Biomethane Potential of Sludges from a Brackish Water Fish Hatchery
Abstract
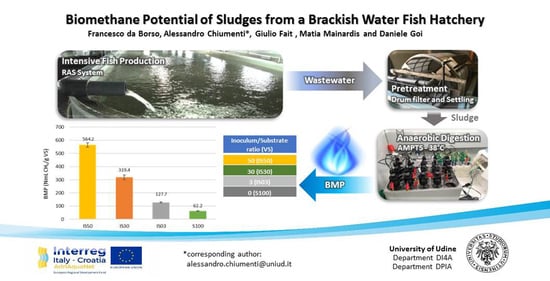
:Featured Application
Abstract
1. Introduction
2. Material and Methods
2.1. Substrate and Inoculum
2.2. Analytical Methods
2.3. Biochemical Methane Potential Tests
2.4. Kinetic Models
- Y(t) is the estimated cumulative methane yield at time t (mL CH4/g VS)
- Y0 is the calculated BMP of the substrate (mL CH4/g VS)
- k is the hydrolysis rate constant (1/day)
- t is the time (day)
- Rmax is the maximum methane production rate (mL CH4/g VS day)
- λ is the lag phase duration (day)
3. Results and Discussions
3.1. Chemical Composition of Fish Sludges
3.2. Methane Production
3.3. Biochemical Methane Potential (BMP)
3.4. Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of the World Fishery and Aquaculture 2018—Meeting the Sustainable Development Goals; Licence: CC BY-NC-SA 3.0 IGO; FAO: Rome, Italy, 2018. [Google Scholar]
- Price, C.; Black, K.D.; Hargrave, B.T.; Morris, J.A. Marine cage culture and the environment: Effects on water quality and primary production. Aquacult. Environ. Interac. 2015, 6, 151–174. [Google Scholar] [CrossRef] [Green Version]
- Timmons, M.B.; Ebeling, J.M. Recirculating Aquaculture; Cayuga Aqua Ventures, LLC: Ithaca, NY, USA, 2007. [Google Scholar]
- Chiumenti, A.; Fait, G.; Limina, S.; da Borso, F. Performances of Conventional and Hybrid Fixed Bed Anaerobic Reactors for the Treatment of Aquaculture Sludge. Bioengineering 2020, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Chiumenti, A.; Limina, S.; Fait, G.; da Borso, F. Anaerobic Digestion of Thickened Sludge from Fish Production in CSTR and Hybrid Pilot Scale Digesters. In Proceedings of the ASABE Annual Meeting, Virtual Edition, Lincoln, NE, USA, 13–15 July 2020. [Google Scholar]
- Kiel, M.; Dobslaw, D.; Engesser, K.H. Comparison of biological and chemical treatment processes as cost-effective methods for elimination of benzoate in saline wastewaters. Water Res. 2014, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Uygur, A. Specific nutrient removal rates in saline wastewater treatment using sequencing batch reactor. Process Biochem. 2006, 41, 61–66. [Google Scholar] [CrossRef]
- Tal, Y.; Schreier, H.J.; Sowers, K.R.; Stubblefield, J.D.; Place, A.R.; Zohar, Y. Environmentally sustainable land-based marine aquaculture. Aquaculture 2009, 286, 28–35. [Google Scholar] [CrossRef]
- Mirzoyan, N.; Tal, Y.; Gross, A. Anaerobic digestion of sludge from intensive recirculating aquaculture systems: Review. Aquaculture 2010, 306, 1–6. [Google Scholar] [CrossRef]
- Mirzoyan, N.; McDonald, R.C.; Gross, A. Anaerobic Treatment of Brackishwater Aquaculture Sludge: An Alternative to Waste Stabilization Ponds. J. World Aquac. Soc. 2012, 43, 238–248. [Google Scholar] [CrossRef]
- Chiumenti, R.; Chiumenti, A.; da Borso, F.; Limina, S.; Landa, A. Anaerobic Digestion of Swine Manure in Conventional and Hybrid Pilot Scale Plants: Performance and Gaseous Emissions Reduction. In Proceedings of the ASABE Annual Meeting, Reno, NV, USA, 21–24 June 2009; pp. 21–24. [Google Scholar]
- Wong, B.; Show, K.Y.; Lee, D.J.; Lai, J.Y. Carbon balance of anaerobic granulation process: Carbon credit. Bioresour. Technol. 2009, 100, 1734–1739. [Google Scholar] [CrossRef]
- Reed, S.C.; Crites, R.W.; Middlebrooks, E.J. Natural Systems for Waste Management and Treatment; McGraw-Hill: New York, NY, USA, 1995. [Google Scholar]
- Lefebvre, O.; Quentin, S.; Torrijos, M.; Godon, J.J.; Delgenès, J.P.; Moletta, R. Impact of increasing NaCl concentrations on the performance and community composition of two anaerobic reactors. Appl. Microbiol. Biotechnol. 2007, 75, 61–69. [Google Scholar] [CrossRef]
