2014, 230271. [Google Scholar] [CrossRef]
- Abdel-Salam, O.M.; Youness, E.R.; Khadrawy, Y.A.; Sleem, A.A. Acetylcholinesterase, butyrylcholinesterase and paraoxonase 1 activities in rats treated with cannabis, tramadol or both. Asian Pac. J. Trop. Med. 2016, 9, 1089–1094. [Google Scholar] [CrossRef]
- Gouédard, C.; Barouki, R.; Morel, Y. Dietary Polyphenols Increase Paraoxonase 1 Gene Expression by an Aryl Hydrocarbon Receptor-Dependent Mechanism. Mol. Cell Biol. 2004, 24, 5209–5222. [Google Scholar] [CrossRef] [PubMed]
- Curtin, B.F.; Seetharam, K.I.; Dhoieam, P.; Gordon, R.K.; Doctor, B.P.; Nambiar, M.P. Resveratrol induces catalytic bioscavenger paraoxonase 1 expression and protects against chemical warfare nerve agent toxicity in human cell lines. J. Cell Biochem. 2008, 103, 1524–1535. [Google Scholar] [CrossRef] [PubMed]
- Rock, W.; Rosenblat, M.; Miller-Lotan, R.; Levy, A.P.; Elias, M.; Aviram, M. Consumption of wonderful variety pomegranate juice and extract by diabetic patients increases paraoxonase 1 association with high-density lipoprotein and stimulates its catalytic activities. J. Agric. Food Chem. 2008, 56, 8704–8713. [Google Scholar] [CrossRef] [PubMed]
- Boesch-Saadatmandi, C.; Egert, S.; Schrader, C.; Coumoul, X.; Barouki, R.; Muller, L.J.; Wolffram, S.; Rimbach, G. Effect of quercetin on paraoxonase 1 activity—Studies in cultured cells, mice and humans. J. Physiol. Pharmacol. 2010, 61, 99–105. [Google Scholar]
- Rosenblat, M.; Volkova, N.; Attias, J.; Mahamid, R.; Aviram, M. Consumption of polyphenolic-rich beverages (mostly pomegranate and black currant juices) by healthy subjects for a short term increased serum antioxidant status, and the serum’s ability to attenuate macrophage cholesterol accumulation. Food Funct. 2010, 1, 99–109. [Google Scholar] [CrossRef]
- Martini, D.; Del Bo, C.; Porrini, M.; Ciappellano, S.; Riso, P. Role of polyphenols and polyphenol-rich foods in the modulation of PON1 activity and expression. J. Nutr. Biochem. 2017, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Wine Flavonoids in Health and Disease Prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Rios-Corripio, G.; Guerrero-Beltrán, J.A. Antioxidant and physicochemical characteristics of unfermented and fermented pomegranate (Punica granatum L.) beverages. J. Food Sci. Technol. 2019, 56, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Sarandöl, E.; Serdar, Z.; Dirican, M.; Şafak, Ö. Effects of red wine consumption on serum paraoxonase/arylesterase activities and on lipoprotein oxidizability in healthy-men. J. Nutr. Biochem. 2003, 14, 507–512. [Google Scholar] [CrossRef]
- Leckey, L.C.; Garige, M.; Varatharajalu, R.; Gong, M.; Nagata, T.; Spurney, C.F.; Lakshman, R.M. Quercetin & ethanol attenuate the progression of atherosclerotic plaques with concomitant up regulation of paraoxonase1 (PON1) gene expression and PON1 activity in LDLR-/- mice. Alcohol. Clin. Exp. Res. 2010, 34, 1535–1542. [Google Scholar]
- Lakshman, R.; Garige, M.; Gong, M.; Leckey, L.; Varatharajalu, R.; Zakhari, S. Is alcohol beneficial or harmful for cardioprotection? Genes Nutr. 2010, 5, 111–120. [Google Scholar] [CrossRef]
- Gupta, N.; Kandimalla, R.; Priyanka, K.; Singh, G.; Gill, K.D.; Singh, S. Effect of Resveratrol and Nicotine on PON1 Gene Expression: In Vitro Study. Indian J. Clin. Biochem. 2014, 29, 69–73. [Google Scholar] [CrossRef]
