Effect of Sucrose on Physicochemical Properties of High-Protein Meringues Obtained from Whey Protein Isolate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Production of Meringues
2.3. Viscosity, Surface Tension and Zeta Potential of Pre-Foam Solutions
2.4. Foam Overrun
2.5. Oscillatory Rheology of the Liquid Foams
2.6. Foam Stability Measured by Turbiscan
2.7. Surface Properties of the Obtained Meringues (Roughness, Contact Angles, Apparent Free Surface Energy and Microstructure)
2.8. Statistical Analysis
3. Results and Discussion
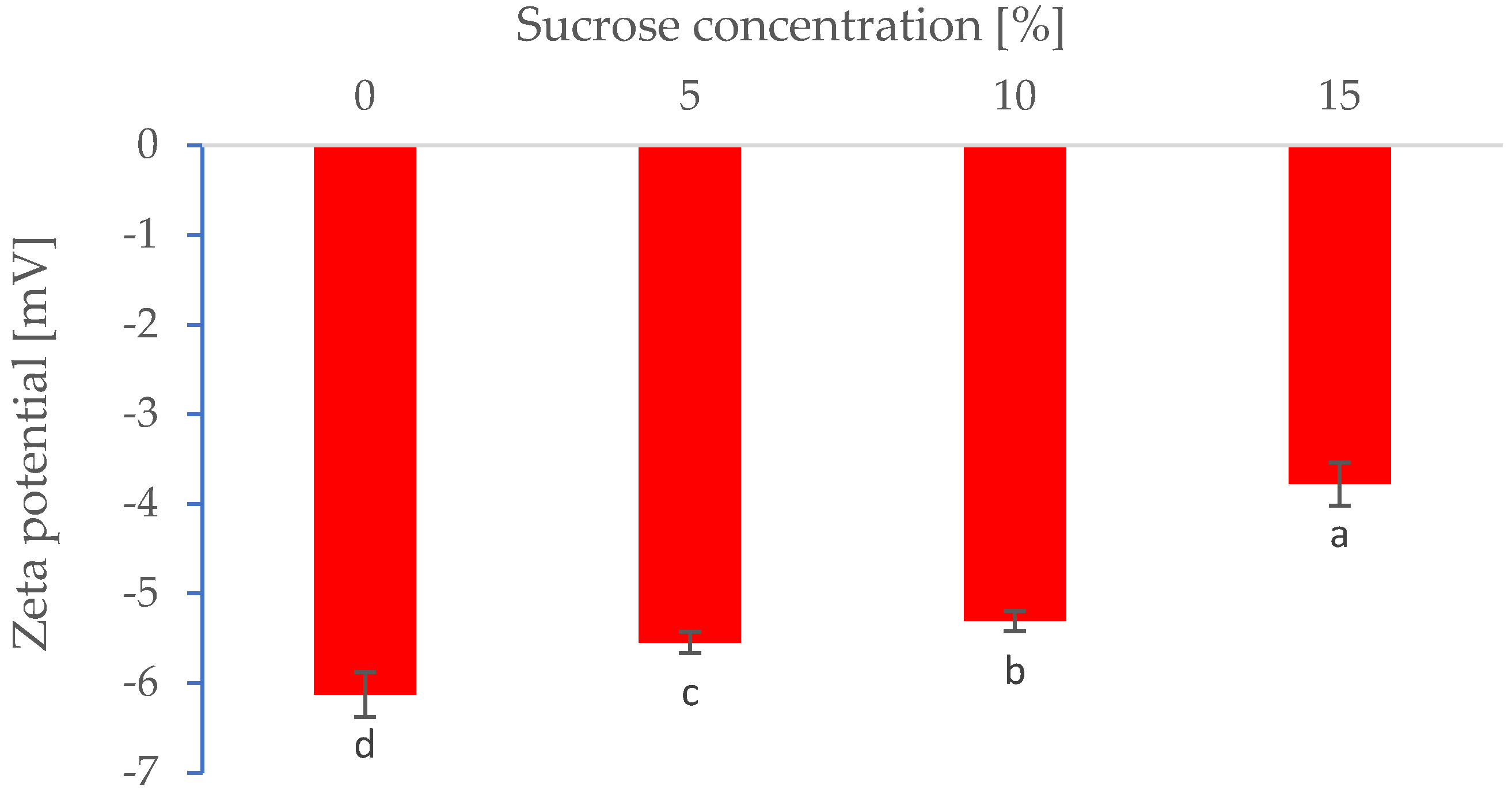
3.1. Properties of Pre-Foam Solutions
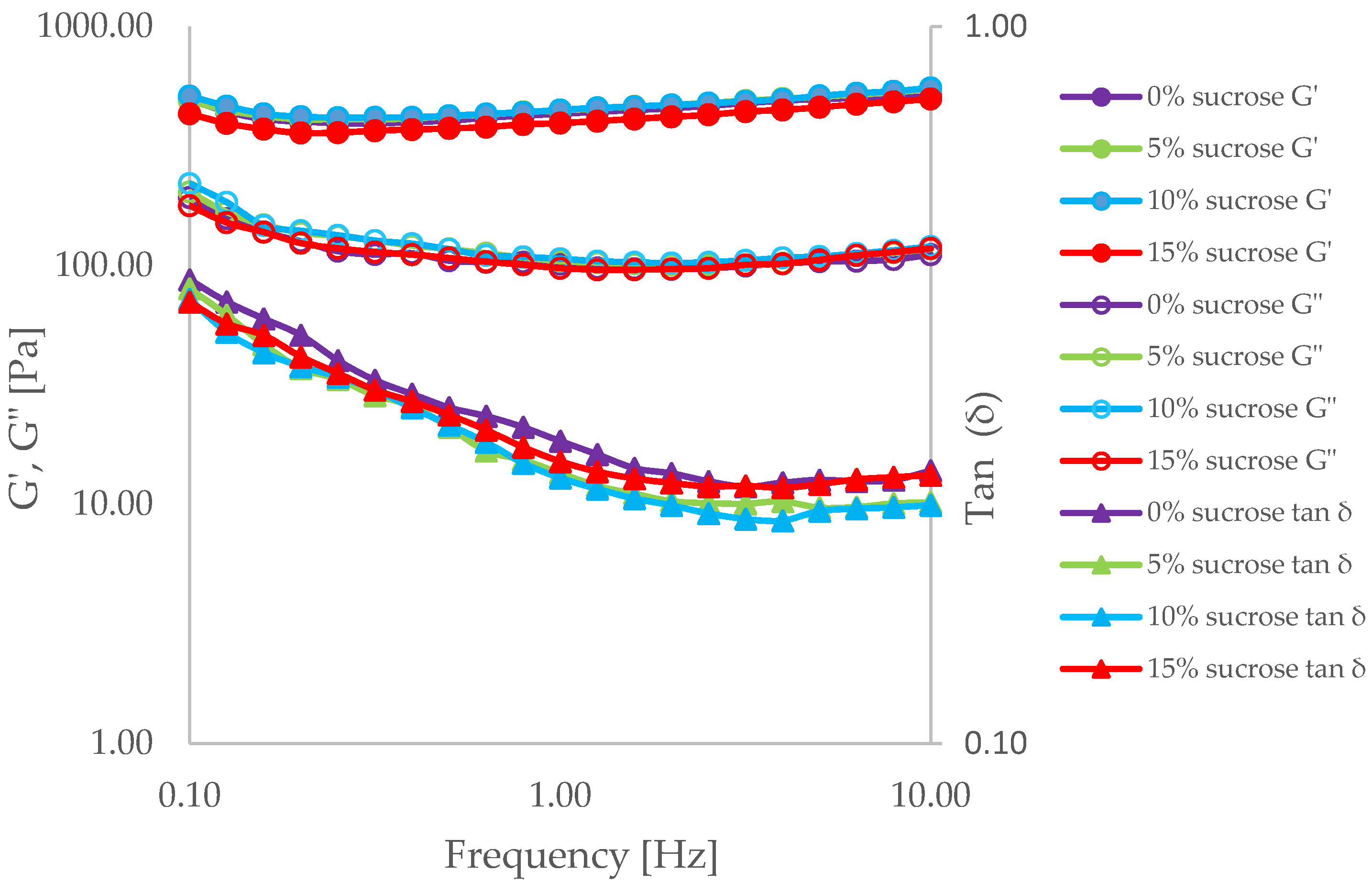
3.2. Overrun, Air Phase Fraction and Rheology of Liquid Foams
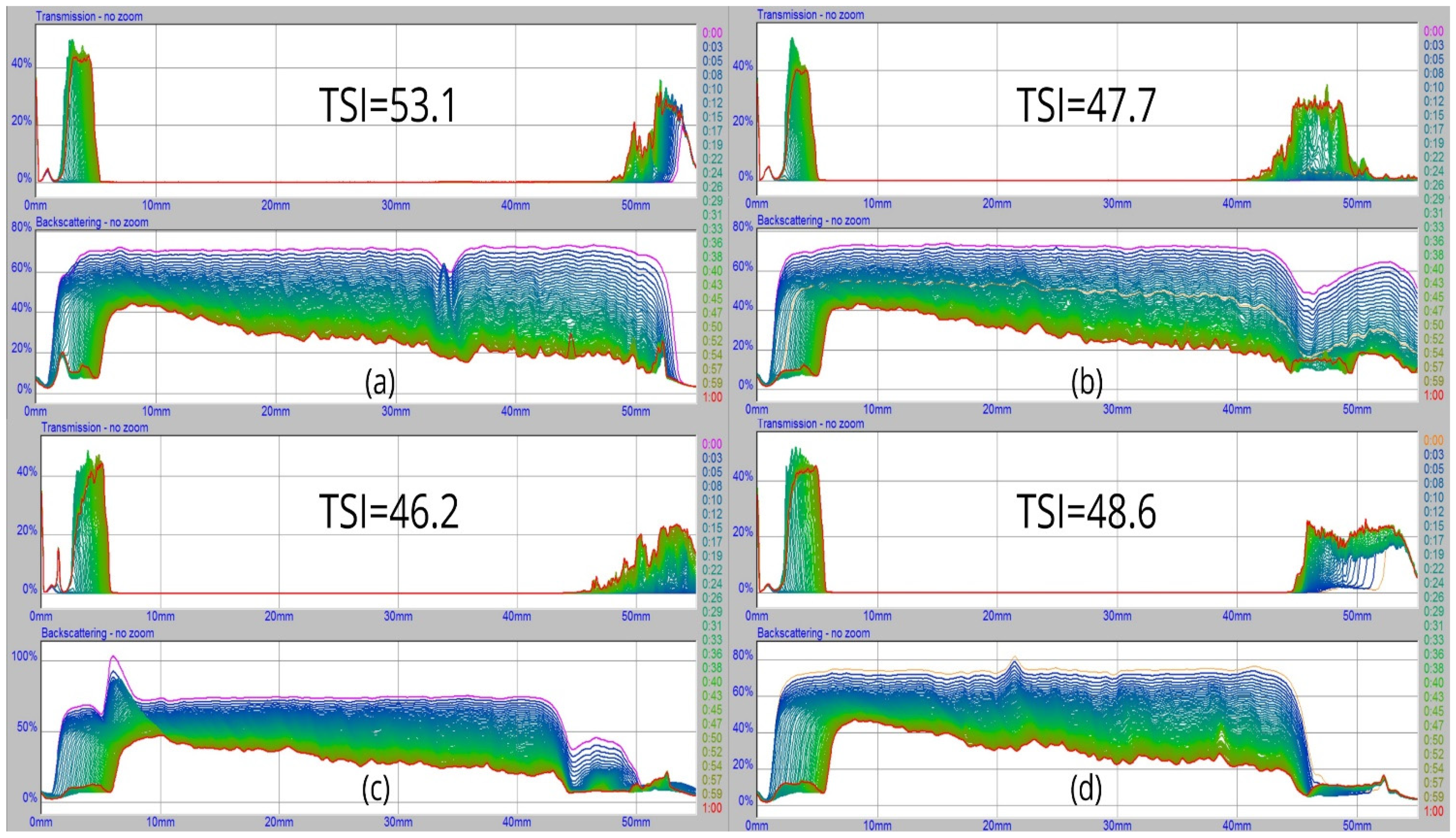
3.3. Foam Stability
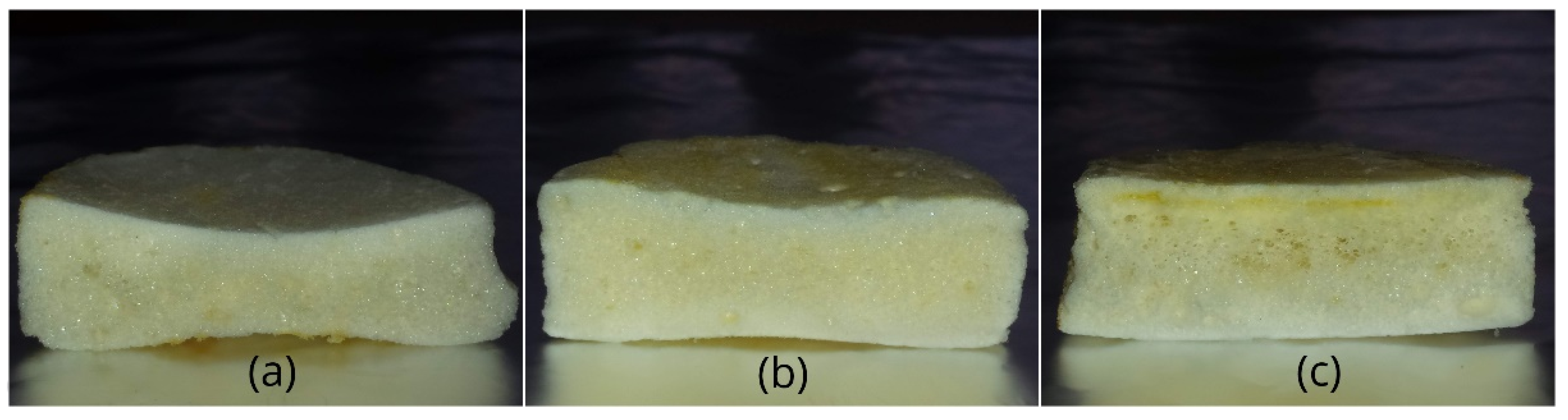
3.4. Surface Properties of Meringues
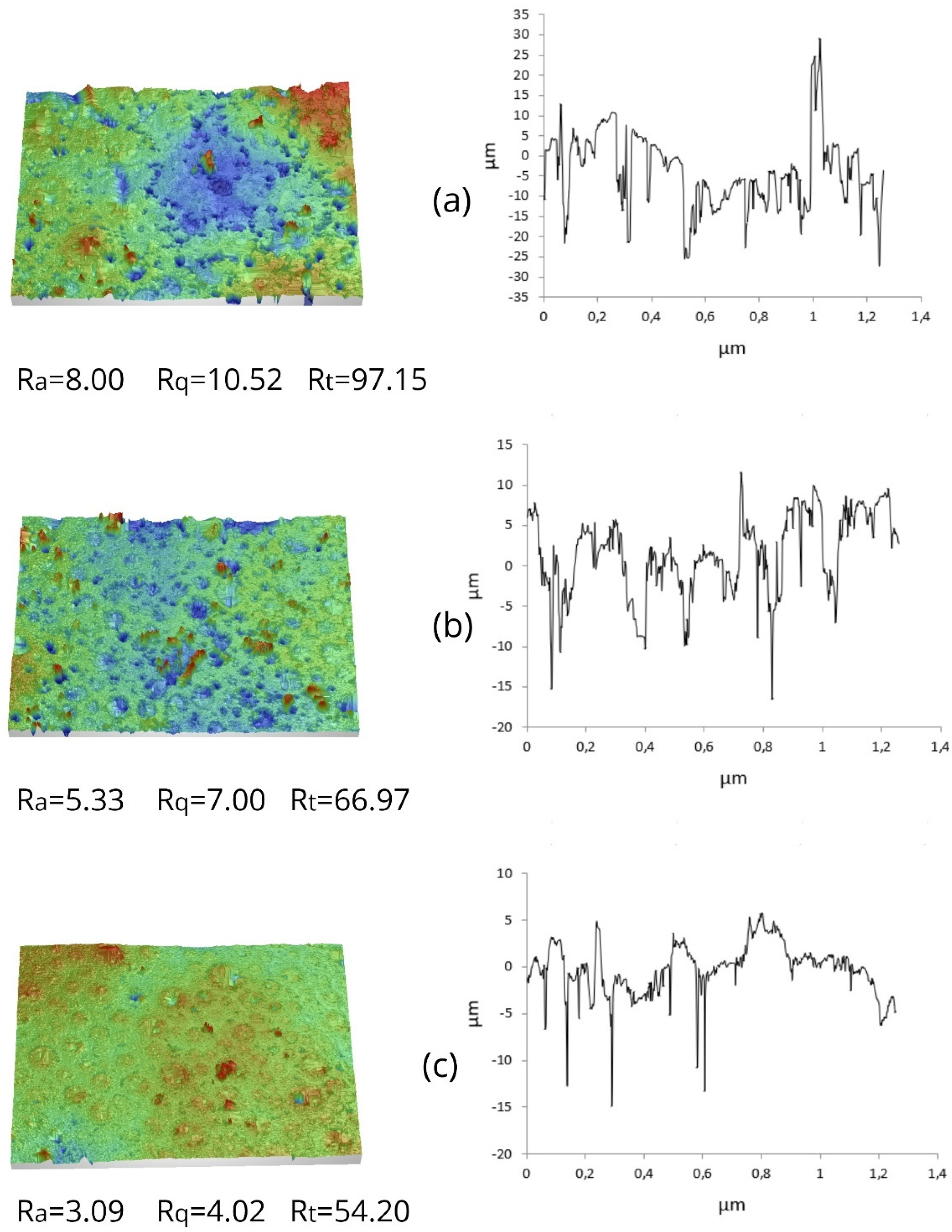
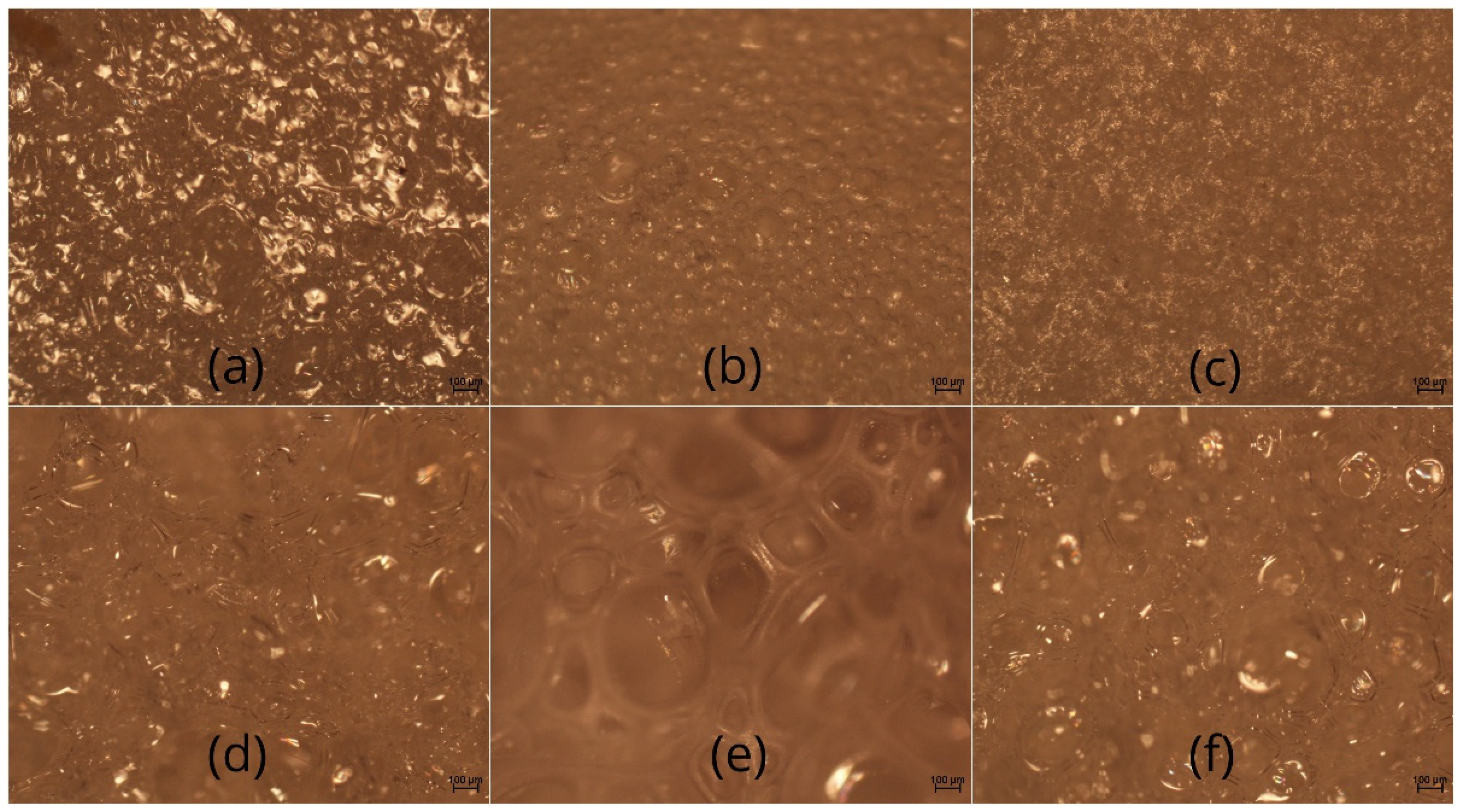
3.5. Microstructure of Meringues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nastaj, M.; Sołowiej, B.G. Effect of various pH values on foaming properties of whey protein preparations. Int. J. Dairy Technol. 2020, 73, 683–694. [Google Scholar] [CrossRef]
- Campbell, G.M.; Mougeot, E. Creation and characterization of aerated food products. Trends Food Sci.Technol. 1999, 10, 283–296. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, F.; Huang, W.; Tang, X.; Zou, Q.; Li, Z.; Ogawa, A. Sucrose substitution by polyols in sponge cake and their effects on the foaming and thermal properties of egg protein. Food Hydrocoll. 2016, 57, 153–159. [Google Scholar] [CrossRef]