- Misson, G.; Mainardis, M.; Incerti, G.; Goi, D.; Peressotti, A. Preliminary evaluation of potential methane production from anaerobic digestion of beach-cast seagrass wrack: The case study of high-Adriatic coast. J. Clean. Prod. 2020, 254C, 120131. [Google Scholar] [CrossRef]
- Gebauer, R. Mesophilic anaerobic treatment of sludge from saline fish farm effluents with biogas production. Bioresour. Technol. 2004, 93, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Dobslaw, D.; Engesser, K.H.; Stork, H.; Gerl, T. Low-cost process for emission abatement of biogas internal combustion engines. J. Clean. Prod. 2019, 227, 1079–1092. [Google Scholar] [CrossRef]
- Mulbry, W.; Selmer, K.; Lansing, S. Effect of liquid surface area on hydrogen sulfide oxidation during micro-aeration in dairy manure digesters. PLoS ONE 2017, 12, e0185738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiumenti, A.; Pezzuolo, A.; Boscaro, B.; da Borso, F. Exploitation of Mowed Grass from Green Areas by Means of Anaerobic Digestion: Effects of Grass Conservation Methods (Drying and Ensiling) on Biogas and Biomethane Yield. Energies 2019, 12, 3244. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Ferreira, R.B.; Hu, J.; Spanjers, H.; van Lier, J.B. Improving methane production and phosphorus release in anaerobic digestion of particulate saline sludge from a brackish aquaculture recirculation system. Bioresour. Technol. 2014, 162, 384–388. [Google Scholar] [CrossRef]
- Pearse, L.F.; Hettiaratchi, J.P.; Kumar, S. Towards developing a representative biochemical methane potential (BMP) assay for landfilled municipal solid waste—A review. Bioresour. Technol. 2018, 254, 312–324. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar]
- Mainardis, M.; Flaibani, S.; Trigatti, M.; Goi, D. Techno-economic feasibility of anaerobic digestion of cheese whey in small Italian dairies and effect of ultrasound pre-treatment on methane yield. J. Environ. Manag. 2019, 246, 557–563. [Google Scholar] [CrossRef]
- Sun, C.; Liu, F.; Song, Z.; Wang, J.; Li, Y.; Pan, Y.; Sheng, T.; Li, L. Feasibility of dry anaerobic digestion of beer lees for methane production and biochar enhanced performance at mesophilic and thermophilic temperature. Bioresour. Technol. 2019, 276, 65–73. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Suja, F.B.; Porhemmat, M.; Pramanik, B.K. Performance and Kinetic Model of a Single-Stage Anaerobic Digestion System Operated at Different Successive Operating Stages for the Treatment of Food Waste. Processes 2019, 7, 600. [Google Scholar] [CrossRef] [Green Version]
- Mirzoyan, N.; Parnes, S.; Singer, A.; Tal, Y.; Sowers, K.; Gross, A. Quality of brackish aquaculture sludge and its suitability for anaerobic digestion and methane production in an upflow anaerobic sludge blanket (UASB) reactor. Aquaculture 2008, 279, 35–41. [Google Scholar] [CrossRef]
- Luo, G.Z.; Li, P.; Tan, H.X.; Du, J.; Liang, W.Y. The start-upand saline adaptation of mesophilic anaerobic sequencing batch reactor treating sludge from recirculating aquaculture systems. Aquac. Eng. 2013, 54, 9–15. [Google Scholar] [CrossRef]
- Chiumenti, A.; da Borso, F.; Guercini, S.; Pezzuolo, A.; Zanotto, M.; Sgorlon, S.; Delle Vedove, G.; Miceli, F.; Stefanon, B. The Impact of the Dairy Cow Diet on Anaerobic Digestion of Manure. In Proceedings of the 2019 ASABE Annual International Meeting, Boston, MA, USA, 7–10 July 2019. [Google Scholar] [CrossRef]