- Rizzi, F.; Conti, C.; Dogliotti, E.; Terranegra, A.; Salvi, E.; Braga, D.; Ricca, F.; Lupoli, S.; Mingione, A.; Pivari, F.; et al. Interaction between polyphenols intake and PON1 gene variants on markers of cardiovascular disease: A nutrigenetic observational study. J. Transl. Med. 2016, 14, 186. [Google Scholar] [CrossRef]
- Schwedhelm, C.; Nimptsch, K.; Bub, A.; Pischon, T.; Linseisen, J. Association between alcohol consumption and serum paraoxonase and arylesterase activities: A cross-sectional study within the Bavarian population. Br. J. Nutr. 2016, 115, 730–736. [Google Scholar] [CrossRef][Green Version]
- Hernández-Ávila, M.; Romieu, I.; Parra, S.; Hernández-Ávila, J.; Madrigal, H.; Willett, W. Validity and reproducibility of a food frequency questionnaire to assess dietary intake of women living in Mexico City. Salud Publica Mex. 1998, 40, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Eckerson, H.W.; Wyte, C.M.; La Du, B.N. The human serum paraoxonase/arylesterase polymorphism. Am. J. Hum. Genet. 1983, 35, 1126–1138. [Google Scholar] [PubMed]
- Richter, R.J.; Jarvik, G.P.; Furlong, C.E. Determination of paraoxonase 1 status without the use of toxic organophosphate substrates. Circ. Cardiovasc. Genet. 2008, 1, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Billecke, S.; Draganov, D.; Counsell, R.; Stetson, P.; Watson, C.; Hsu, C.; La Du, B.N. Human serum paraoxonase (PON1) isozymes Q and R hydrolyze lactones and cyclic carbonate esters. Drug Metab. Dispos. 2000, 28, 1335–1342. [Google Scholar]
- Furlong, C.E.; Richter, R.J.; Seidel, S.L.; Motulsky, A.G. Role of genetic polymorphism of human plasma paraoxonase/arylesterase in hydrolysis of the insecticide metabolites chlorpyrifos oxon and paraoxon. Am. J. Hum. Genet. 1988, 43, 230–238. [Google Scholar]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 27 November 2020).
- Spectra Search Mass Spectrum. Available online: https://foodb.ca/spectra/ms/search (accessed on 24 September 2021).
- Spectra Search Mass Spectrum. Available online: https://hmdb.ca/spectra/ms/search (accessed on 20 August 2021).
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Sparascio, F.; Maffia, M. HPLC analysis of phenols in Negroamaro and primitivo red wines from salento. Foods 2019, 8, 45. [Google Scholar] [CrossRef]
- Granato, D.; Katayama, F.C.U.; De Castro, I.A. Phenolic composition of South American red wines classified according to their antioxidant activity, retail price and sensory quality. Food Chem. 2011, 129, 366–373. [Google Scholar] [CrossRef]
- Giovinazzo, G.; Grieco, F. Functional Properties of Grape and Wine Polyphenols. Plant Foods Hum. Nutr. 2015, 70, 454–462. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; González-Centeno, M.R.; Chira, K.; Jourdes, M.; Teissedre, P.-L. Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. Front. Cell Neurosci. 2018, 23, 373. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Pennathur, S.; Afshinnia, F. Link of dietary patterns with metabolic syndrome: Analysis of the National Health and Nutrition Examination Survey. Nutr. Diabetes 2017, 7, e255. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.J.; Baer, D.J.; Albert, P.S.; Castonguay, T.W.; Dorgan, J.F.; Dawsey, S.M.; Brown, E.D.; Hartman, T.J.; Campbell, W.S.; Giffen, C.A.; et al. Relationship between serum leptin levels and alcohol consumption in a controlled feeding and alcohol ingestion study. J. Natl. Cancer Inst. 2003, 95, 1722–1725. [Google Scholar] [CrossRef]