- Licciardello, F.; Frisullo, P.; Laverse, J.; Muratore, G.; Del Nobile, M.A. Effect of sugar, citric acid and egg white type on the microstructural and mechanical properties of meringues. J. Food Eng. 2012, 108, 453–462. [Google Scholar] [CrossRef]
- Berry, T.K.; Yang, X.; Foegeding, E.A. Foams prepared from whey protein isolate and egg white protein: 1. Changes associated with angel food cake functionality. J. Food Sci. 2009, 74, 269–277. [Google Scholar] [CrossRef]
- McGee, H. On Food and Cooking: The Science and Lore of the Kitchen; MacMillian: New York, NY, USA, 1984; ISBN 1-4165-5637-0. [Google Scholar]
- Di Monaco, R.; Miele, N.A.; Cabisidan, E.K.; Cavella, S. Strategies to reduce sugars in food. Curr. Opin. Food Sci. 2018, 19, 92–97. [Google Scholar] [CrossRef]
- Nastaj, M.; Sołowiej, B.G.; Terpiłowski, K.; Mleko, S. Effect of erythritol on physicochemical properties of reformulated high protein meringues obtained from whey protein isolate. Int. Dairy J. 2020, 105, 104672. [Google Scholar] [CrossRef]
- Mleko, S.; Kristinsson, H.G.; Liang, Y.; Gustaw, W. Rheological properties of foams generated from egg albumin after pH treatment. LWT Food Sci. Technol. 2007, 40, 908–914. [Google Scholar] [CrossRef]
- Mine, Y. Recent advances in understanding of egg white protein functionality. Trends Food Sci. Technol. 1995, 6, 225–232. [Google Scholar] [CrossRef]
