Tissue Engineering 3D Porous Scaffolds Prepared from Electrospun Recombinant Human Collagen (RHC) Polypeptides/Chitosan Nanofibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of the Chitosan/RHC Nanofibers
2.3. Fabrication of the 3D Nanofibrous Scaffolds
2.4. Characterization
2.4.1. Morphology Analysis of the Nanofibers and Scaffolds
2.4.2. Open Porosity
2.4.3. Swelling Ability
2.4.4. Degradation
2.4.5. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4.6. Mechanical Properties
2.5. In Vitro Cell Studies
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Results and Discussion
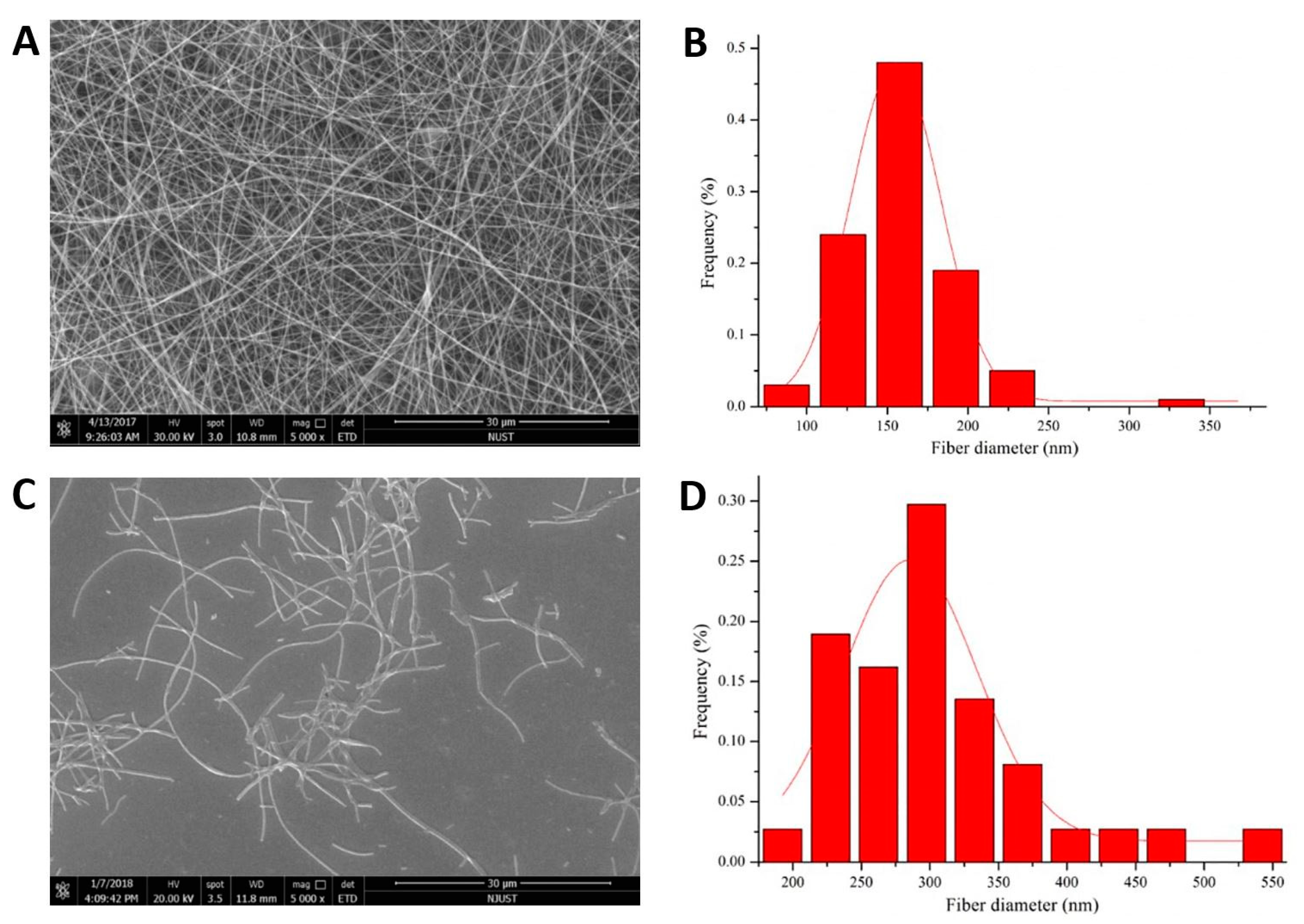
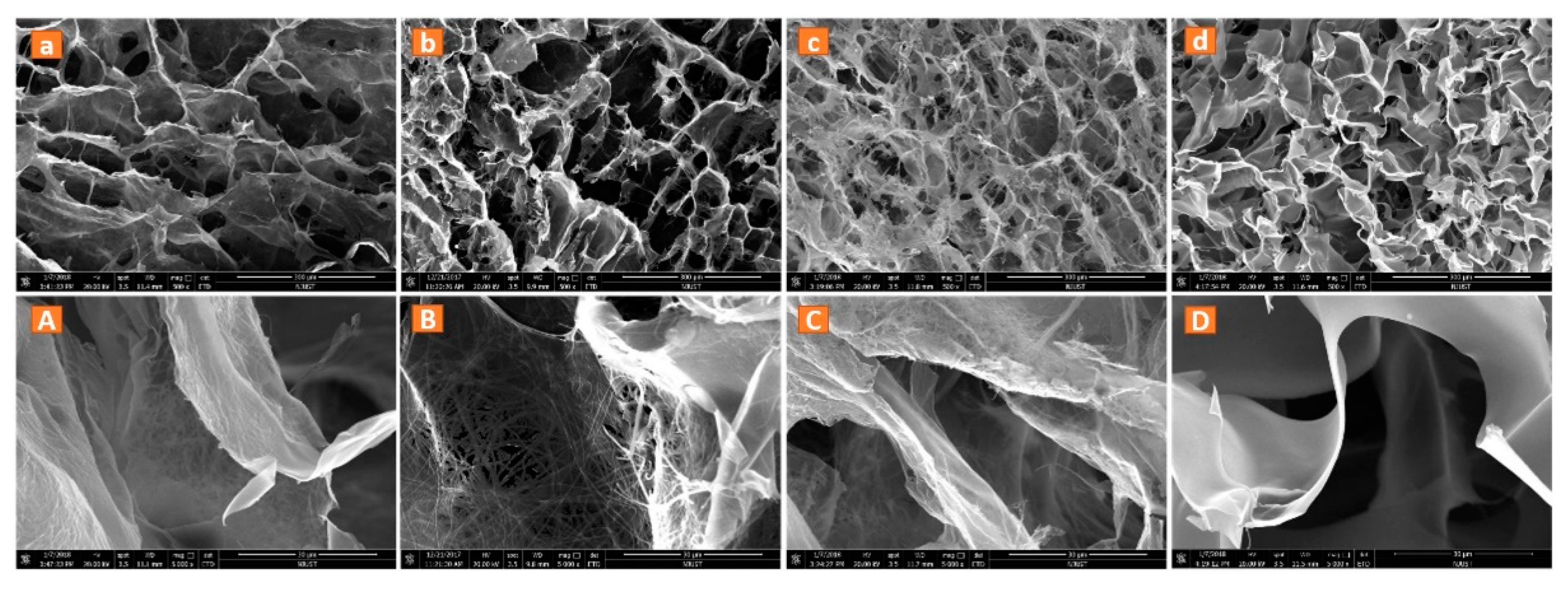
3.1. Structure of the Nanofibers and 3D Nanofibrous Scaffolds
3.2. Swelling Ability and Degradation
3.3. Fourier Transform Infrared Spectroscopy (FT-IR)
3.4. Mechanical Properties of the 3D Nanofibrous Scaffolds
3.5. Cell Proliferation and Morphology
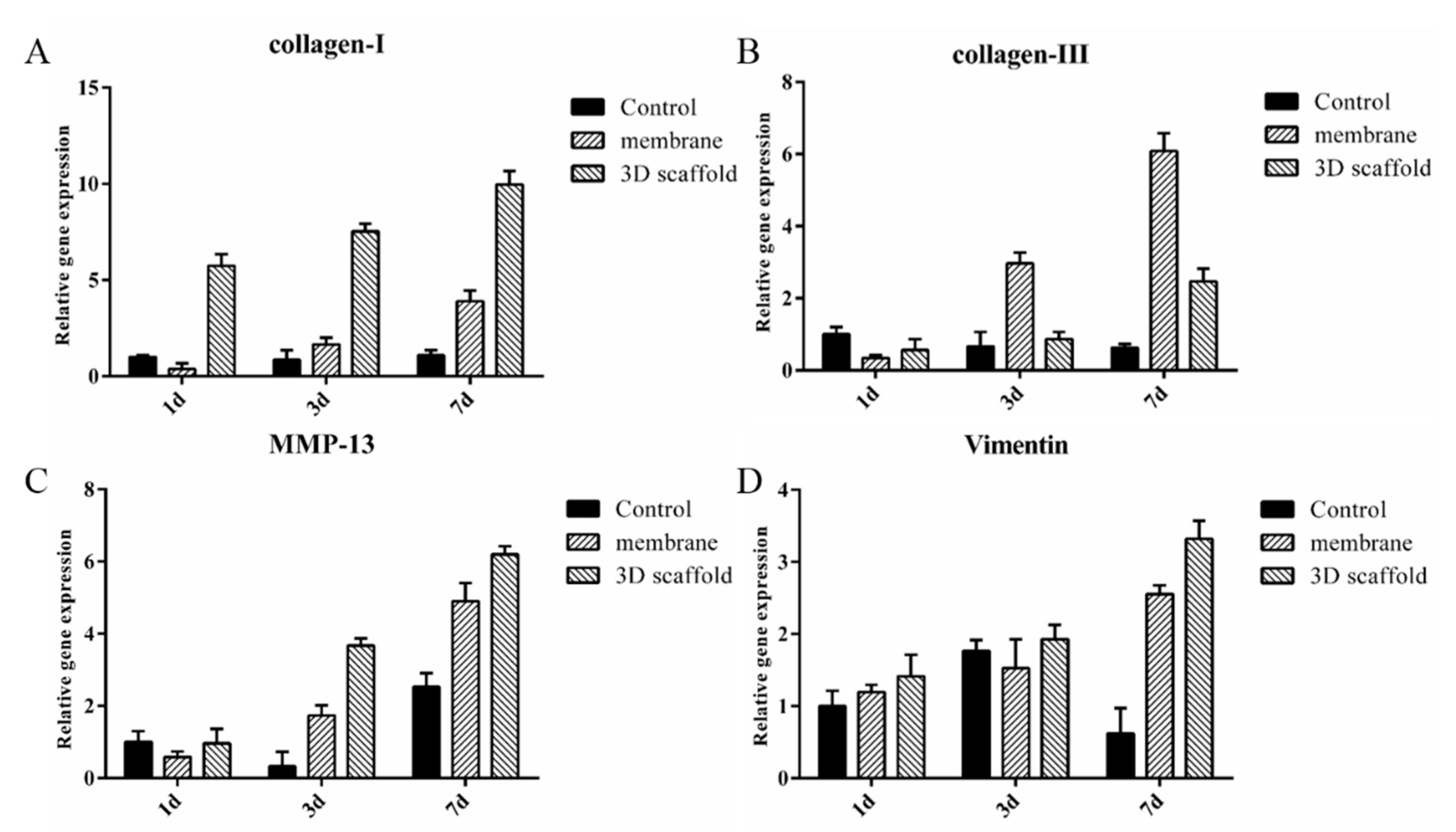
3.6. Gene Expression Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, M.; Khadim, R.R.; Nagayama, M.; Shinohara, M.; Inamura, K.; Danoy, M.; Nishikawa, M.; Furukawa, K.; Sakai, Y.; Niino, T. Fabrication of a Porous Three-Dimensional Scaffold with Interconnected Flow Channels: Co-Cultured Liver Cells and In Vitro Hemocompatibility Assessment. Appl. Sci. 2021, 11, 2473. [Google Scholar] [CrossRef]
- Yao, Q.Q.; Cosme, J.G.L.; Xu, T.; Miszuk, J.M.; Picciani, P.H.S.; Fong, H.; Sun, H.L. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials 2017, 115, 115–127. [Google Scholar] [CrossRef] [Green Version]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011. [Google Scholar] [CrossRef]
- Wang, X.; Ding, B.; Li, B. Biomimetic electrospun nanofibrous structures for tissue engineering. Mater. Today 2013, 16, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, S.; Singh, J.; Sehgal, S.S.; Sharma, N.K. Biomaterials for tissue engineered bone Scaffolds: A review. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Mao, Y.; Guidoin, R.; Li, Y.; Brochu, G.; Zhang, Z.; Wang, L. Soybean-derived phospholipids complexed poly (lactic-co-glycolic acid) nanofibrous scaffolds for tissue engineering applications. Mater. Des. 2021, 205, 109737. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Schubert, D.W.; Boccaccini, A.R.; Roether, J.A.; Santos, H.A. An organic-inorganic hybrid scaffold with honeycomb-like structures enabled by one-step self-assembly-driven electrospinning. Mater. Sci. Eng. C 2021, 124, 112079. [Google Scholar] [CrossRef] [PubMed]
- Bandegi, A.; Moghbeli, M.R. Effect of solvent quality and humidity on the porous formation and oil absorbency of SAN electrospun nanofibers. J. Appl. Polym. Sci. 2018, 135, 45586. [Google Scholar] [CrossRef]
- Bhowmick, S.; Scharnweber, D.; Koul, V. Co-cultivation of keratinocyte-human mesenchymal stem cell (hMSC) on sericin loaded electrospun nanofibrous composite scaffold (cationic gelatin/hyaluronan/chondroitin sulfate) stimulates epithelial differentiation in hMSCs: In vitro study. Biomaterials 2016, 88, 83–96. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhou, Y.; Wang, M. Three-dimensional endothelial cell incorporation within bioactive nanofibrous scaffolds through concurrent emulsion electrospinning and coaxial cell electrospraying. Acta Biomater. 2021, 123, 312–324. [Google Scholar] [CrossRef]
- Al-Kaabi, W.J.; Albukhaty, S.; Al-Fartos, Y.A.; Al-Karagoly, H.K.; Soliman, D.A. Development of Inula graveolens (L.) Plant Extract Electrospun/Polycaprolactone Nanofibers: A Novel Material for Biomedical Application. Appl. Sci. 2021, 11, 828. [Google Scholar] [CrossRef]
- Chen, H.; Peng, Y.; Wu, S.; Tan, L. Electrospun 3D Fibrous Scaffolds for Chronic Wound Repair. Materials 2016, 9, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahjour, S.B.; Sefat, F.; Polunin, Y.; Wang, L.C.; Wang, H.J. Improved cell infiltration of electrospun nanofiber mats for layered tissue constructs. J. Biomed. Mater. Res. Part A 2016, 104, 1479–1488. [Google Scholar] [CrossRef] [Green Version]
