Thermodynamic Study on the Dissociation and Complexation of Coumarinic Acid with Neodymium(III) and Dioxouranium(VI) in Aqueous Media
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Potentiometry and Spectrophotometry
2.3. Synthesis of the Complexes
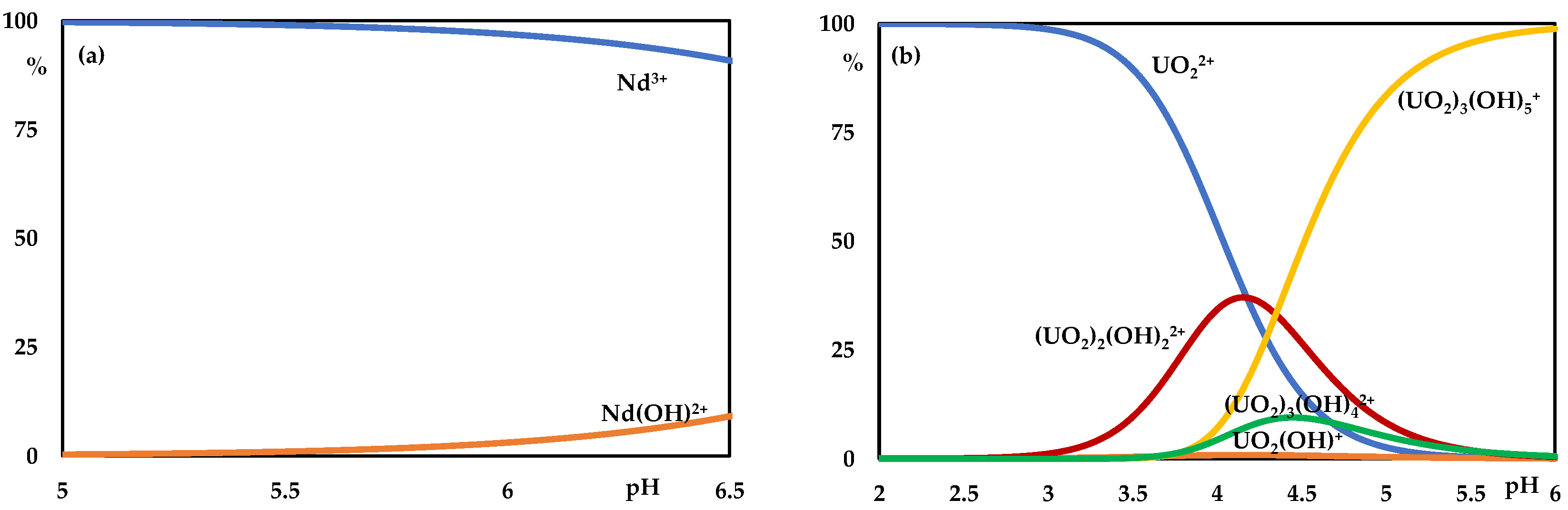
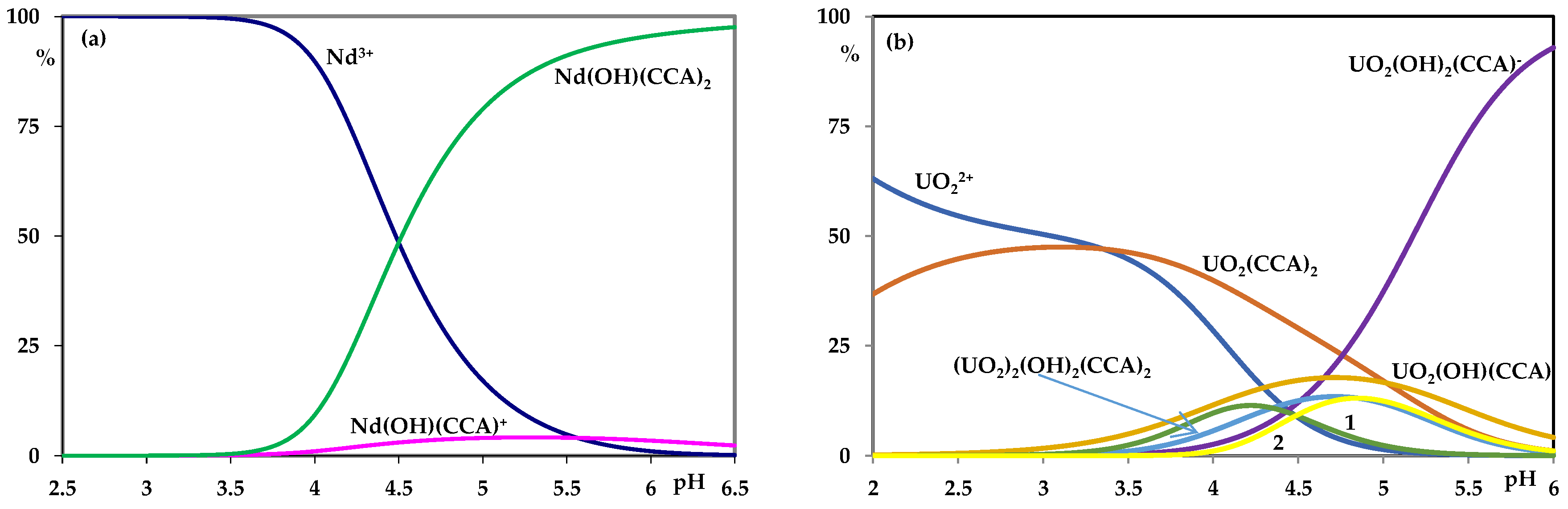
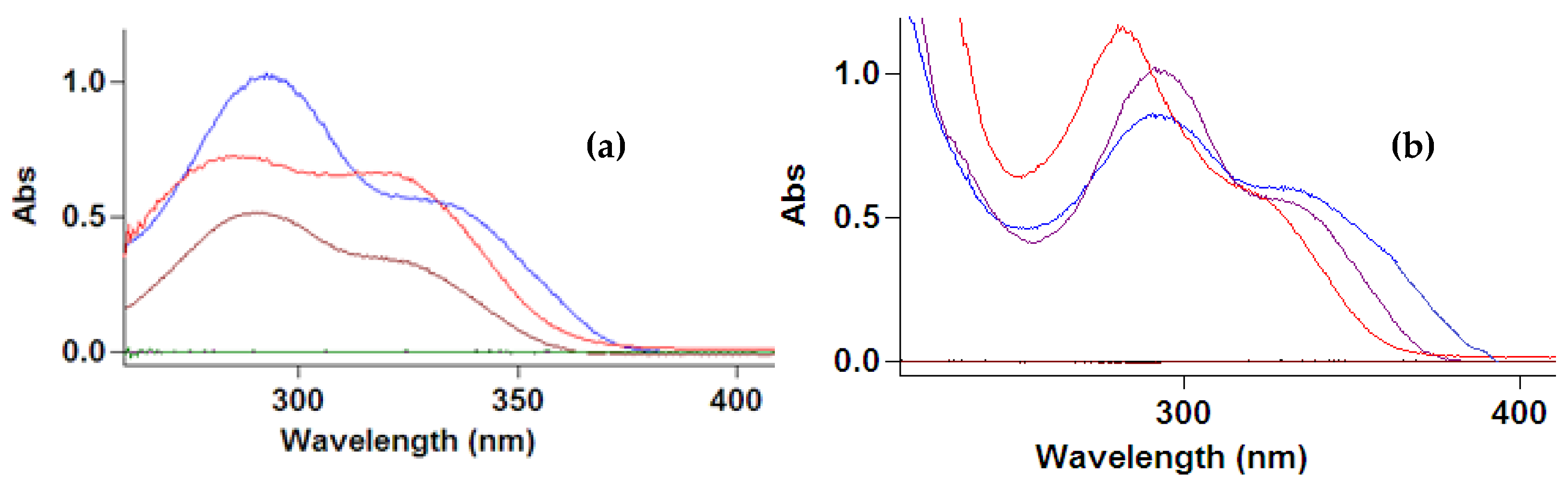
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malacaria, L.; Corrente, G.A.; Beneduci, A.; Furia, E.; Marino, T.; Mazzone, G. A review on coordination properties of Al(III) and Fe(III) towards natural antioxidant molecules: Experimental and theoretical insights. Molecules 2021, 26, 2603. [Google Scholar] [CrossRef] [PubMed]
- Ritacca, A.G.; Malacaria, L.; Algieri, V.; De Nino, A.; Russo, N.; Furia, E.; Maiuolo, L.; Sicilia, E. Sequestering ability of a synthetic chelating agent towards copper(II) and iron(III): A detailed theoretical and experimental analysis. Chem. Asian J. 2020, 15, 3266–3274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, B.; Yang, X.; Zhuo, L.; ·Mu, W.; Chen, Y.; ·Yang, Y.; ·Wei, H.; Li, X. Complexation of 1,3 Diamino 2 hydroxypropane N,N,N’,N’ tetraacetic Acid (DHPTA) with Heavy Lanthanides (Tb3+, Ho3+, Lu3+) in Aqueous Solution. J. Sol. Chem. 2020, 49, 166–178. [Google Scholar] [CrossRef]
- Karaliota, A.; Kretsi, O.; Tzougraki, C. Synthesis and characterization of a binuclear coumarin-3-carboxylate copper (II) complex. J. Inorg. Biochem. 2001, 84, 33–37. [Google Scholar] [CrossRef]
- Creaven, B.S.; Devereux, M.; Karcz, D.; Kellett, A.; McCann, M.; Noble, A.; Walsh, M. Copper (II) complexes of coumarin-derived Schiff bases and their anti-Candida activity. J. Inorg. Biochem. 2009, 103, 1196–1203. [Google Scholar] [CrossRef] [Green Version]