- Raikos, V.; Campbell, L.; Euston, S.R. Effects of sucrose and sodium chloride on foaming properties of egg white proteins. Food Res. Int. 2007, 40, 347–355. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Luck, P.J.; Davis, J.P. Factors determining the physical properties of protein foams. Food Hydrocoll. 2006, 20, 284–292. [Google Scholar] [CrossRef]
- Peram, M.R.; Loveday, S.M.; Ye, A.; Singh, H. In vitro gastric digestion of heat-induced aggregates of b-lactoglobulin. J. Dairy Sci. 2013, 96, 63–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, M.; Han, Y.; Ahn, K. The influence of the time and temperature of heat treatment on the allergenicity of egg white proteins. Allergy Asthma Immunol. Res. 2013, 5, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Geveke, D.J.; Jones, D.R.; Tilman, E.D. Can acceptable quality angel food cakes be made using pasteurized shell eggs? The effects of mixing factors on functional properties of angel food cakes. Food Sci. Nutr. 2020, 7, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Nastaj, M.; Sołowiej, B.G.; Gustaw, W.; Perez-Huertas, S.; Mleko, S.; Wesołowska-Trojanowska, M. Physicochemical properties of High-Protein-Set Yoghurts obtained with the addition of whey protein preparations. Int. J. Dairy Technol. 2019, 72, 395–402. [Google Scholar] [CrossRef]
- Nastaj, M.; Terpiłowski, K.; Sołowiej, B.G. The effect of native and polymerised whey protein isolate addition on surface and microstructural properties of processed cheeses and their meltability determined by Turbiscan. Int. J. Food Sci. Technol. 2020, 55, 2179–2187. [Google Scholar] [CrossRef]