- Ameer, J.M.; Pr, A.K.; Kasoju, N. Strategies to Tune Electrospun Scaffold Porosity for Effective Cell Response in Tissue Engineering. J. Funct. Biomater. 2019, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Aghajanpoor, M.; Hashemi-Najafabadi, S.; Baghaban-Eslaminejad, M.; Bagheri, F.; Mousavi, S.M.; Sayyahpour, F.A. The effect of increasing the pore size of nanofibrous scaffolds on the osteogenic cell culture using a combination of sacrificial agent electrospinning and ultrasonication. J. Biomed. Mater. Res. Part A 2017, 105, 1887–1899. [Google Scholar] [CrossRef]
- Tornello, P.; Caracciolo, P.C.; Rosello, J.I.I.; Abraham, G.A. Electrospun scaffolds with enlarged pore size: Porosimetry analysis. Mater. Lett. 2018, 227, 191–193. [Google Scholar] [CrossRef]
- Gupte, M.J.; Swanson, W.B.; Hu, J.; Jin, X.; Ma, H.; Zhang, Z.; Liu, Z.; Feng, K.; Feng, G.; Xiao, G. Pore Size Directs Bone Marrow Stromal Cell Fate and Tissue Regeneration in Nanofibrous Macroporous Scaffolds by Mediating Vascularization. Acta Biomater. 2018, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, A.; Deng, A.; Yang, Y.; Gao, L.; Zhong, Z.; Yang, S. Pore architecture and cell viability on freeze dried 3D recombinant human collagen-peptide (RHC)–chitosan scaffolds. Mater. Sci. Eng. C 2015, 49, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Yan, X.; Yin, S.; Liu, L.; Liu, X.; Zhao, G.; Ma, W.; Qi, W.; Ren, Z.; Liao, H.; et al. Influence of the pore size and porosity of selective laser melted Ti6Al4V ELI porous scaffold on cell proliferation, osteogenesis and bone ingrowth. Mater. Sci. Eng. C 2020, 106, 110289. [Google Scholar] [CrossRef]
- Mochane, M.J.; Motsoeneng, T.S.; Sadiku, E.R.; Mokhena, T.C.; Sefadi, J.S. Morphology and Properties of Electrospun PCL and Its Composites for Medical Applications: A Mini Review. Appl. Sci. 2019, 9, 2205. [Google Scholar] [CrossRef] [Green Version]
