Quantifying the Effect of Catalysts on the Lifetime of Transformer Oil
Abstract
:1. Introduction
2. A Brief Overview of Methods for Assessing the Condition of Transformer Insulation
3. Materials and Methods
3.1. Tested Oil and Thermo-Oxidative Aging
3.2. Method Description
4. Results
4.1. Changes in the UV-Vis Region of the Electromagnetic Spectrum
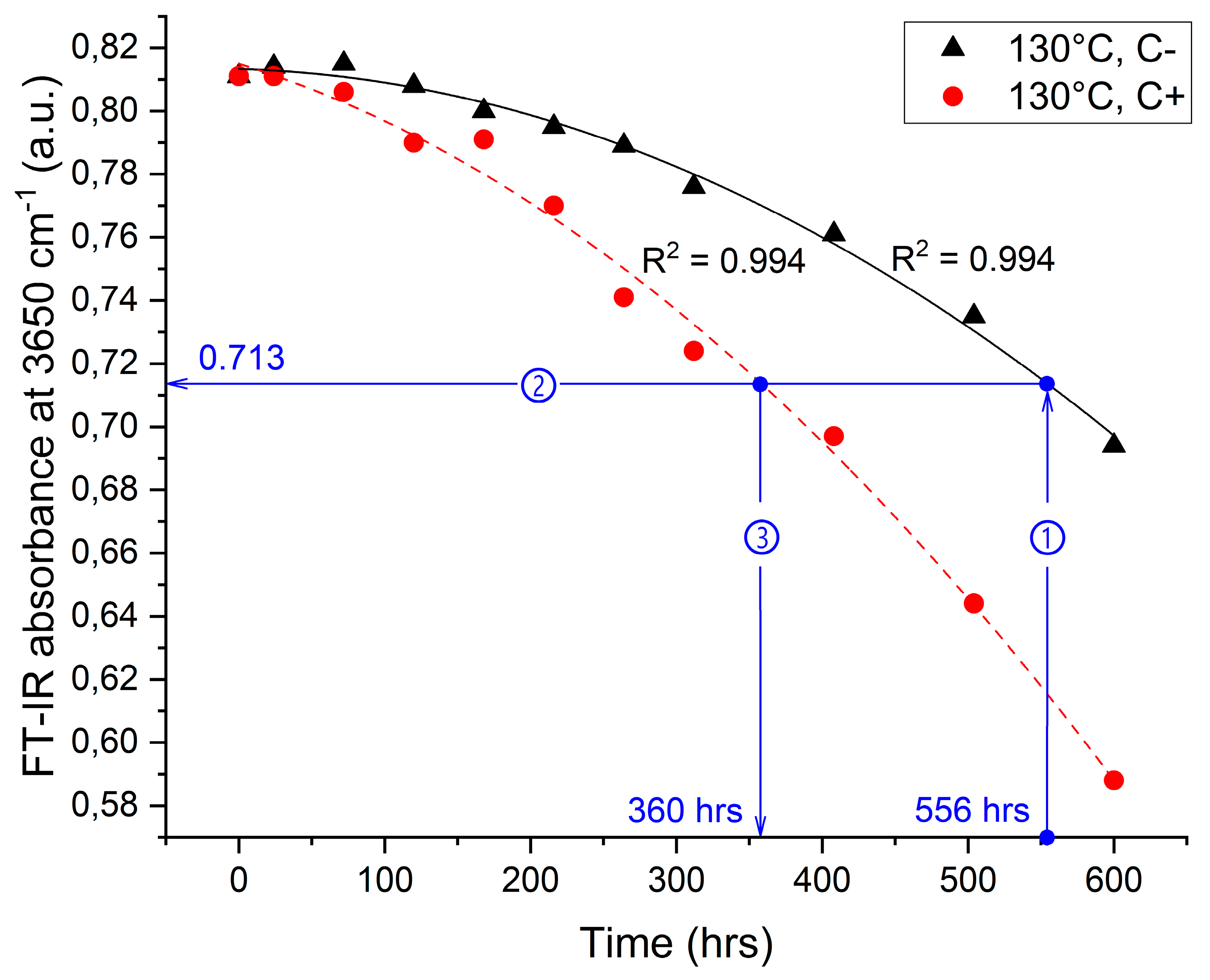
4.2. Changes in the IR Region of the Electromagnetic Spectrum
5. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- The Electric Power Research Institute. Considerations for a Power Transformer Emergency Spare Strategy for the Electric Utility Industry; EPRI: Palo Alto, CA, USA, 2014. [Google Scholar]
- Dumitran, L.M.; Setnescu, R.; Notingher, P.V.; Badicu, L.V.; Setnescu, T. Method for lifetime estimation of power transformer mineral oil. Fuel 2014, 117, 756–762. [Google Scholar] [CrossRef]
- Saha, T.K. Review of Modern Diagnostic Techniques for Assessing Insulation Condition in Aged Transformers. IEEE Trans. Dielectr. Electr. Insul. 2003, 10, 903–917. [Google Scholar] [CrossRef] [Green Version]
- Khayam, U.; Tsuchie, M.; Thein, M.; Hikita, M.; Saito, T. Study on Dissolved Gas Due Tue Thermally Degraded Insulating Paper in Transformer Oil. Procedia Technol. 2013, 11, 257–262. [Google Scholar]
- Kaplan, I.R.; Rasco, J.; Lu, S.-T. Chemical Characterization of Transformer Mineral-Insulating Oils. Environ. Forensics 2010, 11, 117–145. [Google Scholar] [CrossRef]
- Shiri, A.; Gholami, A.; Shoulaie, A. Investigation of the ambient temperature effects on transformer’s insulation life. Electr. Eng. 2011, 93, 193–197. [Google Scholar] [CrossRef]
- Yehye, W.A.; Rahman, N.A.; Ariffin, A.; Abd Hamid, S.B.; Alhadi, A.A.; Kadir, F.A.; Yaeghoobi, M. Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): A review. Eur. J. Med. Chem. 2015, 101, 295–312. [Google Scholar] [CrossRef]