- Szafrańska, J.O.; Sołowiej, B.G. Cheese sauces: Characteristics of ingredients, manufacturing methods, microbiological and sensory aspects. J. Food Process Eng. 2020, 43, e13364. [Google Scholar] [CrossRef]
- Smithers, G.W. Whey and whey proteins—From ‘gutter-to-gold’. Int. Dairy J. 2008, 18, 695–704. [Google Scholar] [CrossRef]
- Yang, X.; Foegeding, E.A. The stability and physical properties of egg white and whey protein foams explained based on microstructure and interfacial properties. Food Hydrocoll. 2011, 25, 1687–1701. [Google Scholar] [CrossRef]
- Díaz-Ramírez, M.; Calderón-Domínguez, G.; García-Garibay, M.; Jiménez-Guzmán, J.; Villanueva-Carvajal, A.; de la Paz Salgado-Cruz, M.; Arizmendi-Cotero, D.; Del Moral-Ramírez, E. Effect of whey protein isolate addition on physical, structural and sensory properties of sponge cake. Food Hydrocoll. 2016, 61, 233–239. [Google Scholar] [CrossRef]
- Nastaj, M.; Sołowiej, B.G.; Gustaw, W. Physicochemical properties of high protein meringues made from different whey protein preparations. Żywność Nauka Technol. Jakość 2014, 2, 33–47. [Google Scholar]
- Inoue, K.; Fu, W.; Nakamura, T. Explaining the different textures of commercial processed cheese from fractured structures. Int. Dairy J. 2019, 97, 40–48. [Google Scholar] [CrossRef]
- Magens, O.M.; Liu, Y.; Hofmans, J.F.A.; Nelissen, J.A.; Wilson, I.D. Adhesion and cleaning of foods with complex structure: Effect of oil content and fluoropolymer coating characteristics on the detachment of cake from baking surfaces. J. Food Eng. 2017, 197, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Razi, S.M.; Motamedzadegan, A.; Shahidi, A.; Rashidinejad, A. The effect of basil seed gum (BSG) on the rheological and physicochemical properties of heat-induced egg albumin gels. Food Hydrocoll. 