- Zarei, M.; Samimi, A.; Khorram, M.; Abdi, M.M.; Golestaneh, S.I. Fabrication and characterization of conductive polypyrrole/chitosan/collagen electrospun nanofiber scaffold for tissue engineering application. Int. J. Biol. Macromol. 2021, 168, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Korpayev, S.; Kaygusuz, G.; En, M.; Orhan, K.; Karakeili, A. Chitosan/collagen based biomimetic osteochondral tissue constructs: A growth factor-free approach. Int. J. Biol. Macromol. 2020, 156, 681–690. [Google Scholar] [CrossRef]
- Yang, Y.; Campbell Ritchie, A.; Everitt, N.M. Recombinant human collagen/chitosan-based soft hydrogels as biomaterials for soft tissue engineering. Mater. Sci. Eng. C 2021, 121, 111846. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Zhang, J.; Xu, M.; Wang, L. Low-temperature 3D printing of collagen and chitosan composite for tissue engineering. Mater. Sci. Eng. C 2021, 123, 111963. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef] [Green Version]
- Fertala, A. Three Decades of Research on Recombinant Collagens: Reinventing the Wheel or Developing New Biomedical Products? Bioengineering 2020, 7, 155. [Google Scholar] [CrossRef]
- Deng, A.; Yang, Y.; Shimei, D.; Shulin, Y. Electrospinning of in situ crosslinked recombinant human collagen peptide/chitosan nanofibers for wound healing. Biomater. Sci. 2018, 6, 2197–2208. [Google Scholar] [CrossRef]
- Liu, B.; Lei, Y.T.; Zhang, J.; Hu, L.; Yang, S.L. Expression, Purification and Characterization of Recombinant Human Gelatin in Pichia pastoris. Adv. Mater. Res. 2011, 236–238, 2905–2912. [Google Scholar] [CrossRef]
- Oh, S.H.; Park, I.K.; Kim, J.M.; Lee, J.H. In vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 2007, 28, 1664–1671. [Google Scholar] [CrossRef]
- Nam, K.; Kimura, T.; Kishida, A. Controlling Coupling Reaction of EDC and NHS for Preparation of Collagen Gels Using Ethanol/Water Co-Solvents. Macromol. Biosci. 2007, 8, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.X.; Shen, Y.; Ao, P.; Dai, L.B.; Liu, Z.H.; Zhou, C.R. The improvement of hemostatic and wound healing property of chitosan by halloysite nanotubes. RSC Adv. 2014, 4, 23540–23553. [Google Scholar] [CrossRef]