- Otaka, M.; Konishi, N.; Odashima, M.; Jin, M.; Wada, I.; Matsuhashi, T.; Ohba, R.; Watanabe, S. Effect of alcohol consumption on leptin level in serum, adipose tissue, and gastric mucosa. Dig. Dis. Sci. 2007, 52, 3066–3069. [Google Scholar] [CrossRef]
- Bach, P.; Koopmann, A.; Kiefer, F. The Impact of Appetite-Regulating Neuropeptide Leptin on Alcohol Use, Alcohol Craving and Addictive Behavior: A Systematic Review of Preclinical and Clinical Data. Alcohol Alcohol. 2021, 56, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Rojdmark, S.; Calissendorff, J.; Brismar, K. Alcohol ingestion decreases both diurnal and nocturnal secretion of leptin in healthy individuals. Clin. Endocrinol. 2001, 55, 639–647. [Google Scholar] [CrossRef]
- Yeomans, M.R. Alcohol, appetite and energy balance: Is alcohol intake a risk factor for obesity? Physiol. Behav. 2010, 100, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Schrieks, I.C.; Stafleu, A.; Griffioen-Roose, S.; de Graaf, C.; Witkamp, R.F.; Boerrigter-Rijneveld, R.; Hendriks, H.F.J. Moderate alcohol consumption stimulates food intake and food reward of savoury foods. Appetite 2015, 89, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Schanze, A.; Gröschl, M.; Bayerlein, K.; Hillemacher, T.; Reulbach, U.; Kornhuber, J.; Bleich, S. Ghrelin levels are increased in alcoholism. Alcohol Clin. Exp. Res. 2005, 29, 2154–2157. [Google Scholar] [CrossRef]
- Hennink, S.D.; Maljaars, P.W.J. Fats and satiety. In Satiation, Satiety and the Control of Food Intake. Theory and Practice; Blundell, J.E., Bellisle, F., Eds.; Woodhead Publishing Limited: Sawston, UK, 2013; pp. 143–165. [Google Scholar]
- Aviram, M.; Kaplan, M.; Rosenblat, M.; Fuhrman, B. Dietary Antioxidants and Paraoxonases against LDL Oxidation and Atherosclerosis Development. In Atherosclerosis: Diet and Drugs; von Eckardstein, A., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 263–300. [Google Scholar]
- Bernal-Hernández, Y.Y.; Medina-Díaz, I.M.; Barrón-Vivanco, B.S.; Robledo-Marenco, M.L.; Girón-Pérez, M.I.; Pérez-Herrera, N.E.; Quintanilla-Vega, B.; Cerda-Flores, R.; Rojas-García, A.E. Paraoxonase 1 and its relationship with pesticide biomarkers in indigenous Mexican farmworkers. J. Occup. Environ. Med. 2014, 56, 281–290. [Google Scholar] [CrossRef]
- García-González, I.; Mendoza-Alcocer, R.; Pérez-Mendoza, G.J.; Rubí-Castellanos, R.; González-Herrera, L. Distribution of genetic variants of oxidative stress metabolism genes: Paraoxonase 1 (PON1) and Glutathione S-transferase (GSTM1/GSTT1) in a population from Southeastern Mexico. Ann. Hum. Biol. 2016, 43, 554–562. [Google Scholar] [CrossRef]
- González, F.E.M.; Ponce-Ruíz, N.; Rojas-García, A.E.; Bernal-Hernández, Y.Y.; Mackness, M.; Ponce-Gallegos, J.; Cardoso-Saldaña, G.; Jorge-Galarza, E.; Torres-Tamayo, M.; Medina-Díaz, I.M. PON1 concentration and high-density lipoprotein characteristics as cardiovascular biomarkers. Arch. Med. Sci. Atheroscler. Dis. 2019, 4, e47–e54. [Google Scholar] [CrossRef]
