Application of Polyacrylamide Flocculant for Stabilization of Anaerobic Digestion under Conditions of Excessive Accumulation of Volatile Fatty Acids
Abstract
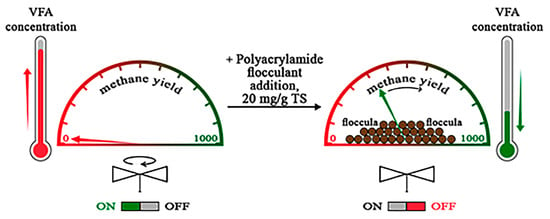
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate and Inoculum
2.2. Experimental Setup
- a low inoculum to substrate ratio (ISR = 30%/70% on VS basis);
- a high proportion of readily degradable co-substrate, s-OFMSW, which was 45% of the total VS of the digesting mixture (a mixture of inoculum, co-substrates and tap water). Accordingly, the second co-substrate, namely SS, represented 25% of the total VS of the mixture;
- a high concentration of VS in the mixture (40 g/kg).
2.3. Analytical Methods
3. Results
3.1. Toxicity of the PAM and its Possible Degradation under Anaerobic Conditions
3.1.1. Toxicity of the PAM
3.1.2. PAM Biodegradability
3.2. Application of the Flocculant to Restore Methanogenesis in Destabilized Bioreactors
4. Discussion
4.1. Toxicity of the PAM and its Possible Degradation under Anaerobic Conditions
4.2. Application of the Flocculant to Restore Methanogenesis in Destabilized Bioreactors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Werle, S.; Sobek, S. Gasification of sewage sludge within a circular economy perspective: A Polish case study. Environ. Sci. Pollut. Res. 2019, 26, 35422–35432. [Google Scholar] [CrossRef] [Green Version]
- Yadav, P.; Samadder, S.R. A global prospective of income distribution and its effect on life cycle assessment of municipal solid waste management: A review. Environ. Sci. Pollut. Res. 2017, 24, 9123–9141. [Google Scholar] [CrossRef] [Green Version]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef]
- Bolzonella, D.; Battistoni, P.; Susinii, C.; Cecchi, F. Anaerobic codigestion of waste activated sludge and OFMSW: The experiences of viareggio and treviso plants (Italy). Water Sci. Technol. 2006, 53, 203–211. [Google Scholar] [CrossRef]
- Stams, A.J.; Sousa, D.Z.; Kleerebezem, R.; Plugge, C.M. Role of syntrophic microbial communities in high-rate methanogenic bioreactors. Water Sci. Technol. 2012, 66, 352–362. [Google Scholar] [CrossRef]
- Schnürer, A. Biogas production: Microbiology and technology. In Anaerobes in Biotechnology: Advances in Biochemical Engineering/Biotechnology; Hatti-Kaul, R., Mamo, G., Mattiasson, B., Eds.; Springer: Cham, Switzerland, 2016; Volume 156. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Macé, S.; Astals, S. Codigestion of solid wastes: A review of its uses and perspectives including modeling. Crit. Rev. Biotechnol. 2011, 31, 99–111. [Google Scholar] [CrossRef]
- Schnurer, A.; Jarvis, A. Microbiological Handbook for Biogas Plant; Swedish Waste Management U2009:03; Swedish Gas Centre Report 207; Swedish Gas Centre: Malmö, Sweden, 2010; p. 138. [Google Scholar]
- Fotidis, I.A.; Karakashev, D.; Angelidaki, I. Bioaugmentation with an acetate-oxidising consortium as a tool to tackle ammonia inhibition of anaerobic digestion. Bioresour. Technol. 2013, 146, 57–62. [Google Scholar] [CrossRef] [Green Version]
