Facile Method by Bentonite Treated with Heat and Acid to Enhance Pesticide Adsorption
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of Modified Bentonite
2.3. Characterisation
2.4. Batch Adsorption
2.5. Effect of pH
2.6. Simultaneous Adsorption of Various Pesticides in Solutions
2.7. Adsorption Isotherm
2.8. Kinetic and Thermodynamics Adsorption Study
3. Results and Discussion
3.1. Characterisation of Bentonite and Modified Bentonite
3.1.1. XRF and CEC Analysis
3.1.2. XRD Analysis
3.1.3. Thermogravimetric Analysis
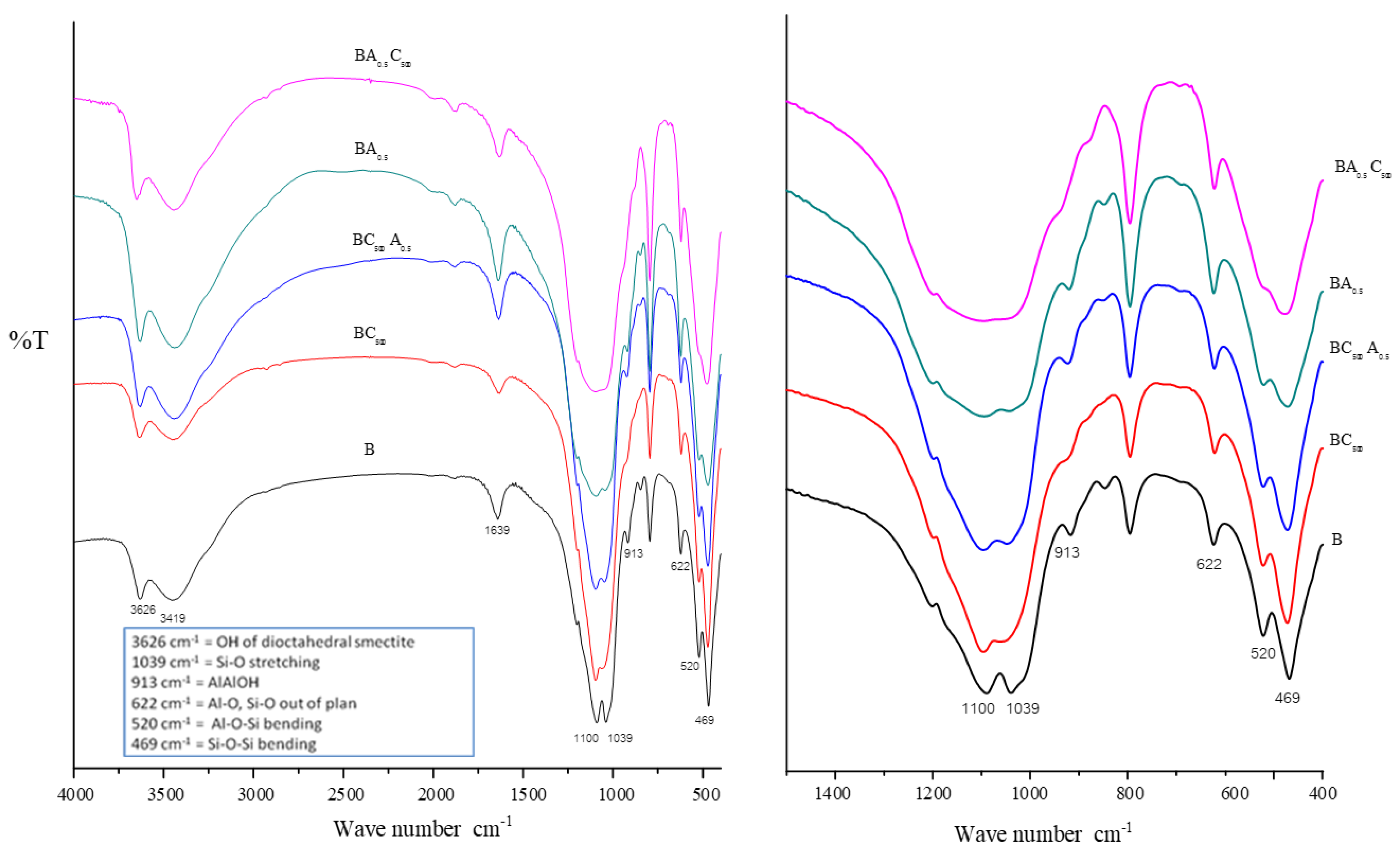
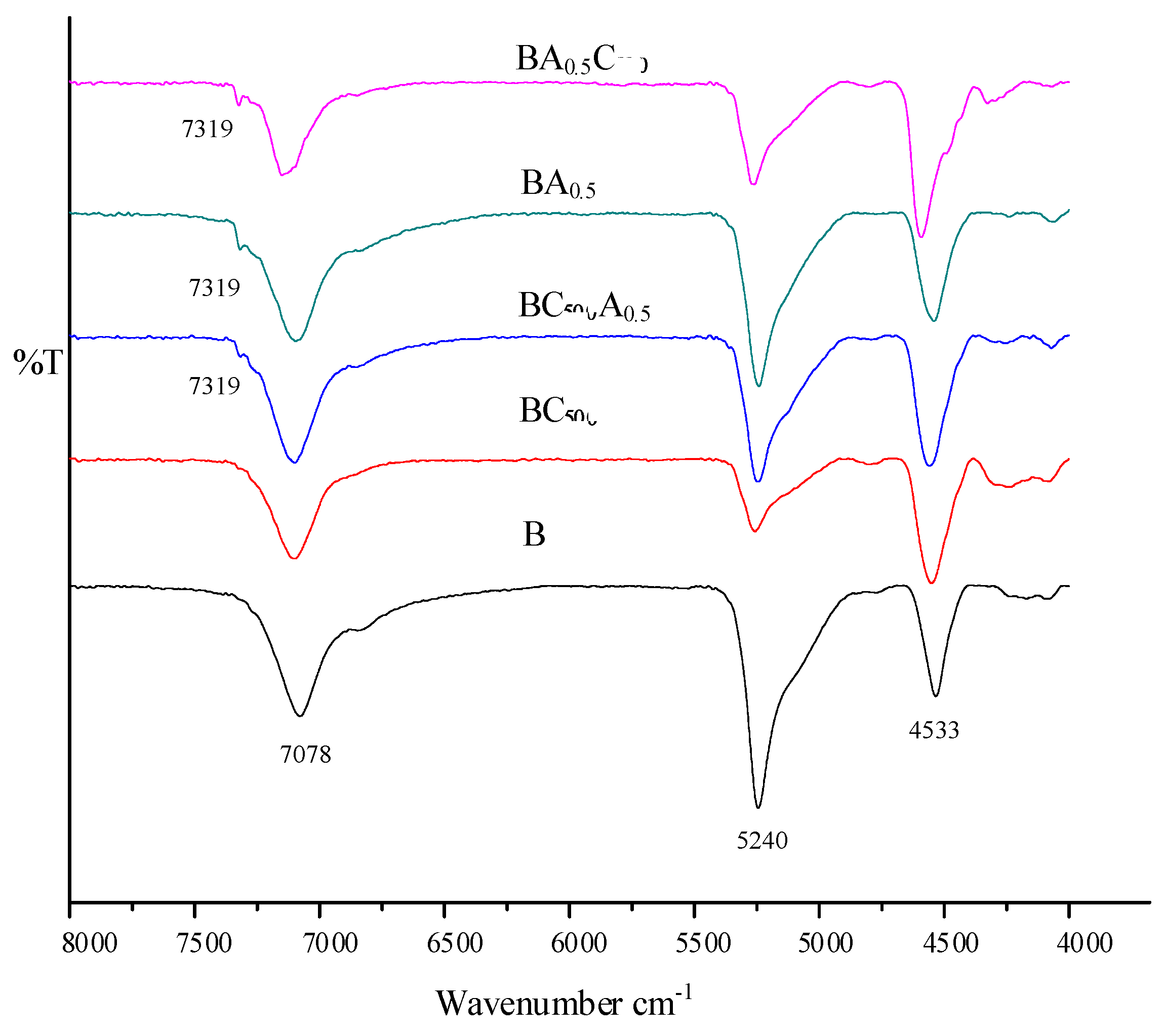
3.1.4. FT-IR Analysis
3.1.5. Temperature-Programmed Desorption of Ammonium (NH3-TPD)
3.1.6. NMR Analysis
3.1.7. Textural Properties Study
3.1.8. Particle Size Analysis
3.2. Adsorption of Pesticides onto Bentonite and Modified Bentonite
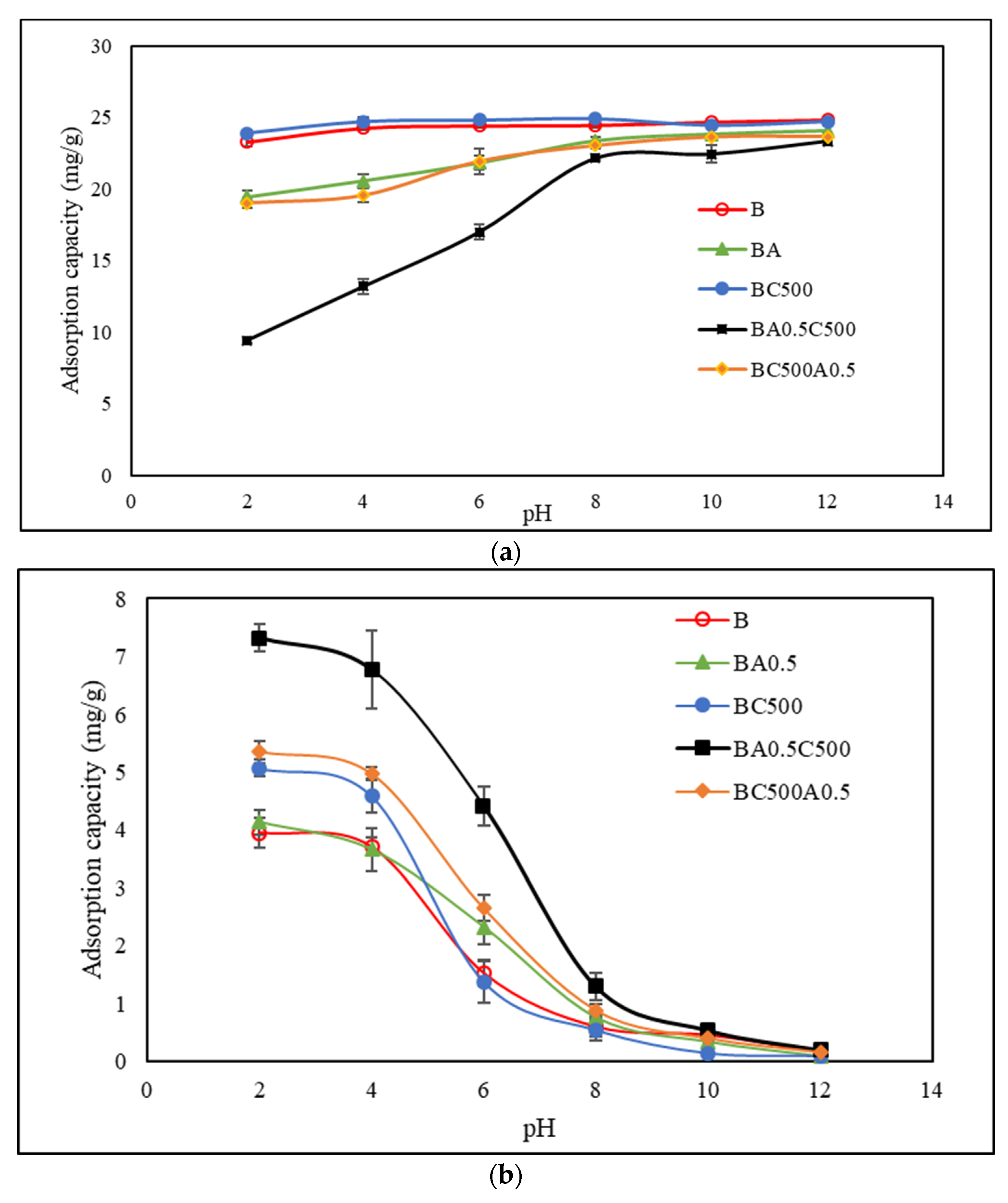
3.2.1. The Effect of pH
3.2.2. Simultaneous Adsorption of Various Pesticides in Solutions
3.3. Adsorption Isotherms
3.4. Kinetic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Amaral, B.D.; de Araujo, J.A.; Peralta-Zamora, P.G.; Nagata, N. Simultaneous determination of atrazine and metabolites (DIA and DEA) in natural water by multivariate electronic spectroscopy. Microchem. J. 2014, 117, 262–267. [Google Scholar] [CrossRef]
- Jamil, T.S.; Gad-Allah, T.A.; Ibrahim, H.S.; Saleh, T.S. Adsorption and isothermal models of atrazine by zeolite prepared from Egyptian kaolin. Solid State Sci. 2011, 13, 198–203. [Google Scholar] [CrossRef]
- Leite, M.P.; dos Reis, L.G.T.; Robaina, N.F.; Pacheco, W.F.; Cassella, R.J. Adsorption of paraquat from aqueous medium by Amberlite XAD-2 and XAD-4 resins using dodecylsulfate as counter ion. Chem. Eng. J. 2013, 215–216, 691–698. [Google Scholar] [CrossRef]
- Li, K.; Wu, J.Q.; Jiang, L.L.; Shen, L.Z.; Li, J.Y.; He, Z.H.; Wei, P.; Lv, Z.; He, M.F. Developmental toxicity of 2,4-dichlorophenoxyacetic acid in zebrafish embryos. Chemosphere 2017, 198, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.F.; Chu, W. Degradation of linuron by UV, ozonation, and UV/O3 processes—Effect of anions and reaction mechanism. J. Hazard. Mater. 2010, 180, 514–523. [Google Scholar] [CrossRef]