- Mota, A.; Hemati-dinarvand, M.; Akbar Taheraghdam, A.; Reza Nejabati, H.; Ahmadi, R.; Ghasemnejad, T.; Hasanpour, M.; Valilo, M. Association of Paraoxonse1 (PON1) Genotypes with the Activity of PON1 in Patients with Parkinson’s Disease. Acta Neurol. Taiwan 2019, 28, 66–74. [Google Scholar]
- Costa, L.G.; Giordano, G.; Furlong, C.E. Pharmacological and dietary modulators of paraoxonase 1 (PON1) activity and expression: The hunt goes on. Biochem. Pharmacol. 2011, 81, 337–344. [Google Scholar] [CrossRef]
- Costa, L.G.; Vitalone, A.; Cole, T.B.; Furlong, C.E. Modulation of paraoxonase (PON1) activity. Biochem. Pharmacol. 2005, 69, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.; Silaste, M.L.; Tuominen, A.; Kaikkonen, J.; Salonen, J.T.; Alfthan, G.; Aro, A.; Kesäniemi, Y.A. Dietary modifications and gene polymorphisms alter serum paraoxonase activity in healthy women. J. Nutr. 2002, 132, 3012–3017. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar] [PubMed]
- Nadeem, N.; Woodside, J.V.; Kelly, S.; Allister, R.; Young, I.S.; McEneny, J. The two faces of alpha- and gamma-tocopherols: An in vitro and ex vivo investigation into VLDL, LDL and HDL oxidation. J. Nutr. Biochem. 2012, 23, 845–851. [Google Scholar] [CrossRef]
- Wade, L.; Nadeem, N.; Young, I.S.; Woodside, J.V.; McGinty, A.; McMaster, C.; McEneny, J. α-Tocopherol induces proatherogenic changes to HDL2 & HDL3: An in vitro and ex vivo investigation. Atherosclerosis 2013, 226, 392–397. [Google Scholar]
- Durrington, P.N.; Mackness, B.; Mackness, M.I. The hunt for nutritional and pharmacological modulators of paraoxonase. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1248–1250. [Google Scholar] [CrossRef]
- Thomàs-Moyà, E.; Gianotti, M.; Proenza, A.M.; Llado, I. Paraoxonase 1 response to a high-fat diet: Gender differences in the factors involved. Mol. Med. 2007, 13, 203–209. [Google Scholar] [CrossRef]
- Hoefel, A.L.; Hansen, F.; Rosa, P.D.; Assis, A.M.; da Silveira, S.L.; Denardin, C.C.; Pettenuzzo, L.; Augusti, P.R.; Somacal, S.; Emanuelli, T.; et al. The effects of hypercaloric diets on glucose homeostasis in the rat: Influence of saturated and monounsaturated dietary lipids. Cell Biochem. Funct. 2011, 29, 569–576. [Google Scholar] [CrossRef]
- Kim, D.S.; Burt, A.A.; Ranchalis, J.E.; Richter, R.J.; Marshall, J.K.; Nakayama, K.S.; Jarvik, E.R.; Eintracht, J.F.; Rosenthal, E.A.; Furlong, C.E.; et al. Dietary cholesterol increases paraoxonase 1 enzyme activity. J. Lipid Res. 2012, 53, 2450–2458. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, F.K.; Pedrosa, M.L.; Paula, H.; Santos, R.C.; Silva, M.E. Evaluation of biological and biochemical quality of whey protein. J. Med. Food. 2010, 13, 1505–1509. [Google Scholar] [CrossRef]
- Dirican, M.; Tas, S.; Sarandöl, E. High-dose taurine supplementation increases serum paraoxonase and arylesterase activities in experimentalhypothyroidism. Clin. Exp. Pharmacol. Physiol. 2007, 34, 833–837. [Google Scholar] [CrossRef] [PubMed]
- Ponce-Ruíz, N.; Murillo-González, F.E.; Rojas-García, A.E.; Bernal-Hernández, Y.Y.; Mackness, M.; Ponce-Gallegos, J.; Barrón-Vivanco, B.S.; Hernández-Ochoa, I.; González-Arias, C.A.; Ortega-Cervantes, L.; et al. Phenotypes and concentration of PON1 in cardiovascular disease: The role of nutrient intake. Nutr. Metab. Cardiovasc. Dis. 2019, 30, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Powell, S.R. The antioxidant properties of zinc. J. Nutr. 2000, 130, 1447S–1454S. [Google Scholar] [CrossRef]