- Sanghi, R. Chemistry behind the life of a transformer. Resonance 2003, 8, 17–23. [Google Scholar] [CrossRef]
- Meshkatoddini, M.R. Aging Study and Lifetime Estimation of Transformer Mineral Oil. Am. J. Eng. Appl. Sci. 2008, 1, 384–388. [Google Scholar] [CrossRef] [Green Version]
- Loiselle, L.; Fofana, I.; Hume, D. Influence of Transformer Oil Decay Products on its Thermal Conductivity. In Proceedings of the 31st International Thermal Conductivity Conference (ITCC) and the 19th International Thermal Expansion Symposium (ITES), Chicoutimi, QC, Canada, 26–30 June 2011; pp. 1–10. [Google Scholar]
- Kalantar, A.; Levin, M. Factors affecting the dissolution of copper in transformer oils. Lubr. Sci. 2008, 20, 223–240. [Google Scholar] [CrossRef]
- Amimoto, T.; Nagao, E.; Tanimura, J.; Toyama, S.; Fujita, Y.; Kawarai, H.; Yamada, N. Identification of affecting factors of copper sulfide deposition on insulating paper in oil. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 265–272. [Google Scholar] [CrossRef]
- The International Council on Large Electric Systems. Technical brochure CIGRE WG A2-32. In Copper Sulphide in Transformer Insulation; CIGRE: Paris, France, 2009. [Google Scholar]
- Hao, J.; Liao, R.; Yang, L.; Gao, S.; Liao, Q.; Gao, J. Copper Catalytic Effect on the Thermal Deterioration and Surface Morphology Performance of Transformer Oil—Paper Insulation. IEEJ Trans. Electr. Electron. Eng. 2018, 13, 373–381. [Google Scholar] [CrossRef]
- International Electrotechnical Commission. IEC 60216 Guide for the Determination of Thermal Endurance Properties of Electrical Insulating Materials; Parts 1–7; IEC: Geneva, Switzerland, 2002. [Google Scholar]
- Darveniza, M.; Saha, T.K.; Hill, D.J.T.; Le, T.T. Investigations into effective methods for assessing the condition of insulation in aged power transformers. IEEE Trans. Power Deliv. 1998, 13, 1214–1223. [Google Scholar] [CrossRef]