- Salman, J.M.; Njoku, V.O.; Hameed, B.H. Adsorption of pesticides from aqueous solution onto banana stalk activated carbon. Chem. Eng. J. 2011, 174, 41–48. [Google Scholar] [CrossRef]
- Tomkins, B.A.; Ilgner, R.H. Determination of atrazine and four organophosphorus pesticides in ground water using solid phase microextraction (SPME) followed by gas chromatography with selected-ion monitoring. J. Chromatogr. A 2002, 972, 183–194. [Google Scholar] [CrossRef]
- Castro, C.S.; Guerreiro, M.C.; Goncalves, M.; Oliveira, L.C.A.; Anastacio, A.S. Activated carbon/iron oxide composites for the removal of atrazine from aqueous medium. J. Hazard. Mater. 2009, 164, 609–614. [Google Scholar] [CrossRef]
- Chen, G.C.; Shan, X.Q.; Zhou, Y.Q.; Shen, X.E.; Huang, H.L.; Khan, S.U. Adsorption kinetics, isotherms and thermodynamics of atrazine on surface oxidized multiwalled carbon nanotube. J. Hazard. Mater. 2009, 169, 912–918. [Google Scholar] [CrossRef]
- Kovaios, I.D.; Paraskeva, C.A.; Koutsoukos, P.G. Adsorption of atrazine from aqueous electrolyte solutions on humic acid and silica. J. Colloid Interface Sci. 2011, 356, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Salvestrini, S.; Sagliano, P.; Iovino, P.; Capasso, S.; Colella, C. Atrazine adsorption by acid-activated zeolite-rich tuffs. Appl. Clay Sci. 2010, 49, 330–335. [Google Scholar] [CrossRef]
- Zadaka, D.; Nir, S.; Radian, A.; Mishael, Y.G. Atrazine removal from water by polycation-clay composites: Effect of dissolved organic matter and comparison to activated carbon. Water Res. 2009, 43, 677–683. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, J.; Zhang, C.; Yang, Z. Enhanced adsorption of atrazine from aqueous solution by molecularly imprinted TiO2 film. Solid State Sci. 2012, 14, 777–781. [Google Scholar] [CrossRef]
- Miricioiu, M.G.; Niculescu, V.-C. Fly Ash, from Recycling to Potential Raw Material for Mesoporous Silica Synthesis. Nanomaterials 2020, 10, 474. [Google Scholar] [CrossRef] [Green Version]