- Water Environment Federation. Operation of Municipal Wastewater Treatment Plants: Manual of Practice No. 11, 6th ed.; Water Environment Federation: Alexandria, VA, USA, 2008; p. 1980. [Google Scholar]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Nikitina, A.A.; Kevbrina, M.V.; Kallistova, A.Y.; Nekrasova, V.K.; Litti, Y.V.; Nozhevnikova, A.N. Intensification of microbial decomposition of organic fraction of municipal waste: Laboratory and field experiments. Appl. Biochem. Microbiol. 2015, 51, 393–401. [Google Scholar] [CrossRef]
- Campos, E.; Almirall, M.; Mtnez-Almela, J.; Palatsi, J.; Flotats, X. Feasibility study of the anaerobic digestion of dewatered pig slurry by means of polyacrylamide. Bioresour. Technol. 2008, 99, 387–395. [Google Scholar] [CrossRef]
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process. Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Lu, L.; Pan, Z.; Hao, N.; Peng, W. A novel acrylamide-free flocculant and its application for sludge dewatering. Water Res. 2014, 57, 304–312. [Google Scholar] [CrossRef]
- Litti, Y.; Nikitina, A.; Kovalev, D.; Ermoshin, A.; Mahajan, R.; Goel, G.; Nozhevnikova, A. Influence of cationic polyacrilamide flocculant on high-solids’ anaerobic digestion of sewage sludge under thermophilic conditions. Environ. Technol. 2019, 40, 1146–1155. [Google Scholar] [CrossRef]
- Wang, D.; Liu, X.; Zeng, G.; Zhao, J.; Liu, Y.; Wang, Q.; Chen, F.; Li, X.; Yang, Q. Understanding the impact of cationic polyacrylamide on anaerobic digestion of waste activated sludge. Water Res. 2018, 130, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Luo, F.; Yi, J.; He, Q.; Dong, B. Biodegradation of polyacrylamide by anaerobic digestion under mesophilic condition and its performance in actual dewatered sludge system. Bioresour. Technol. 2014, 153, 55–61. [Google Scholar] [CrossRef]
- Pfennig, N. Anreicherungskulturen für rote und grüne Schwefelbakterien. Zbl. Bakt. I. Abt. Orig. Suppl. 1965, 1, 179–189. [Google Scholar]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- McInerney, M.J.; Sieber, J.R.; Gunsalus, R.P. Syntrophy in anaerobic global carbon cycles. Curr. Opin. Biotechnol. 2009, 20, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Chu, C.P.; Lee, D.J.; Chang, B.V.; You, C.H.; Liao, C.S.; Tay, J.H. Anaerobic digestion of polyelectrolyte flocculated waste activated sludge. Chemosphere 2003, 53, 757–764. [Google Scholar] [CrossRef]
- Song, W.; Zhang, Y.; Gao, Y.; Chen, D.; Yang, M. Cleavage of the main carbon chain backbone of high molecular weight polyacrylamide by aerobic and anaerobic biological treatment. Chemosphere 2017, 189, 277–283. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Q.; Wang, D.; Wu, Y.; Yang, Q.; Liu, Y.; Wang, Q.; Li, X.; Li, H.; Zeng, G.; et al. Unveiling the mechanisms of how cationic polyacrylamide affects short-chain fatty acids accumulation during long-term anaerobic fermentation of waste activated sludge. Water Res. 2019, 155, 142–151. [Google Scholar] [CrossRef]
- Dai, X.; Luo, F.; Zhang, D.; Dai, L.; Chen, Y.; Dong, B. Waste-activated sludge fermentation for polyacrylamide biodegradation improved by anaerobic hydrolysis and key microorganisms involved in biological polyacrylamide removal. Sci. Rep. 2015, 5, 11675. [Google Scholar] [CrossRef]
- Pullammanappallil, P.C.; Chynoweth, D.P.; Lyberatos, G.; Sveronos, S.A. Stable performance of anaerobic digestion in the presence of a high concentration of propionicacid. Bioresour. Technol. 2001, 78, 165–169. [Google Scholar] [CrossRef]