- Taslak, E.; Arikan, O.; Fadil, C.; Kalenderli, O. Analyses of the insulating characteristics of mineral oil at operating conditions. Electr. Eng. 2018, 100, 321–331. [Google Scholar] [CrossRef]
- Abdi, S.; Boubakeur, A.; Haddad, A.; Harid, N. Influence of Artificial Thermal Aging on Transformer Oil Properties. Electr. Power Compon. Syst. 2011, 39, 1701–1711. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Huang, B.; Hao, J.; Chen, G. Review of Research Progress on the Electrical Properties and Modification of Mineral Insulating Oils Used in Power Transformers. Energies 2018, 11, 487. [Google Scholar] [CrossRef] [Green Version]
- Sikorski, W.; Walczak, K.; Przybylek, P. Moisture Migration in an Oil-Paper Insulation System in Relation to Online Partial Discharge Monitoring of Power Transformers. Energies 2016, 9, 1082. [Google Scholar] [CrossRef] [Green Version]
- Leong, S.Y.; Ker, P.J.; Jamaludin, M.Z.; Nomanbhay, S.M.; Ismail, A.; Abdullah, F.; Looe, H.M.; Lo, C.K. UV-Vis Spectroscopy : A New Approach for Assessing the Color Index of Transformer Insulating Oil. Sensors 2018, 18, 2175. [Google Scholar] [CrossRef] [Green Version]
- Fofana, I.; Bouaicha, A.; Farzaneh, M.; Sabau, J.; Bussieres, D.; Robertson, E.B. Decay products in the liquid insulation of power transformers. IET Electr. Power Appl. 2010, 4, 177–184. [Google Scholar] [CrossRef]
- Fofana, I.; Bouaicha, A.; Farzaneh, M. Characterization of aging transformer oil—Pressboard insulation using some modern diagnostic techniques. Eur. Trans. Electr. Power 2011, 21, 1110–1127. [Google Scholar] [CrossRef]
- Hadjadj, Y.; Fofana, I.; Sabau, J.; Briosso, E. Assessing Insulating Oil Degradation by Means of Turbidity and UV/Vis Spectrophotometry Measurements. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2653–2660. [Google Scholar] [CrossRef]