- Choo, K.Y.; Bai, K. The effect of the mineralogical composition of various bentonites on CEC values determined by three different analytical methods. Appl. Clay Sci. 2016, 126, 153–159. [Google Scholar] [CrossRef]
- Murray, H.H. Chapter 2 Structure and Composition of the Clay Minerals and their Physical and Chemical Properties. Dev. Clay Sci. Elsevier 2006, 2, 7–31. [Google Scholar] [CrossRef]
- Park, J.-H.; Shin, H.-J.; Kim, M.-H.; Kim, J.-S.; Kang, N.; Lee, J.-Y.; Kim, K.-T.; Lee, J.-I.; Kim, D.-D. Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. J. Pharm. Investig. 2016, 46, 363–375. [Google Scholar] [CrossRef]
- Slaný, M.; Jankovič, L.; Madejová, J. Structural characterization of organo-montmorillonites prepared from a series of primary alkylamines salts: Mid-IR and near-IR study. Appl. Clay Sci. 2019, 176, 11–20. [Google Scholar] [CrossRef]
- Tong, D.S.; Zheng, Y.M.; Yu, W.H.; Wu, L.M.; Zhou, C.H. Catalytic cracking of rosin over acid-activated montmorillonite catalysts. Appl. Clay Sci. 2014, 100, 123–128. [Google Scholar] [CrossRef]
- Gates, W.P.; Anderson, J.S.; Raven, M.D.; Churchman, G.J. Mineralogy of a bentonite from Miles, Queensland, Australia and characterisation of its acid activation products. Appl. Clay Sci. 2002, 20, 189–197. [Google Scholar] [CrossRef]
- Bieseki, L.; Treichel, H.; Araujo, A.S.; Pergher, S.B.C. Porous materials obtained by acid treatment processing followed by pillaring of montmorillonite clays. Appl. Clay Sci. 2013, 85, 46–52. [Google Scholar] [CrossRef]
- Pentrák, M.; Czímerová, A.; Madejová, J.; Komadel, P. Changes in layer charge of clay minerals upon acid treatment as obtained from their interactions with methylene blue. Appl. Clay Sci. 2012, 55, 100–107. [Google Scholar] [CrossRef]
- Bojemueller, E.; Nennemann, A.; Lagaly, G. Enhanced pesticide adsorption by thermally modified bentonites. Appl. Clay Sci. 2001, 18, 277–284. [Google Scholar] [CrossRef]