- Creaven, B.S.; Egan, D.A.; Karcz, D.; Kavanagh, K.; McCann, M.; Mahon, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis, characterisation and antimicrobial activity of copper(II) and manganese(II) complexes of coumarin-6,7-dioxyacetic acid (cdoaH2) and 4-methylcoumarin-6,7-dioxyacetic acid (4-MecdoaH2): X-ray crystal structures of [Cu(cdoa)(phen)2]·8.8H2O and [Cu(4-Mecdoa)(phen)2]·13H2O (phen = 1,10-phenanthroline). J. Inorg. Biochem. 2007, 101, 1108–1119. [Google Scholar]
- Creaven, B.S.; Egan, D.A.; Kavanagh, K.; McCann, M.; Mahon, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis and antimicrobial activity of copper(II) and silver(I) complexes of hydroxynitrocoumarins: X-ray crystal structures of [Cu(hnc)2(H2O)2]·2H2O and [Ag(hnc)] (hncH = 4-hydroxy-3-nitro-2H-chromen-2-one). Polyhedron 2005, 24, 949–957. [Google Scholar] [CrossRef]
- Creaven, B.S.; Egan, D.A.; Kavanagh, K.; McCann, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis, characterization and antimicrobial activity of a series of substituted coumarin-3-carboxylatosilver (I) complexes. Inorg. Chim. Acta 2006, 359, 3976–3984. [Google Scholar] [CrossRef]
- Thati, B.; Noble, A.; Rowan, R.; Creaven, B.S.; Walsh, M.; McCann, M.; Egan, D.; Kavanagh, K. Mechanism of action of coumarin and silver (I)–coumarin complexes against the pathogenic yeast Candida albicans. Toxicol. Vitr. 2007, 21, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Grazul, M.; Budzisz, E. Biological activity of metal ions complexes of chromones, coumarins and flavones. Coord. Chem. Rev. 2009, 253, 2588–2598. [Google Scholar] [CrossRef]