- Gao, X.; Qin, W.; Wang, P.; Wang, L.; Weir, M.D.; Reynolds, M.A.; Zhao, L.; Lin, Z.; Xu, H.H.K. Nano-Structured Demineralized Human Dentin Matrix to Enhance Bone and Dental Repair and Regeneration. Appl. Sci. 2019, 9, 1013. [Google Scholar] [CrossRef] [Green Version]
- Winter, G.D. Transcutaneous implants: Reactions of the skin-implant interface. J. Biomed. Mater. Res. 1974, 8, 99–113. [Google Scholar] [CrossRef]
- Chen, W.M.; Ma, J.; Zhu, L.; Morsi, Y.; Ei-Hamshary, H.; Al-Deyab, S.S.; Mo, X.M. Superelastic, superabsorbent and 3D nanofiber-assembled scaffold for tissue engineering. Colloids Surf. B-Biointerfaces 2016, 142, 165–172. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, X.; Li, G.; Wang, P.; Yang, Y. Tailoring degradation rates of silk fibroin scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 104–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staroszczyk, H.; Sztuka, K.; Wolska, J.; Wojtasz-Pająk, A.; Kołodziejska, I. Interactions of fish gelatin and chitosan in uncrosslinked and crosslinked with EDC films: FT-IR study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 707–712. [Google Scholar] [CrossRef]
- Harley, B.A.; Leung, J.H.; Silva, E.C.C.M.; Gibson, L.J. Mechanical characterization of collagen–glycosaminoglycan scaffolds. Acta Biomater. 2007, 3, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ritchie, A.C.; Everitt, N.M. Comparison of glutaraldehyde and procyanidin cross-linked scaffolds for soft tissue engineering. Mater. Sci. Eng. C 2017, 80, 263–273. [Google Scholar] [CrossRef]
- Alberti, T.B.; Coelho, D.S.; Pra, M.; Maraschin, M.; Veleirinho, B. Electrospun PVA nanoscaffolds associated with propolis nanoparticles with wound healing activity. J. Mater. Sci. 2020, 55, 9712–9727. [Google Scholar] [CrossRef]