- Maqueda, C.; dos Santos Afonso, M.; Morillo, E.; Torres Sánchez, R.M.; Perez-Sayago, M.; Undabeytia, T. Adsorption of diuron on mechanically and thermally treated montmorillonite and sepiolite. Appl. Clay Sci. 2013, 72, 175–183. [Google Scholar] [CrossRef]
- Komadel, P. Acid activated clays: Materials in continuous demand. Appl. Clay Sci. 2016, 131, 84–99. [Google Scholar] [CrossRef]
- Komadel, P.; Madejová, J. Chapter 7.1 Acid Activation of Clay Minerals. Dev. Clay Sci. Elsevier 2006, 1, 263–287. [Google Scholar] [CrossRef]
- Gupta, V.K.; Sharma, M.; Vyas, R.K. Hydrothermal modification and characterization of bentonite for reactive adsorption of methylene blue: An ESI-MS study. J. Environ. Chem. Eng. 2015, 3, 2172–2179. [Google Scholar] [CrossRef]
- Heller-Kallai, L. Chapter 7.2 Thermally Modified Clay Minerals. Dev. Clay Sci. Elsevier 2006, 289–308. [Google Scholar] [CrossRef]
- Nones, J.; Nones, J.; Riella, H.G.; Poli, A.; Trentin, A.G.; Kuhnen, N.C. Thermal treatment of bentonite reduces aflatoxin b1 adsorption and affects stem cell death. Mater. Sci. Eng. C 2015, 55, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wu, H.; Li, R. The microstructural study of thermal treatment montmorillonite from Heping, China. Spectrochim. Acta A. 2005, 61, 3020–3025. [Google Scholar] [CrossRef]
- Chorom, M.; Rengasamy, P. Effect of heating on swelling and dispersion of different cationin forms of a smectite. Clays Clay Miner. 1996, 44, 783–790. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, S.J. Expansion characteristics of organoclay as a precursor to nanocomposites. Colloids Surf. A 2002, 211, 19–26. [Google Scholar] [CrossRef]
- Boudiaf, H.; Boutahala, M.; Sahnoun, S.; Tiar, C.; Gomri, F. Adsorption characteristics, isotherm, kinetics, and diffusion of modified natural bentonite for removing the 2,4,5-trichlorophenol. Appl. Clay Sci. 2014, 90, 81–87. [Google Scholar] [CrossRef]
- Olalekan, S.T.; Qudsieh, I.Y.; Kabbashi, N.A.; Alkhatib, M.A.; Muyibi, S.A.; Yusof, F.; Shah, Q.H. Effect of modification on the physicochemical and thermal properties of organophilic clay modified with octadecylamine. Int. J. Eng. Tech. IJET IJENS. 2010, 10, 27–35. [Google Scholar]