2018, 82, 268–277. [Google Scholar] [CrossRef]
- Lau, C.K.; Dickinson, E. Instability and structural change in an aerated system containing egg albumen and invert sugar. Food Hydrocoll. 2015, 19, 111–121. [Google Scholar] [CrossRef]
- Davis, J.P.; Foegeding, E.A. Comparisons of the foaming and interfacial properties of whey protein isolate and egg white proteins. Colloids Surf. B 2007, 54, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Belyakova, L.E.; Antipova, A.S.; Semenova, M.G.; Dickinson, E.; Merino, L.; Tsapkina, E.N. Effect of sucrose on molecular and interaction parameters of sodium caseinate in aqueous solution: Relationship to protein gelation. Colloids Surf. B 2003, 31, 31–46. [Google Scholar] [CrossRef]
- Cano-Sarmiento, C.; Téllez-Medina, D.I.; Viveros-Contreras, R.; Cornejo-Mazón, M.; Figueroa-Hernández, C.Y.; García-Armenta, E.; Alamilla-Beltrán, L.; García, H.S.; Gutiérrez-López, G.F. Zeta Potential of Food Matrices. Food Eng. Rev. 2018, 10, 113–138. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and zeta potential—What they are and what they are not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Foegeding, E.A. Effects of sucrose on egg white protein and whey protein isolate foams: Factors determining properties of wet and dry foams (cakes). Food Hydrocoll. 2010, 24, 227–238. [Google Scholar] [CrossRef]