- Kulkarni, A.; Patil, S.A.; Badami, P.S. Synthesis, characterization, DNA cleavage and in vitro antimicrobial studies of La (III), Th (IV) and VO (IV) complexes with Schiff bases of coumarin derivatives. Eur. J. Med. Chem. 2009, 44, 2904–2912. [Google Scholar] [CrossRef]
- Roh, S.-G.; Baek, N.S.; Hong, K.-S.; Kim, H.K. Synthesis and Photophysical Properties of Luminescent Lanthanide Complexes Based on Coumarin-3-carboxylic Acid for Advanced Photonic Applications. Bull. Korean Chem. Soc. 2004, 25, 343–344. [Google Scholar]
- Georgieva, I.; Trendafilova, N.; Aquino, A.J.A.; Lischka, H. Theoretical study of metal− ligand interaction in Sm(III), Eu(III), and Tb(III) complexes of coumarin-3-carboxylic acid in the gas phase and solution. Inorg. Chem. 2007, 46, 10926–10936. [Google Scholar] [CrossRef] [PubMed]
- Mihaylov, T.; Trendafilova, N.; Kostova, I.; Georgieva, I.; Bauer, G. DFT modeling and spectroscopic study of metal–ligand bonding in La (III) complex of coumarin-3-carboxylic acid. Chem. Phys. 2006, 327, 209–219. [Google Scholar] [CrossRef]
- Georgieva, I.; Trendafilova, N.; Kiefer, W.; Rastogi, V.K.; Kostova, I. Vibrational and theoretical study of coumarin-3-carboxylic acid binding mode in Ce (III) and Nd (III) complexes. Vibr. Spectr. 2007, 44, 78–88. [Google Scholar] [CrossRef]
- Kostova, I.; Manolov, I.; Nicolova, I.; Konstantinov, S.; Karaivanova, M. New lanthanide complexes of 4-methyl-7-hydroxycoumarin and their pharmacological activity. Eur. J. Med. Chem. 2001, 36, 339–347. [Google Scholar] [CrossRef]
- Kostova, I.; Manolov, I.; Konstantinov, S.; Karaivanova, M. Synthesis, physicochemical characterisation and cytotoxic screening of new complexes of cerium, lanthanum and neodymium with Warfarin and Coumachlor sodium salts. Eur. J. Med. Chem. 1999, 34, 63–68. [Google Scholar] [CrossRef]