- Komadel, P.; Madejová, J. Acid Activation of Clay Minerals. Dev. Clay Sci. Elsevier 2013, 5, 385–409. [Google Scholar]
- Alver, B.E.; Alver, Ö. The investigation of the effect of thermal treatment on bentonites from Turkey with Fourier transform infrared and solid state nuclear magnetic resonance spectroscopic methods. Spectrochim. Acta A 2012, 94, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Brus, J.; Kobera, L.; Schoefberger, W.; Urbanová, M.; Klein, P.; Sazama, P.; Tabor, E.; Sklenak, S.; Fishchuk, A.V.; Dědeček, J. Structure of framework aluminum Lewis sites and perturbed aluminum atoms in zeolites as determined by 27Al{1H} REDOR (3Q) MAS NMR spectroscopy and DFT/molecular mechanics. Angew. Chem. 2015, 54, 541–545. [Google Scholar]
- Takahashi, T.; Ohkubo, T.; Suzuki, K.; Ikeda, Y. High resolution solid-state NMR studies on dissolution and alteration of Na-montmorillonite under highly alkaline conditions. Microporous Mesoporous Mater. 2007, 106, 284–297. [Google Scholar] [CrossRef]
- Calvet, R.; Prost, R. Cation migration into empty octahedral sites and surface properties of clays. Clays Clay Miner. 1971, 19, 175–186. [Google Scholar] [CrossRef]
- Au, P.-I.; Leong, Y.-K. Rheological and zetapotential behaviour of kaolin and bentonite composite slurries. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 530–541. [Google Scholar] [CrossRef]
- Moradi, S.E. Microwave assisted preparation of sodium dodecyl sulphate (SDS) modified ordered nanoporous carbon and its adsorption for MB dye. J. Ind. Eng. Chem. 2014, 20, 208–215. [Google Scholar] [CrossRef]
- Nejati, K.; Davary, S.; Saati, M. Study of 2,4-dichlorophenoxyacetic acid (2,4-D) removal by Cu-Fe-layered double hydroxide from aqueous solution. Appl. Surf. Sci. 2013, 280, 67–73. [Google Scholar] [CrossRef]
- Nithaya, K.M.; Arnepalli, D.N.; Gandhi, S.R. In Proccedings of the 6th International Congress on Environmental Geotechnics, New Delhi, India, 8–12 November 2010.