- Tabilo-Munizaga, G.; Barbosa-Canovas, G.V. Rheology for the food industry. J. Food Eng. 2005, 67, 147–156. [Google Scholar] [CrossRef]
- Poole, R.J. The Deborah and Weissenberg numbers. Br. Soc. Rheol. Rheol. Bull. 2012, 53, 32–39. [Google Scholar]
- Luck, P.J.; Bray, N.; Foegeding, E.A. Factors determining yield stress and overrun of whey protein foams. J. Food Sci. 2006, 65, 1677–1681. [Google Scholar] [CrossRef]
- Thakur, R.K.; Vial, C.; Djelveh, G. Effect of composition and process parameters on elasticity and solidity of foamed food. Chem. Eng. Process. 2008, 47, 474–483. [Google Scholar] [CrossRef]
- Sadahira, M.S.; Rodrigues, M.I.; Akhtar, M.; Murray, B.S.; Netto, F.M. Effect of egg white protein-pectin electrostatic interactions in a high sugar content system on foaming and foam rheological properties. Food Hydrocoll. 2016, 58, 1–10. [Google Scholar] [CrossRef]
- Martínez-Padilla, L.P.; García-Mena, V.; Casas-Alencáster, N.B.; Sosa-Herrera, M.G. Foaming properties of skim milk powder fortified with milk proteins. Int. Dairy J. 2014, 36, 21–28. [Google Scholar] [CrossRef]
- Rodríguez Patino, J.M.; Naranjo Delgado, M.D.; Linares Fernández, J.A. Stability and mechanical strength of aqueous foams containing food proteins. Colloids Surf. A 1995, 99, 65–78. [Google Scholar] [CrossRef]
- Yankov, S.; Panchev, I. Foaming properties of sugar-egg mixtures with milk protein concentrates. Food Res. Int. 1996, 29, 521–525. [Google Scholar] [CrossRef]
- Arunepanlop, B.; Morr, C.V.; Karleskind, D.; Laye, I. Partial replacement of egg white proteins with whey proteins in angel food cakes. J. Food Sci. 1996, 61, 1085–1093. [Google Scholar] [CrossRef]