- Sánchez, C.; López-Jurado, M.; Aranda, P.; Llopis, J. Plasma levels of copper, manganese and selenium in an adult population in southern Spain: Influence of age, obesity and lifestyle factors. Sci. Total Environ. 2010, 408, 1014–1020. [Google Scholar] [CrossRef]
- Azadmanesh, J.; Borgstahl, G.E.O. A Review of the Catalytic Mechanism of Human Manganese Superoxide Dismutase. Antioxidants 2018, 7, 25. [Google Scholar] [CrossRef]
- Begcevic, I.; Simundic, A.M.; Nikolac, N.; Dobrijevic, S.; Rajkovic, M.G.; Tesija-Kuna, A. Can cranberry extract and vitamin C + Zn supplements affect the In vivo activity of paraoxonase 1, antioxidant potential, and lipid status? Clin. Lab. 2013, 59, 1053–1060. [Google Scholar] [CrossRef]
- Manolescu, B.N.; Berteanu, M.; Cinteza, D. Effect of the nutritional supplement ALAnerv® on the serum PON1 activity in post-acute stroke patients. Pharmacol. Rep. 2013, 65, 743–750. [Google Scholar] [CrossRef]
- Ardalić, D.; Stefanović, A.; Kotur-Stevuljević, J.; Vujović, A.; Spasić, S.; Spasojević-Kaliomanvska, V.; Jelić-Ivanović, Z.; Mandić-Marković, V.; Miković, Z.; Cerović, N. The influence of maternal smoking habits before pregnancy and antioxidative supplementation during pregnancy on oxidative stress status in a non-complicated pregnancy. Adv. Clin. Exp. Med. 2014, 23, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Taş, S.; Sarandöl, E.; Dirican, M. Vitamin B6 supplementation improves oxidative stress and enhances serum paraoxonase/arylesterase activities in streptozotocin-induced diabetic rats. Sci. World J. 2014, 2014, 351598. [Google Scholar] [CrossRef] [PubMed]
- Freese, R.; Alfthan, G.; Jauhiainen, M.; Basu, S.; Erlund, I.; Salminen, I.; Aro, A.; Mutanen, M. High intakes of vegetables, berries, and apples combined with a high intake of linoleic or oleic acid only slightly affect markers of lipid peroxidation and lipoprotein metabolism in healthy subjects. Am. J. Clin. Nutr. 2002, 76, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Kleemola, P.; Freese, R.; Jauhiainen, M.; Pahlman, R.; Alfthan, G.; Mutanen, M. Dietary determinants of serum paraoxonase activity in healthy humans. Atherosclerosis 2002, 160, 425–432. [Google Scholar] [CrossRef]
- Shahidi, F.; de Camargo, A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef] [PubMed]
- Mielgo-Ayuso, J.; Aparicio-Ugarriza, R.; Olza, J.; Aranceta-Bartrina, J.; Gil, A.; Ortega, R.M.; Serra-Majem, L.; Varela-Moreiras, G.; González-Gross, M. Dietary Intake and Food Sources of Niacin, Riboflavin, Thiamin and Vitamin B6 in a Representative Sample of the Spanish Population. The Anthropometry, Intake, and Energy Balance in Spain (ANIBES) Study dagger. Nutrients 2018, 10, 846. [Google Scholar] [CrossRef] [PubMed]
- Spectra Search Mass Spectrum. Available online: https://hmdb.ca/metabolites/HMDB0033908 (accessed on 21 August 2021).
- Spectra Search Mass Spectrum. Available online: https://hmdb.ca/metabolites/HMDB0003747 (accessed on 21 August 2021).
- Spectra Search Mass Spectrum. Available online: https://hmdb.ca/metabolites/HMDB0005807 (accessed on 21 August 2021).
- Spectra Search Mass Spectrum. Available online: https://hmdb.ca/spectra/ms_ms/252054 (accessed on 22 August 2021).
- Spectra Search Mass Spectrum. Available online: https://foodb.ca/compounds/FDB006783 (accessed on 25 August 2021).