- Godinho, M.S.; Blanco, M.R.; Gambarra Neto, F.F.; Lião, L.M.; Sena, M.M.; Tauler, R.; de Oliveira, A.E. Evaluation of transformer insulating oil quality using NIR, fluorescence, and NMR spectroscopic data fusion. Talanta 2014, 129, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Polansky, R.; Prosr, P.; Vik, R.; Moravcova, D.; Pihera, J. Comparison of the mineral oil lifetime estimates obtained by differential scanning calorimetry, infrared spectroscopy, and dielectric dissipation factor measurements. Thermochim. Acta 2017, 647, 86–93. [Google Scholar] [CrossRef]
- Nynas AB High Grade Nytro Lyra X—High Performance Insulating Oil. Available online: https://www.nynas.com/en/product-areas/transformer-oils/oils/nytro-lyra-x/ (accessed on 24 July 2019).
- ASTM International. ASTM D6802-02 Test Method for Determination of the Relative Content Of Dissolved Decay Products in Mineral Insulating Oils by Spectrophotometry; ASTM: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Hadjadj, Y.; Fofana, I.; Jalbert, J. Insulating Oil Decaying Assessment by FTIR and UV-Vis Spectrophotometry Measurements. In Proceedings of the 2013 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Shenzhen, China, 20–23 October 2013; pp. 1310–1313. [Google Scholar]
- ASTM International. ASTM D1934—95 Standard Test Method for Oxidative Aging of Electrical Insulating Petroleum Oils by Open-Beaker Method; ASTM: West Conshohocken, PA, USA, 2012. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989; ISBN 0813815614. [Google Scholar]
- International Electrotechnical Commission. Insulating Liquids—Measurement of Relative Permittivity, Dielectric Dissipation Factor (tan d) and d.c. Resistivity; EN 60247:2004; IEC: Geneva, Switzerland, 2004. [Google Scholar]
- Yadav, L.D.S. Organic Spectroscopy; Springer: Dordrecht, The Netherlands, 2005; ISBN 978-94-017-2508-8. [Google Scholar]
- Bley, T.; Pignanelli, E.; Schütze, A. Multi-channel IR sensor system for determination of oil degradation. J. Sens. Sens. Syst. 2014, 3, 121–132. [Google Scholar] [CrossRef]