- Mensink, M.A.; Frijlink, H.W.; Maarschalk, K.V.; Hinrichs, W.L.J. How sugars protect proteins in the solid state and during drying (review): Mechanisms of stabilization in relation to stress conditions. Eur. J. Pharm. Biopharm. 2017, 114, 288–295. [Google Scholar] [CrossRef]
- Terpiłowski, K.; Rymuszka, D.; Goncharuk, O.V.; Sulym, I.Y.; Gun’ko, V.M. Wettability of modified silica layers deposited on glass support activated by plasma. Appl. Surf. Sci. 2015, 353, 843–850. [Google Scholar] [CrossRef]
- Drelich, J.W.; Boinovich, L.; Chibowski, E.; Della Volpe, C.; Marmur, A.; Siboni, S. Contact angles: History of over 200 years of open questions. Surf. Innov. 2020, 8, 3–27. [Google Scholar] [CrossRef] [Green Version]
- Wilderjans, E.; Luyts, A.; Brijs, K.; Delcour, J.A. Ingredient functionality in batter type cake making. Trends Food Sci. Technol. 2013, 30, 6–15. [Google Scholar] [CrossRef]
- Rodríguez Patino, J.M.; Rodríguez Niño, M.R.; Sánchez, C.C.; Navarro García, J.M.; Rodríguez Mateo, G.R.; Cejudo Fernández, M. The effect of temperature on food emulsifiers at fluid–fluid interfaces. Colloids Surf. B 2001, 21, 87–99. [Google Scholar] [CrossRef]

| Sample | γ (mN/m2) |
|---|---|
| 20% WPI/0% sucrose | 44.68 c ± 0.02 |
| 20% WPI/5% sucrose | 48.83 b ± 0.26 |
| 20% WPI/10% sucrose | 49.79 a ± 0.19 |
| 20% WPI/15% sucrose | 49.67 a ± 0.14 |
| Sample | OR (%) | Φ |
|---|---|---|
| 20% WPI/0% sucrose | 661.82 c ± 8.44 | 0.867 c ± 0.001 |