- Kay-Shoemake, J.L.; Watwood, M.E.; Lentz, R.D.; Sojka, R.E. Polyacrylamide as an organic nitrogen source for soil microorganisms with potential effects on inorganic soil nitrogen in agricultural soil. Soil Biol. Biochem. 1998, 30, 1045–1052. [Google Scholar] [CrossRef]
- El-Mamouni, R.; Frigon, J.C.; Hawari, J.; Marroni, D.; Guiot, S.R. Combining photolysis and bioprocesses for mineralization of high molecular weight polyacrylamides. Biodegradation 2002, 13, 221–227. [Google Scholar] [CrossRef]
- Stroot, P.G.; McMahon, K.D.; Mackie, R.I.; Raskin, L. Anaerobic codigestion of municipal solid waste and biosolids under various mixing conditions—I. digester performance. Water Res. 2001, 35, 1804–1816. [Google Scholar] [CrossRef]
- Vavilin, V.A.; Angelidaki, I. Anaerobic degradation of solid material: Importance of initiation centers for methanogenesis, mixing intensity, and 2D distributed model. Biotechnol. Bioeng. 2004, 89, 113–122. [Google Scholar] [CrossRef]
- Bolzonella, D.; Pavan, P.; Battistoni, P.; Cecchi, F. Influence of the cationic flocculant praestol K233L on the mesophilic anaerobic digestion of waste activated sludge. J. Residuals Sci. Technol. 2005, 2, 133–141. [Google Scholar] [CrossRef]
- El-Mamouni, R.; Leduc, R.; Guiot, S.R. Influence of synthetic and natural polymers on anaerobic granulation process. Water Sci. Technol. 1998, 38, 341–347. [Google Scholar] [CrossRef]




| Sample | Day of Incubation | VFA Concentration | Ammonium Concentration, mg/L | Methane Content, % | Methane Yield, mL | |||
|---|---|---|---|---|---|---|---|---|
| Acetate, mmol/L | Propionate, mmol/L | Butyrate, mmol/L | Total VFA, g/L | |||||
| Control T-0 | 0 | 44.871 | 6.965 | 22.783 | 5.20 | 35.2 | 0 | 0 |
| 6 | 18.617 | 3.289 | 0.159 | 1.35 | 36.5 | 77.29 | 85.98 | |
| 22 | 1.705 | 0.015 | 0.025 | 0.10 | – | 79.22 | 144.63 | |
| 42 | 0.512 | 0.003 | 0.007 | 0.03 | 76.5 | 23.27 | 146.47 | |
| T-5 | 0 | 44.871 | 6.965 | 22.783 | 5.20 | 35.2 | 0 | 0 |
| 6 | 24.986 | 4.186 | 0.696 | 1.84 | 58.4 | 76.48 | 76.4 | |
| 22 | 3.343 | 0.020 | 0.051 | 0.20 | – | 77.59 | 131.37 | |
| 42 | 0.011 | 0.003 | 0.004 | 0 | 89.6 | 25.07 | 138.44 | |
| T-40 | 0 | 44.871 | 6.965 | 22.783 | 5.20 | 35.2 | 0 | 0 |
| 6 | 35.412 | 6.566 | 4.472 | 2.96 | 44.2 | 76.73 | 60.93 | |
| 22 | 0.429 | 6.712 | 0.053 | 0.52 | – | 80.01 | 136.41 | |
| 42 | 6.170 | 0.026 | 0.007 | 0.37 | 100.3 | 41.39 | 147.56 | |
| T-80 | 0 | 44.871 | 6.965 | 22.783 | 5.20 | 35.2 | 0 | 0 |
| 6 | 33.418 | 6.386 | 8.896 | 3.21 | 68.2 | 75.46 | 42.13 | |
| 22 | 0.524 | 6.610 | 0.030 | 0.52 | – | 78.51 | 129.48 | |
| 42 | 4.822 | 2.884 | 0.005 | 0.49 | 103.2 | 48.70 | 136.49 | |
| T-200 | 0 | 44.871 | 6.965 | 22.783 | 5.20 | 35.2 | 0 | 0 |
| 6 | 42.764 | 9.203 | 24.309 | 5.31 | 61.8 | 73.23 | 20.24 | |
| 22 | 0.362 | 7.214 | 0.070 | 0.55 | – | 78.16 | 106.60 | |
| 42 | 3.211 | 4.444 | 0.012 | 0.51 | 104.8 | 33.59 | 111.46 | |
| T-400 | 0 | 44.871 | 6.965 | 22.783 | 5.20 | 35.2 | 0 | 0 |
| 6 | 44.671 | 8.234 | 26.129 | 5.51 | 101.4 | 70.94 | 11.12 | |
| 22 | 12.164 | 8.250 | 0.192 | 1.34 | – | 79.07 | 104.09 | |
| 42 | 0.475 | 8.307 | 0.004 | 0.63 | 131.7 | 31.33 | 117.29 | |
| Sample | Day of Incubation | Volatile Fatty Acids Concentration | Ammonium Concentration, mg/L | Methane Content in Biogas, % | Methane Yield, mL | |||
|---|---|---|---|---|---|---|---|---|