- Naskar, D.; Ghosh, A.K.; Mandal, M.; Das, P.; Nandi, S.K.; Kundu, S.C. Dual growth factor loaded nonmulberry silk fibroin/carbon nanofiber composite 3D scaffolds for in vitro and in vivo bone regeneration. Biomaterials 2017, 136, 67–85. [Google Scholar] [CrossRef]
- Ryoo, S.R.; Kim, Y.K.; Kim, M.H.; Min, D.H. Behaviors of NIH-3T3 Fibroblasts on Graphene/Carbon Nanotubes: Proliferation, Focal Adhesion, and Gene Transfection Studies. ACS Nano 2010, 4, 6587–6598. [Google Scholar] [CrossRef] [PubMed]
- Zubair, A.R.; Shahrom, A.W.; Swarhib, M.; Nurliza, A. Determination of age of skin wound by measuring collagen type I and III using picrosirius polarization method. Int. J. Med. Toxicol. Leg. Med. 2018, 21, 1. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Wolter, W.R.; Anderson, B.; Schroeder, V.A.; Gao, M.; Gooyit, M.; Suckow, M.A.; Chang, M. Limitations of Knockout Mice and Other Tools in Assessment of the Involvement of Matrix Metalloproteinases in Wound Healing and the Means to Overcome Them. ACS Pharmacol. Transl. Sci. 2020, 3, 489–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, S.D.; Farrugia, B.L.; Dargaville, T.R.; Dhara, S. Chitosan-collagen scaffolds with nano/microfibrous architecture for skin tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 3482–3492. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, A.; Yang, Y.; Du, S. Tissue Engineering 3D Porous Scaffolds Prepared from Electrospun Recombinant Human Collagen (RHC) Polypeptides/Chitosan Nanofibers. Appl. Sci. 2021, 11, 5096. https://doi.org/10.3390/app11115096
Deng A, Yang Y, Du S. Tissue Engineering 3D Porous Scaffolds Prepared from Electrospun Recombinant Human Collagen (RHC) Polypeptides/Chitosan Nanofibers. Applied Sciences. 2021; 11(11):5096. https://doi.org/10.3390/app11115096
Chicago/Turabian StyleDeng, Aipeng, Yang Yang, and Shimei Du. 2021. "Tissue Engineering 3D Porous Scaffolds Prepared from Electrospun Recombinant Human Collagen (RHC) Polypeptides/Chitosan Nanofibers" Applied Sciences 11, no. 11: 5096. https://doi.org/10.3390/app11115096