- Kostova, I.; Momekov, G.; Zaharieva, M.; Karaivanova, M. Cytotoxic activity of new lanthanum (III) complexes of bis-coumarins. Eur. J. Med. Chem. 2005, 40, 542–551. [Google Scholar] [CrossRef]
- Creaven, B.S.; Devereux, M.; Georgieva, I.; Karcz, D.; McCann, M.; Trendafilova, N.; Walsh, M. Molecular structure and spectroscopic studies on novel complexes of coumarin-3-carboxylic acid with Ni(II), Co(II), Zn(II) and Mn(II) ions based on density functional theory. Spectrochim. Acta Part A 2011, 84, 275–285. [Google Scholar] [CrossRef]
- Furia, E.; Beneduci, A.; Russo, N.; Marino, T. Structural characterization of aluminium(III) and iron(III) complexes of coumarinic acid in aqueous solutions from combined experimental and theoretical investigations. New J. Chem. 2018, 42, 11006–11012. [Google Scholar] [CrossRef]
- Furia, E.; Sindona, G. Complexation of l-Cystine with Metal Cations. J. Chem. Eng. Data 2010, 55, 2985–2989. [Google Scholar] [CrossRef]
- Furia, E.; Porto, R. 2-Hydroxybenzamide as a Ligand. Complex Formation with Dioxouranium(VI), Aluminum(III), Neodymium(III), and Nickel(II) Ions. J. Chem. Eng. Data 2008, 53, 2739–2745. [Google Scholar] [CrossRef]
- Biedermann, G.; Sillén, L.G. Studies on the hydrolysis of metal ions. IV. Liquid junction potentials and constancy of activity factors in NaClO4–HClO4 ionic medium. Arkiv Kemi. 1953, 5, 425–440. [Google Scholar]
- Gran, G. Determination of the equivalent point in potentiometric titrations. Acta Chem. Scand. 1950, 4, 559–577. [Google Scholar] [CrossRef] [Green Version]
- Gran, G. Determination of the equivalence point in potentiometric titrations. Part II. Analyst 1952, 77, 661–670. [Google Scholar] [CrossRef]