- Zakharich, M.P.; Zaitsev, I.I.; Komar, V.P.; Nikonovich, F.N.; Ryzhkov, M.P.; Skornyakov, I.V. Analysis of Transformer Oil Using IR Analyzers. J. Appl. Spectrosc. 2001, 68, 61–65. [Google Scholar] [CrossRef]
- Hao, J.; Liao, R.; Liang, S.; Yang, L.; Zhu, M. Choice of antioxidants and their synergistic effect study towards new insulation oil. In Proceedings of the 2009 IEEE 9th International Conference on the Properties and Applications of Dielectric Materials, Harbin, China, 19–23 July 2009; pp. 53–56. [Google Scholar]
- Prosr, P.; Polanský, R.; Pihera, J.; Hahn, P. Classification of Insulating Liquids Thermal Treatment Using Infrared Spectroscopy and Multivariate Statistical Method. In Proceedings of the 37th Electrical Insulation Conference, Calgary, AB, Canada, 16–20 June 2019; pp. 291–294. [Google Scholar]
- Polanský, R.; Prosr, P.; Čermák, M. Determination of the thermal endurance of PCB FR4 epoxy laminates via thermal analyses. Polym. Degrad. Stab. 2014, 105, 107–115. [Google Scholar] [CrossRef]
- Rabelo Neto, R.C.; Lima, D.O.; Pinheiro, T.D.S.; Almeida, R.F.; Castro Dantas, T.N.; Dantas, M.S.G.; Araújo, M.A.S.; Cavalcante, C.L.; Azevedo, D.C.S. Thermo-Oxidative Stability of Mineral Naphthenic Insulating Oils: Combined Effect of Antioxidants and Metal Passivator. Ind. Eng. Chem. Res. 2004, 43, 7428–7434. [Google Scholar] [CrossRef]
- Sierota, A.; Rungis, J. Electrical insulating oils. I. Characterization and pre-treatment of new transformer oils. IEEE Electr. Insul. Mag. 1995, 11, 8–20. [Google Scholar] [CrossRef]
- Maleville, X.; Faure, D.; Legros, A.; Hipeaux, J.C. Oxidation of mineral base oils of petroleum origin: The relationship between chemical composition, thickening, and composition of degradation products. Lubr. Sci. 1996, 9, 1–60. [Google Scholar] [CrossRef]