| Men (n = 19) | Women (n = 26) | p | |
|---|---|---|---|
| Age, years (95% CI) | 28 (25–31) | 28 (25–31) | 1.00 a |
| BMI, kg/m2 (±SD) Underweight (≤18.5), n (%) Normal (>18.5, ≤25), n (%) Overweight (>25, ≤ 30), n (%) Obese (>30), n (%) | 27.4 (±5.8) | 24.5 (±3.8) | 0.05 b |
| 1 (5.3) | --- | 0.27 c | |
| 6 (31.6) | 14 (53.9) | ||
| 7 (36.8) | 9 (34.6) | ||
| 5 (26.3) | 3 (11.5) | ||
| SBP, mmHg (±SD) | 115 (±11) | 106 (±11) | 0.02 b |
| DBP, mmHg (95% CI) | 73 (68–78) | 67 (64–71) | 0.06 a |
| Heart rate, bpm (95% CI) | 70 (66–74) | 72 (69–76) | 0.31 a |
| Physical activity, n (%) | 12 (63.2) | 16 (61.5) | 0.73 d |
| Alcohol consumption, n (%) | 19 (100) | 24 (92.3) | 0.50 c |
| Smokers | |||
| Active, n (%) | 5 (26.3) | 3 (11.5) | 0.24 c |
| Passive, n (%) | 1 (5.3) | 5 (19.2) | |
| Past, n (%) | 6 (31.6) | 5 (19.2) | 0.07 d |
| Drug consumption, n (%) | 2 (10.5) | 2 (7.7) |
| Initial Intake | Final Intake | p | |
|---|---|---|---|
| Carbohydrates (%) | 46.2 (±7.4) | 45.44 (±7.4) | 0.53 a |
| Protein (%) | 11.0 (9.0–13.5) | 10.4 (8.6–12.5) | 0.61 b |
| Fat (%) | 38.0 (±7.11) | 38.2 (±6.9) | 0.78 a |
| Calories (kCal) | 1856.1 (1571.6–2192.0) | 1694.6 (1501.3–1912.7) | <0.001 b |
| Vitamin | Initial Intake | Final Intake | p |
|---|---|---|---|
| Vitamin C (mg) | 132.6 (105.8–166.1) | 83.7 (65.8–106.3) | <0.001 |
| Vitamin B1 (mg) | 1.3 (1.1–1.6) | 1.2 (1.0–1.3 | <0.01 |
| Vitamin B2 (mg) | 1.3 (1.1–1.6) | 1.2 (1.0–1.3) | <0.01 |
| Niacin (mg) | 17.9 (15.1–21.3) | 16.9 (15.0–19.0) | 0.01 |
| Vitamin B5 (mg) | 7.9 (6.2–9.9) | 7.0 (5.4–9.0) | >0.05 |
| Vitamin B6 (mg) | 1.7 (1.5–2.1) | 1.6 (1.4–1.8) | <0.01 |
| Glycosylated vitamin B6 (mg) | 4.1 (2.5–6.6) | 4.3 (3.3–5.7) | >0.05 |
| Folate (μg) | 481.7 (363.5–638.5) | 380.5 (277.9–521.1) | 0.01 |
| Vitamin B12 (μg) | 7.1 (5.6–8.9) | 6.1 (5.1–7.3) | 0.001 |
| Vitamin K (μg) | 74.3 (58.5–94.6) | 72.2 (59.2–87.9) | >0.05 |
| Retinol (UI) | 1721.8 (1325.3–2236.9) | 1436.6 (1183.0–1744.5) | 0.01 |
| Vitamin D (UI) | 156.5 (123.7–198.1) | 136.9 (114.0–164.4) | 0.02 |
| Vitamin E (μg) | 11.7 (±6.6) | 8.7 (±4.0) | <0.01 a |
| Vitamin E activity (IU) | 12.3 (9.8–15.3) | 9.8 (8.5–11.4) | <0.01 |
| α-Tocopherol (mg) | 11.6 (9.4–14.2) | 9.4 (8.1–10.9) | <0.01 |
| β-Tocopherol (mg) | 0.6 (±0.43) | 0.5 (±0.2) | 0.04 a |
| γ-Tocopherol (mg) | 13.4 (10.5–17.1) | 14.8 (12.5–17.4) | >0.05 |
| δ-Tocopherol (mg) | 2.3 (1.7–3.0) | 2.8 (2.3–3.4) | >0.05 |
| α-Tocopherol (eq/mg) | 13.3 (10.8–16.3) | 11.1 (9.6–12.8) | <0.01 |
| Mineral | Initial Intake | Final Intake | p |
| Calcium (mg) | 538.8 (441.9–656.9) | 486.3 (423.6–558.4) | <0.01 |
| Iron (mg) | 12.6 (10.5–15.0) | 11.9 (10.6–13.5) | 0.02 |
| Magnesium (mg) | 280.9 (233.3–338.2) | 272.7 (243.3–305.6) | 0.04 |
| Phosphorus (mg) | 1077.4 (896.2–1295.2) | 1058.5 (946.1–1184.3) | 0.02 |