- Sillén, L.G. Some Graphical Methods for Determining Equilibrium Constants. II. On "Curve-fitting" Methods for Two-variable Data. Acta Chem. Scand. 1956, 10, 186–202. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Superquad: An improved general program for computation of formation constants from potentiometric data. J. Chem. Soc. Dalton Trans. 1985, 6, 1195–1200. [Google Scholar] [CrossRef]
- Baes, C.F.; Mesmer, R.E. The Hydrolysis of Cations; Wiley Interscience: New York, NY, USA, 1976. [Google Scholar]
- Martin, J.; Mladěnka, P.; Saso, L.; Kostova, I. Lanthanide(III) complexes are more active inhibitors of the Fenton reaction than pure ligands. Redox Rep. 2016, 21, 84–89. [Google Scholar] [CrossRef] [Green Version]
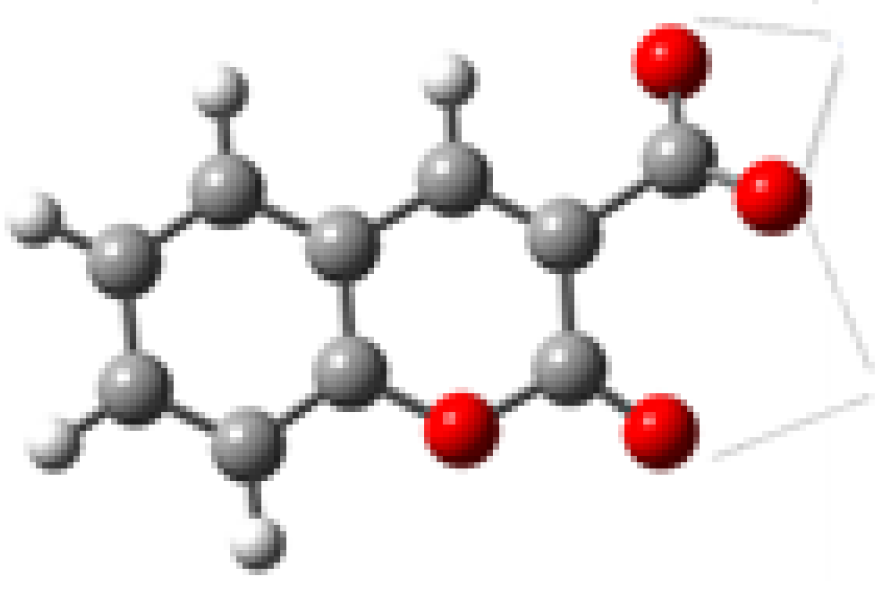


| Metal Ions | Species | log β ± 3σ |
|---|---|---|
| Nd3+ | Nd(OH)(CCA)+ | 9.2 ± 0.1 |
| Nd(OH)(CCA)2 | 11.9 ± 0.3 | |
| UO22+ | UO2(CCA)2 | 8.2 ± 0.1 |
| UO2(OH)(CCA) | 13.0 ± 0.3 | |
| UO2(OH)2(CCA)- | 21.6 ± 0.1 | |
| (UO2)2(OH)2(CCA)2 | 28.9 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malacaria, L.; Corrente, G.A.; Furia, E. Thermodynamic Study on the Dissociation and Complexation of Coumarinic Acid with Neodymium(III) and Dioxouranium(VI) in Aqueous Media. Appl. Sci. 2021, 11, 4475. https://doi.org/10.3390/app11104475
Malacaria L, Corrente GA, Furia E. Thermodynamic Study on the Dissociation and Complexation of Coumarinic Acid with Neodymium(III) and Dioxouranium(VI) in Aqueous Media. Applied Sciences. 2021; 11(10):4475. https://doi.org/10.3390/app11104475
Chicago/Turabian StyleMalacaria, Luana, Giuseppina Anna Corrente, and Emilia Furia. 2021. "Thermodynamic Study on the Dissociation and Complexation of Coumarinic Acid with Neodymium(III) and Dioxouranium(VI) in Aqueous Media" Applied Sciences 11, no. 10: 4475. https://doi.org/10.3390/app11104475