- Ding, L.; Lu, X.; Deng, H.; Zhang, X. Adsorptive Removal of 2,4-Dichlorophenoxyacetic Acid (2,4-D) from Aqueous Solutions Using MIEX Resin. Ind. Eng. Chem. Res. 2012, 51, 11226–11235. [Google Scholar] [CrossRef]
- Bouras, O.; Bollinger, J.-C.; Baudu, M.; Khalaf, H. Adsorption of diuron and its degradation products from aqueous solution by surfactant-modified pillared clays. Appl. Clay Sci. 2007, 37, 240–250. [Google Scholar] [CrossRef]
- Ng, C.; Losso, J.N. Freundlich adsorption isotherms of agricultural by-product-based powdered activated carbons in a geosmin–water system. Bioresour. Technol. 2002, 85, 131–135. [Google Scholar] [CrossRef]
- Yuh-Shan, H. Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 2004, 59, 171–177. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ali, I.; ALOthman, Z.A.; Warthan, A. Sorption, kinetics and thermodynamics studies of atrazine herbicide removal from water using iron nano-composite material. Int. J. Environ. Sci. Technol. 2016, 13, 733–742. [Google Scholar] [CrossRef] [Green Version]
- Cansado, P.T.T.; Mourão, P.A.M.; Gomes, J.A.F.L.; Almodôvar, V. Adsorption of MCPA, 2,4-D and diuron onto activated carbons from wood composites. Ciência Tecnol. Mater. 2017, 29, e224–e228. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Q.; Yang, Z.; Wang, W. Adsorption of 2,4-D on magnetic graphene and mechanism study. Colloids Surf. A Physicochem. Eng. Asp. 2016, 509, 367–375. [Google Scholar] [CrossRef]
- Tsai, W.T.; Hsien, K.J.; Chang, Y.M.; Lo, C.C. Removal of herbicide paraquat from an aqueous solution by adsorption onto spent and treated diatomaceous earth. Bioresour. Technol. 2005, 96, 657–663. [Google Scholar] [CrossRef] [PubMed]







| Sample | Chemical (%Weight) | SiO2/ Al2O3 | CEC (cmol kg−1) | %LOI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na2O | MgO | Al2O3 | SiO2 | P2O5 | K2O | CaO | TiO2 | MnO | Fe2O3 | ||||
| Bentonite (Na,Ca)x(Al,Mg)2Si4O10(OH)2.nH2O | 0.079 | 0.607 | 9.472 | 78.543 | 1.347 | 1.703 | 2.813 | 0.788 | 0.045 | 4.239 | 8.29 | 67.62 | 13.04 |
| BC500 | 0.058 | 0.760 | 9.426 | 78.914 | 1.308 | 1.693 | 2.750 | 0.805 | 0.044 | 4.021 | 8.37 | 47.21 | 4.98 |
| BC500A0.5 | ND | 0.664 | 8.982 | 83.001 | 1.301 | 0.963 | 0.825 | 0.758 | 0.024 | 3.342 | 9.24 | 44.15 | 8.69 |
| BA0.5 | ND | 0.505 | 7.705 | 85.236 | 1.279 | 0.838 | 0.530 | 0.740 | ND | 2.957 | 11.06 | 43.45 | 9.43 |
| BA0.5C500 | ND | 0.642 | 7.355 | 85.001 | 1.274 | 0.832 | 0.558 | 0.740 | 0.020 | 2.687 | 11.56 | 19.11 | 4.29 |
| Samples | d-spacing (Å) | ||||
|---|---|---|---|---|---|
| No Adsorption | Atrazine | Diuron | 2,4-D | Paraquat | |
| Bentonite | 14.02 | 14.59 | 14.01 | 14.21 | 12.27 |