| 20% WPI/5% sucrose | 699.13 b ± 7.63 | 0.874 b ± 0.001 |
| 20% WPI/10% sucrose | 724.21 a ± 6.76 | 0.878 a ± 0.001 |
| 20% WPI/15% sucrose | 642.05 d ± 5.16 | 0.864 d ± 0.001 |
| Sample | Probe Liquid | Contact θa | Angles θr | γs (mJ/m2) |
|---|---|---|---|---|
| 20% WPI/5% sucrose | Water | 120.6 a ± 8.9 | 110.1 a ± 8.0 | 33.4 c ± 1.2 |
| 20% WPI/5% sucrose | Diiodomethane | 74.8 a ± 4.6 | 68.3 a ± 2.4 | 21.2 c ± 3.9 |
| 20% WPI/10% sucrose | Water | 83.2 b ± 5.5 | 65.6 b ± 4.2 | 46.4 b ± 2.8 |
| 20% WPI/10% sucrose | Diiodomethane | 52.7 b ± 5.0 | 45.5 b ± 2.8 | 42.0 b ± 1.0 |
| 20% WPI/15% sucrose | Water | 63.1 c ± 5.1 | 35.9 c ± 3.2 | 60.1 a ± 1.6 |
| 20% WPI/15% sucrose | Diiodomethane | 51.5 b ± 3.3 | 43.8 b ± 2.7 | 42.4 b ± 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nastaj, M.; Mleko, S.; Terpiłowski, K.; Tomczyńska-Mleko, M. Effect of Sucrose on Physicochemical Properties of High-Protein Meringues Obtained from Whey Protein Isolate. Appl. Sci. 2021, 11, 4764. https://doi.org/10.3390/app11114764
Nastaj M, Mleko S, Terpiłowski K, Tomczyńska-Mleko M. Effect of Sucrose on Physicochemical Properties of High-Protein Meringues Obtained from Whey Protein Isolate. Applied Sciences. 2021; 11(11):4764. https://doi.org/10.3390/app11114764
Chicago/Turabian StyleNastaj, Maciej, Stanisław Mleko, Konrad Terpiłowski, and Marta Tomczyńska-Mleko. 2021. "Effect of Sucrose on Physicochemical Properties of High-Protein Meringues Obtained from Whey Protein Isolate" Applied Sciences 11, no. 11: 4764. https://doi.org/10.3390/app11114764