| Acetate, mmol/L | Propionate, mmol/L | Butyrate, mmol/L | Total VFA, g/L | |||||
| Control (B-0) | 0 | 0.121 | 0.009 | 0.008 | 0.01 | 35.2 | 0 | 0 |
| 6 | 0.175 | 0.011 | 0.032 | 0.01 | 72.1 | 67.54 | 8.53 | |
| 22 | 0.203 | 0.240 | 0.012 | 0.03 | – | 68.77 | 17.25 | |
| 42 | 0.176 | 0.042 | 0.015 | 0.01 | 95.8 | 37.33 | 20.54 | |
| B-5 | 0 | 0.121 | 0.009 | 0.008 | 0.01 | 35.2 | 0 | 0 |
| 6 | 0.169 | 0.021 | 0.032 | 0.01 | 92.3 | 66.10 | 8.58 | |
| 22 | 0.351 | 0.062 | 0.054 | 0.03 | – | 69.79 | 16.57 | |
| 42 | 0.224 | 0.009 | 0.013 | 0.01 | 104.5 | 37.67 | 19.95 | |
| B-40 | 0 | 0.121 | 0.009 | 0.008 | 0.01 | 35.2 | 0 | 0 |
| 6 | 0.142 | 0.047 | 0.014 | 0.01 | 55.1 | 64.14 | 7.01 | |
| 22 | 0.429 | 0.015 | 0.042 | 0.03 | – | 68.05 | 14.57 | |
| 42 | 0.154 | 0.011 | 0.008 | 0.01 | 106.1 | 38.12 | 17.67 | |
| B-80 | 0 | 0.121 | 0.009 | 0.008 | 0.01 | 35.2 | 0 | 0 |
| 6 | 0.179 | 0.116 | 0.019 | 0.02 | 83.2 | 66.09 | 7.6 | |
| 22 | 0.556 | 0.018 | 0.037 | 0.04 | – | 68.98 | 14.87 | |
| 42 | 0.171 | 0.020 | 0.007 | 0.01 | 109.4 | 35.84 | 17.81 | |
| B-200 | 0 | 0.121 | 0.009 | 0.008 | 0.01 | 35.2 | 0 | 0 |
| 6 | 0.803 | 0.327 | 0.078 | 0.08 | 112.1 | 67.96 | 8.74 | |
| 22 | 0.158 | 0.020 | 0.047 | 0.01 | – | 66.45 | 17.97 | |
| 42 | 0.128 | 0.011 | 0.013 | 0.01 | 127.1 | 38.05 | 21.24 | |
| B-400 | 0 | 0.121 | 0.009 | 0.008 | 0.01 | 35.2 | 0 | 0 |
| 6 | 1.221 | 0.313 | 0.071 | 0.09 | 125.3 | 50.99 | 5.15 | |
| 22 | 0.689 | 0.362 | 0.029 | 0.07 | – | 74.84 | 15.65 | |
| 42 | 0.141 | 0.078 | 0.009 | 0.01 | 137.4 | 23.71 | 19.67 | |
| The Concentration of PAM in the Experimental Mixture | VFA Concentration, g/L | Ammonium Concentration, mg/L | pH | Methane Yield, mL/g VSinitial | VS Decomposition, % | ||
|---|---|---|---|---|---|---|---|
| Initial | Terminal | Initial | Terminal | ||||
| Control (PAM-0) | 2.23 ± 0.11 | 11.75 ± 0.58 | 60.7 ± 3.0 | 822.1 ± 41.1 | 5.68 | 11.16 ± 0.55 | 35.0 ± 1.7 |
| PAM–20 | 2.23 ± 0.11 | 0.73 ± 0.03 | 60.7 ± 3.0 | 1047.2 ± 52.3 | 7.60 | 189.84 ± 9.54 | 60.4 ± 3.0 |
| PAM–40 | 2.23 ± 0.11 | 8.37 ± 0.41 | 60.7 ± 3.0 | 1033.0 ± 51.6 | 6.89 | 53.41 ± 2.67 | 52.6 ± 2.6 |
| PAM–60 | 2.23 ± 0.11 | 13.09 ± 0.65 | 60.7 ± 3.0 | 836.5 ± 41.8 | 5.96 | 12.45 ± 0.62 | 52.1 ± 2.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikitina, A.A.; Ermoshin, A.A.; Zhuravleva, E.A.; Kovalev, A.A.; Kovalev, D.A.; Panchenko, V.; Litti, Y.V. Application of Polyacrylamide Flocculant for Stabilization of Anaerobic Digestion under Conditions of Excessive Accumulation of Volatile Fatty Acids. Appl. Sci. 2021, 11, 100. https://doi.org/10.3390/app11010100
Nikitina AA, Ermoshin AA, Zhuravleva EA, Kovalev AA, Kovalev DA, Panchenko V, Litti YV. Application of Polyacrylamide Flocculant for Stabilization of Anaerobic Digestion under Conditions of Excessive Accumulation of Volatile Fatty Acids. Applied Sciences. 2021; 11(1):100. https://doi.org/10.3390/app11010100
Chicago/Turabian StyleNikitina, Anna A., Artem A. Ermoshin, Elena A. Zhuravleva, Andrey A. Kovalev, Dmitriy A. Kovalev, Vladimir Panchenko, and Yuri V. Litti. 2021. "Application of Polyacrylamide Flocculant for Stabilization of Anaerobic Digestion under Conditions of Excessive Accumulation of Volatile Fatty Acids" Applied Sciences 11, no. 1: 100. https://doi.org/10.3390/app11010100