| BC500 | 9.65 | 12.38 | 12.29 | 12.90 | 12.05 |
| BA0.5 | 13.00 | 13.71 | 12.47 | 13.36 | 12.16 |
| BC500 A0.5 | 12.08 | 12.76 | 12.17 | 12.54 | 12.38 |
| BA0.5C500 | 9.59 | 9.51 | 9.32 | 9.21 | 9.70 |
| Adsorbents | Weight Loss (%) | |
|---|---|---|
| 50–150 °C | 150–400 °C | |
| B | 7.83 | 2.50 |
| BA0.5 | 5.56 | 2.01 |
| BC500 | 1.03 | 1.41 |
| BA0.5C500 | 1.18 | 1.21 |
| BC500A0.5 | 4.59 | 2.54 |
| Main Characteristics | B | BC500 | BC500A0.5 | BA0.5 | BA0.5 C500 |
|---|---|---|---|---|---|
| Specific surface area (m2/g) | 31.76 | 32.19 | 67.86 | 77.12 | 80.09 |
| Pore surface area (m2g−1) | 30.901 | 31.301 | 55.354 | 64.704 | 69.154 |
| Pore volume (cm3 g−1) | 0.1246 | 0.1247 | 0.1750 | 0.2281 | 0.2456 |
| Zeta potential (mV)-pH 7 | −35.5 | −22.5 | −5.55 | −11.4 | 4.83 |
| Samples | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | n | R2 | |
| (mg g−1) | (L mg−1) | (mg g−1). (L mg−1)1/n | ||||
| B | 4.76 | 18.77 | 0.9972 | 6.69 | 1.81 | 0.8977 |
| BA0.5 | 1.75 | 100.39 | 0.9966 | 3.54 | 1.56 | 0.9266 |
| BC500 | 3.58 | 23.26 | 0.9983 | 5.12 | 1.71 | 0.9101 |
| BA0.5C500 | 0.85 | 52.63 | 0.9987 | 11.83 | 5.26 | 0.9726 |
| BC500A0.5 | 2.11 | 43.90 | 0.9986 | 2.44 | 1.43 | 0.8715 |
| Samples | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | n | R2 | |
| (mg g−1) | (L mg−1) | (mg g−1). (L mg−1)1/n | ||||
| B | 0.32 | 12.19 | 0.9968 | 1.26 | 4.32 | 0.9701 |
| BA0.5 | 0.31 | 14.74 | 0.9976 | 0.96 | 3.06 | 0.9906 |
| BC500 | 0.28 | 15.24 | 0.9972 | 1.17 | 3.06 | 0.9479 |
| BA0.5C500 | 0.39 | 16.57 | 0.9925 | 0.58 | 1.80 | 0.9842 |
| BC500A0.5 | 0.31 | 14.74 | 0.9986 | 1.22 | 3.70 | 0.9946 |
| Samples | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | n | R2 | |
| (mg g−1) | (L mg−1) | (mg g−1). (L mg−1)1/n | ||||
| B | 3.18 | 1.01 | 0.9970 | 0.38 | 2.52 | 0.9822 |
| BA0.5 | 6.37 | 0.52 | 0.9993 | 0.28 | 1.99 | 0.9563 |
| BC500 | 4.52 | 2.05 | 0.9920 | 0.36 | 2.38 | 0.9795 |
| BA0.5C500 | 5.22 | 0.53 | 0.9982 | 0.31 | 2.14 | 0.9651 |
| BC500A0.5 | 15.87 | 3.64 | 0.9970 | 0.31 | 2.04 | 0.9902 |
| Samples | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | n | R2 | |
| (mg g−1) | (L mg−1) | (mg g−1). (L mg−1)1/n | ||||
| B | 0.17 | 31.71 | 0.9820 | 0.39 | 1.87 | 0.9703 |
| BA0.5 | 0.88 | 8.01 | 0.9327 | 2.08 | 3.61 | 0.9668 |
| BC500 | 1.92 | 5.05 | 0.9541 | 4.01 | 5.08 | 0.8312 |
| BA0.5C500 | 1.24 | 7.41 | 0.9715 | 3.01 | 4.04 | 0.8983 |
| BC500A0.5 | 1.19 | 6.38 | 0.9262 | 2.82 | 4.55 | 0.8886 |
| Samples | Temperature (K) | Langmuir Isotherm | ||
|---|---|---|---|---|
| qm (mg g−1) | KL (M−1) | R2 | ||
| Bentonite | 303 | 3.38 | 6.15 | 0.9915 |
| 313 | 3.89 | 5.53 | 0.9964 | |
| 323 | 4.19 | 6.65 | 0.9990 | |
| BC500A0.5 | 303 | 16.64 | 452.14 | 0.9992 |