| Potassium (mg) | 2840.6 (2401.6–3359.9) | 2453.5 (2154.6–2793.9) | <0.001 |
| Sodium (mg) | 1593.4 (1342.2–1891.5) | 1410.9 (1225.1–1624.9) | <0.01 |
| Zinc (mg) | 13.9 (11.1–17.4) | 12.6 (10.3–15.5) | 0.03 |
| Copper (mg) | 1.8 (1.4–2.3) | 1.6 (1.3–2.0) | 0.01 |
| Manganese (mg) | 11.8 (8–17.4) | 11.9 (8.7–16.1) | >0.05 |
| Selenium (μg) | 40.3 (32.3–50.6) | 38.9 (32.6–46.5) | >0.05 |
| AREase | |||
|---|---|---|---|
| Nutrient | OR | 95% CI | p |
| Total fiber (g) | 1.002 | 1.0004–1.004 | 0.02 |
| β-tocopherol (mg) | 0.085 | 0.0088–0.813 | 0.03 |
| γ-tocopherol (mg) | 0.814 | 0.668–0.992 | 0.04 |
| δ-tocopherol (mg) | 0.517 | 0.281–0.950 | 0.03 |
| Polyunsaturated fat (g) | 0.906 | 0.823–0.998 | 0.04 |
| PONase | |||
| Nutrient | β | 95% CI | p |
| Total protein (g) | −0.008 | −0.0139–−0.0013 | 0.02 |
| Animal protein (g) | −0.012 | −0.0214–−0.0032 | 0.01 |
| Calcium (mg) | −0.0007 | −0.0014–−0.00006 | 0.03 |
| Potassium (mg) | −0.0001 | −0.0003–−4.6 × 10−6 | 0.04 |
| Vitamin B2 (mg) | −0.302 | −0.581–−0.0234 | 0.03 |
| Vitamin B3 (mg) | −0.023 | −0.045–−0.0024 | 0.03 |
| Vitamin B6 (mg) | −0.217 | −0.416–−0.0178 | 0.03 |
| Animal fat (g) | −0.008 | −0.016–−0.0003 | 0.04 |
| Saturated fat (g) | −0.017 | −0.032–−0.0011 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-García, F.; Ponce-Ruíz, N.; Rojas-García, A.E.; Ávila-Villarreal, G.; Herrera-Moreno, J.F.; Barrón-Vivanco, B.S.; Bernal-Hernández, Y.Y.; González-Arias, C.A.; Medina-Díaz, I.M. The Role of Nutritional Habits and Moderate Red Wine Consumption in PON1 Status in Healthy Population. Appl. Sci. 2021, 11, 9503. https://doi.org/10.3390/app11209503
Navarro-García F, Ponce-Ruíz N, Rojas-García AE, Ávila-Villarreal G, Herrera-Moreno JF, Barrón-Vivanco BS, Bernal-Hernández YY, González-Arias CA, Medina-Díaz IM. The Role of Nutritional Habits and Moderate Red Wine Consumption in PON1 Status in Healthy Population. Applied Sciences. 2021; 11(20):9503. https://doi.org/10.3390/app11209503
Chicago/Turabian StyleNavarro-García, Fidel, Néstor Ponce-Ruíz, Aurora Elizabeth Rojas-García, Gabriela Ávila-Villarreal, José Francisco Herrera-Moreno, Briscia S. Barrón-Vivanco, Yael Y. Bernal-Hernández, Cyndia Azucena González-Arias, and Irma Martha Medina-Díaz. 2021. "The Role of Nutritional Habits and Moderate Red Wine Consumption in PON1 Status in Healthy Population" Applied Sciences 11, no. 20: 9503. https://doi.org/10.3390/app11209503
APA StyleNavarro-García, F., Ponce-Ruíz, N., Rojas-García, A. E., Ávila-Villarreal, G., Herrera-Moreno, J. F., Barrón-Vivanco, B. S., Bernal-Hernández, Y. Y., González-Arias, C. A., & Medina-Díaz, I. M. (2021). The Role of Nutritional Habits and Moderate Red Wine Consumption in PON1 Status in Healthy Population. Applied Sciences, 11(20), 9503. https://doi.org/10.3390/app11209503