- Priadi, C.; Wulandari, D.; Rahmatika, I.; Sarwanto Moersidik, S. Biogas Production in the Anaerobic Digestion of Paper Sludge. 5th International Conference on Chemical, Biological and Environmental Engineering. APCBEE Procedia 2014, 9, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Mcdonald, A.G. Anaerobic digestion of pre-fermented potato peel wastes for methane production. Waste Manag. 2015, 46, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Achinas, P.; Li, Y.; Achinas, V.; Euverink, G.J.W. Biogas Potential from the Anaerobic Digestion of Potato Peels: Process Performance and Kinetics Evaluation. Energies 2019, 12, 2311. [Google Scholar] [CrossRef] [Green Version]
- Lanari, D.; Franci, C. Biogas production from solid wastes removed from fish farm effluents. Aquat. Living Resour. 1998, 11, 289–295. [Google Scholar] [CrossRef]
- Zhang, X.; Tao, Y.; Hu, J.; Liu, G.; Spanjers, H.; van Lier, J.B. Biomethanation and microbial community changes in a digester treating sludge from a brackish aquaculture recirculation system. Biores. Technol. 2016, 214, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Gebauer, R.; Eikebrokk, B. Mesophilic anaerobic treatment of sludge from salmon smolt hatching. Biores. Technol. 2006, 97, 2389–2401. [Google Scholar] [CrossRef]
- Mirzoyan, N.; Gross, A. Use of UASB for brakish aquaculture sludge digestion under different conditions. Water Res. 2013, 47, 2843–2850. [Google Scholar] [CrossRef]
- IWA Task Group for Mathematical Modelling of Anaerobic Digestion Processes. Anaerobic Digestion Model No. 1 (ADM1). In Scientific and Technical Report No. 13; IWA Publishing: London, UK, 2002. [Google Scholar]
- Kafle, G.K.; Chen, L. Comparison on batch anaerobic digestion of five different livestock manures and prediction of biochemical methane potential (BMP) using different statistical models. Waste Manag. 2016, 48, 492–502. [Google Scholar] [CrossRef] [Green Version]




| Parameter | Units | Fish Sludge (Substrate) | Inoculum |
|---|---|---|---|
| Total solids (TS) | g/L | 14.29 ± 0.3 | 64.27 ± 0.8 |
| Volatile solids (VS) | g/L | 4.70 ± 0.1 | 47.82 ± 0.6 |
| Volatile solids (VS) | % TS | 32.9 ± 1.5 | 74.4 ± 0.9 |
| Total COD (tCOD) | mg/L | 4045 ± 405 | n.d. |
| Soluble COD (sCOD) | mg/L | 3680 ± 220 | 8000 ± 75 |
| Total Nitrogen (TN) | g/L | 0.2 ± 0.07 | 4.53 ± 0.6 |
| Ammoniacal Nitrogen (TAN) | g/L | 0.011 ± 0.002 | 2.95 ± 0.5 |
| Volatile fatty acids (VFA) | mg/L | 596.5 ± 64.3 | 3115 ± 16.5 |
| Total alkalinity (TAC) | mg/L | 815.0 ± 0.1 | 17,058 ± 183 |
| VFA/TAC | - | 0.732 | 0.183 |
| Salinity | g/L | 12 | - |
| Redox potential | mV | −60 ± 8 | −328 ± 9 |
| pH | - | 5.9 ± 0.2 | 7.9 ± 0.0 |
| I/S Ratio | K (1/Day) | λ (Day) | Rmax (NmL/g VS Day) |
|---|---|---|---|
| 50 (IS50) | 0.15 | 14.0 | 63.1 |
| 30 (IS30) | 0.13 | 13.5 | 40.3 |
| 3 (IS03) | 0.11 | 5.5 | 16.0 |
| 0 (S100) | 0.11 | 7.6 | 15.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Borso, F.; Chiumenti, A.; Fait, G.; Mainardis, M.; Goi, D. Biomethane Potential of Sludges from a Brackish Water Fish Hatchery. Appl. Sci. 2021, 11, 552. https://doi.org/10.3390/app11020552
da Borso F, Chiumenti A, Fait G, Mainardis M, Goi D. Biomethane Potential of Sludges from a Brackish Water Fish Hatchery. Applied Sciences. 2021; 11(2):552. https://doi.org/10.3390/app11020552
Chicago/Turabian Styleda Borso, Francesco, Alessandro Chiumenti, Giulio Fait, Matia Mainardis, and Daniele Goi. 2021. "Biomethane Potential of Sludges from a Brackish Water Fish Hatchery" Applied Sciences 11, no. 2: 552. https://doi.org/10.3390/app11020552