| 313 | 17.15 | 632.94 | 0.9995 | |
| 323 | 18.35 | 955.66 | 0.9986 | |
| Samples | Temperature (K) | Langmuir Isotherm | ||
|---|---|---|---|---|
| qm (mg g−1) | KL (M−1) | R2 | ||
| Bentonite | 303 | 5.12 | 0.83 | 0.9580 |
| 313 | 5.10 | 1.06 | 0.9920 | |
| 323 | 5.45 | 1.30 | 0.9608 | |
| BC500 | 303 | 10.34 | 11.85 | 0.8725 |
| 313 | 11.27 | 13.87 | 0.8531 | |
| 323 | 11.95 | 14.86 | 0.8935 | |
| Samples | Temperature (K) | Langmuir Isotherm | ||
|---|---|---|---|---|
| qm (mg g−1) | KL (M−1) | R2 | ||
| Bentonite | 303 | 4.07 | 1.29 | 0.9829 |
| 313 | 4.50 | 1.17 | 0.9618 | |
| 323 | 4.40 | 1.85 | 0.9643 | |
| BA0.5C500 | 303 | 7.37 | 2.66 | 0.9662 |
| 313 | 8.55 | 8.64 | 0.9990 | |
| 323 | 8.66 | 10.45 | 0.9981 | |
| Samples | Temperature (K) | Langmuir Isotherm | ||
|---|---|---|---|---|
| qm (mg g−1) | KL (M−1) | R2 | ||
| Bentonite | 303 | 94.34 | 480.83 | 0.9966 |
| 313 | 92.59 | 563.90 | 0.9970 | |
| 323 | 87.72 | 4023.82 | 0.9697 | |
| BC500 | 303 | 92.59 | 328.58 | 0.9959 |
| 313 | 84.03 | 1446.36 | 0.9671 | |
| 323 | 84.03 | 1446.36 | 0.9671 | |
| Pesticide | Absorbents | qm | pH | Reference |
|---|---|---|---|---|
| Atrazine | Zeolite-X | qm = 11.86 mg g−1 | Not adjust | [2] |
| Biochars (SBB) | qm = 3.05 mg g−1 | [46] | ||
| carbon nanotubes (MWCNTs-O) | qe = 17.35 mg g−1 | 6 | [9] | |
| Iron nanoparticles (INPs) | qm = 11.76 µg g−1 | 4.5 | [49] | |
| Combination with Heat 500 °C and activated 0.5 HCl on Bentonite (BC500A0.5) | qm= 15.87 mg g−1 | 6.5 | This work | |
| Diuron | Activated carbon | qm = 0.97 mmol.g−1 | [50] | |
| CTA-TixHy-montm | qe, exp = 3.08 mg g−1 | [45] | ||
| Heat treatment (500 °C) of bentonite (BC500) | qm = 1.92 mg g−1 | 6.5 | This work | |
| 2,4-D | Graphene (FGN) | qe = 19.95 mg g−1 | [51] | |
| Combination with Heat 500 °C and activated 0.5 HCl on Bentonite (BA0.5C500) | qm = 0.39 qe,exp = 6.25 mg g−1 | 6.5 | This work | |
| Paraquat | Treated diatomaceous earth | qm = 17.54 mg g−1 | [52] | |
| Bentonite | qm = 94.34 mg g−1 | 6.5 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pluangklang, C.; Rangsriwatananon, K. Facile Method by Bentonite Treated with Heat and Acid to Enhance Pesticide Adsorption. Appl. Sci. 2021, 11, 5147. https://doi.org/10.3390/app11115147
Pluangklang C, Rangsriwatananon K. Facile Method by Bentonite Treated with Heat and Acid to Enhance Pesticide Adsorption. Applied Sciences. 2021; 11(11):5147. https://doi.org/10.3390/app11115147
Chicago/Turabian StylePluangklang, Chutima, and Kunwadee Rangsriwatananon. 2021. "Facile Method by Bentonite Treated with Heat and Acid to Enhance Pesticide Adsorption" Applied Sciences 11, no. 11: 5147. https://doi.org/10.3390/app11115147