- Mortier, R.M.; Fox, M.F.; Orszulik, S.T. Chemistry and Technology of Lubricants; Springer: New York, NY, USA, 2010; ISBN 978-1-4020-8662-5. [Google Scholar]
- Migdal, C.A.; Wardlow, A.B.; Ameye, J.L. Oxidation and the Testing of Turbine Oils; ASTM International: West Conshohocken, PA, USA, 2008; ISBN 978-0-8031-3493-5. [Google Scholar]
- Miyagi, K.; Oe, E.; Yamagata, N. Evaluation of Aging for Thermally Upgraded Paper in Mineral Oil. J. Int. Counc. Electr. Eng. 2011, 1, 181–187. [Google Scholar] [CrossRef] [Green Version]


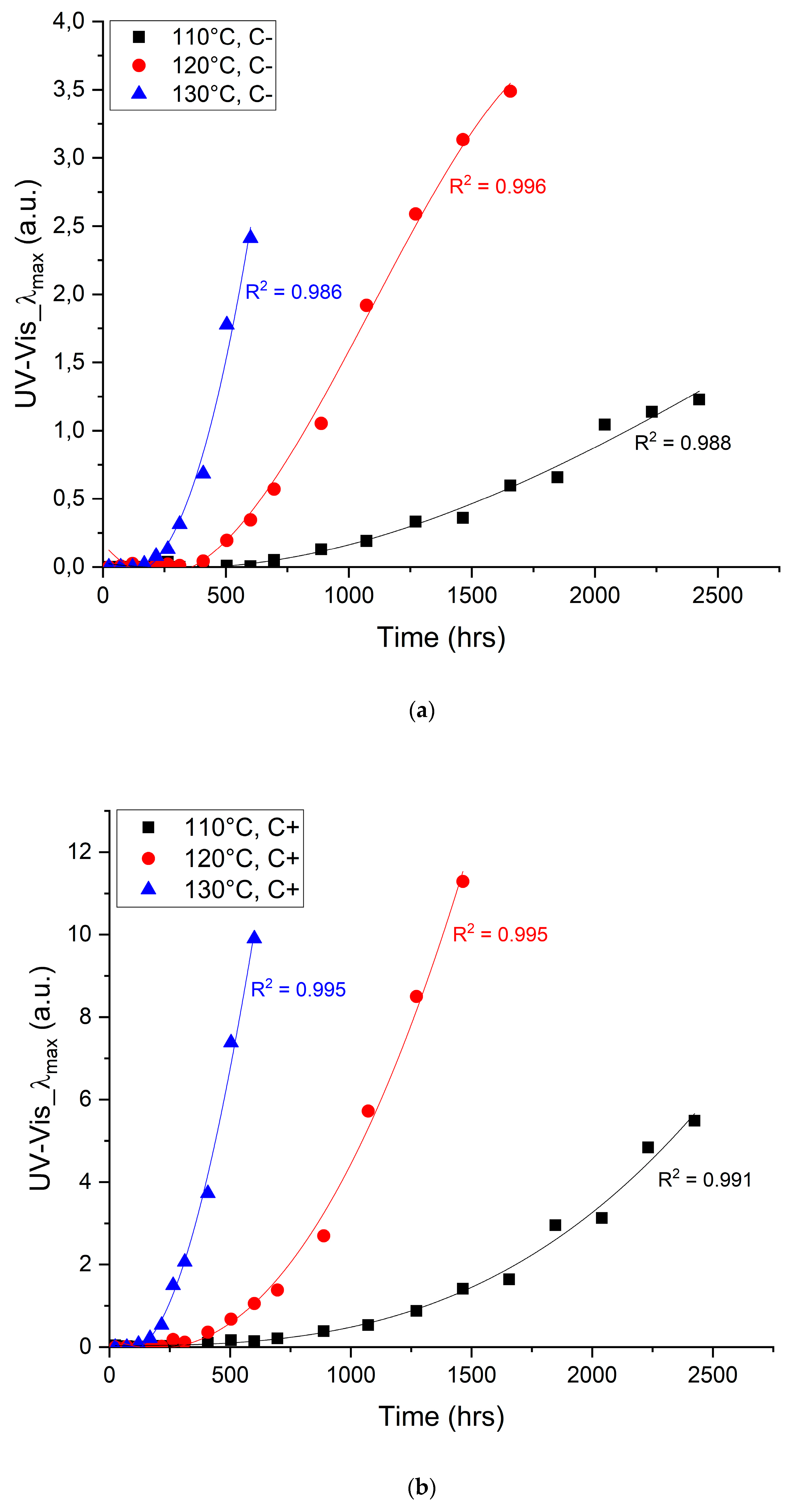



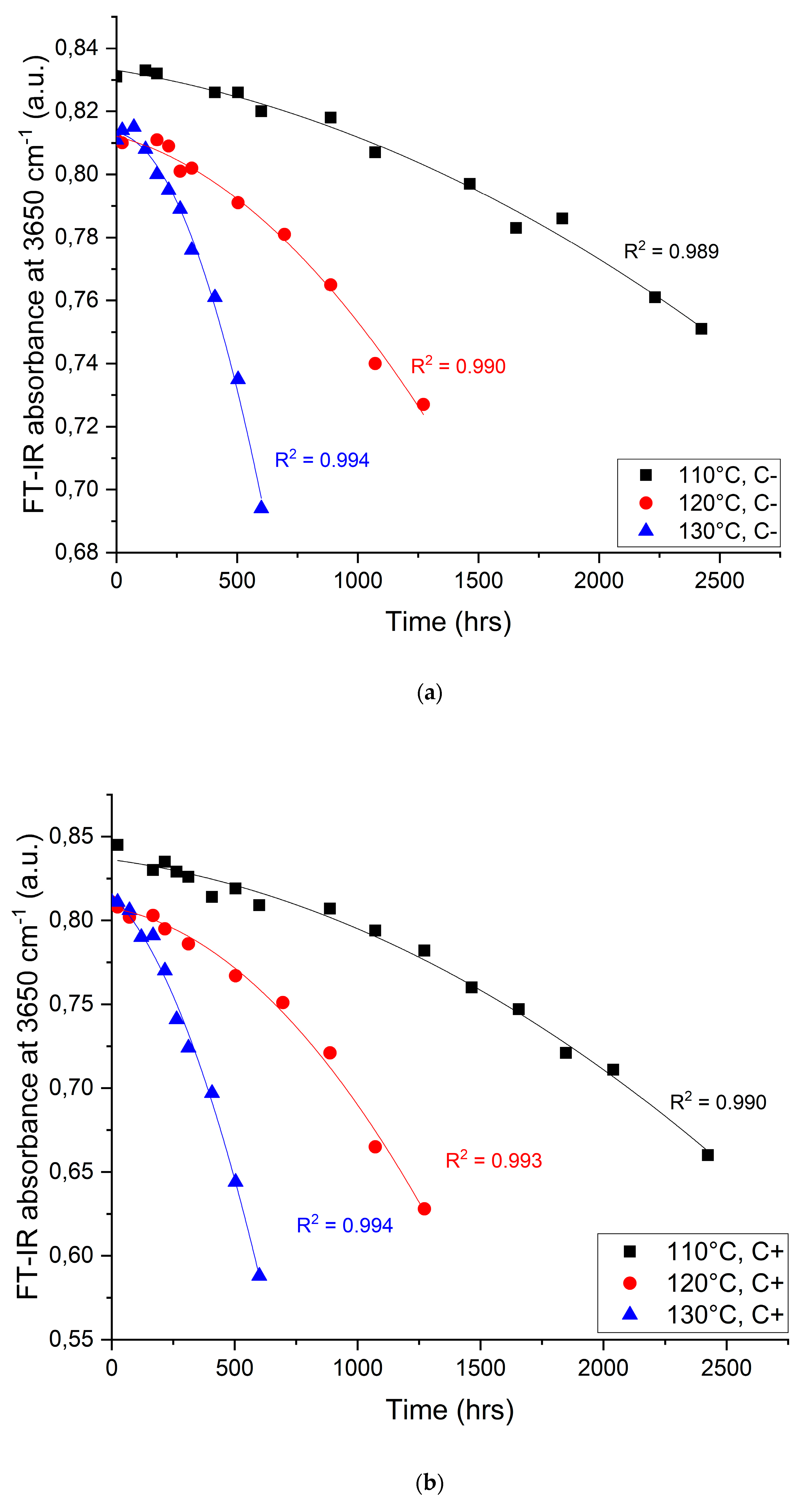
| Temperature (°C) | Catalysts (Yes/No) | Fitting Parameters (y = ax3 + bx2 + cx) | Endpoint Criterion (a.u.) | Time to Criterion (Hours) | Reduction in Lifetime (%) | |||
|---|---|---|---|---|---|---|---|---|
| a | b | c | R2 | |||||
| 110 | No | 3.82 × 10−10 | −1.06 × 10−8 | 1.22 × 10−4 | 0.988 | 1.06 | 2197 | 39.1 |
| Yes | −2.17 × 10−11 | 3.30 × 10−7 | −1.38 × 10−4 | 0.991 | 1338 | |||
| 120 | No | −1.15 × 10−9 | 3.94 × 10−6 | −1.22 × 10−3 | 0.996 | 2.15 | 1177 | 35.3 |
| Yes | 1.31 × 10−9 | 4.31 × 10−6 | −1.22 × 10−3 | 0.995 | 762 | |||
| 130 | No | 5.90 × 10−9 | 5.21 × 10−6 | −1.08 × 10−3 | 0.986 | 2.02 | 556 | 44.8 |
| Yes | −3.96 × 10−9 | 3.85 × 10−5 | −4.86 × 10−3 | 0.995 | 307 | |||
| Temperature (°C) | Catalysts (Yes/No) | Fitting Parameters (y = ax2 + bx) | Endpoint Criterion (a.u.) | Time to Criterion (Hours) | Reduction in Lifetime (%) | ||
|---|---|---|---|---|---|---|---|
| a | b | R2 | |||||
| 110 | No | −8.71 × 10−8 | −1.25 × 10−5 | 0.989 | 0.763 | 2197 | 34.5 |
| Yes | −2.14 × 10−8 | −1.98 × 10−5 | 0.990 | 1439 | |||
| 120 | No | −3.84 × 10−8 | −2.06 × 10−5 | 0.990 | 0.735 | 1177 | 35.3 |
| Yes | −9.18 × 10−8 | −2.55 × 10−5 | 0.993 | 761 | |||
| 130 | No | −3.01 × 10−7 | −1.27 × 10−5 | 0.994 | 0.713 | 556 | 35.3 |
| Yes | 3.94 × 10−7 | −1.41 × 10−4 | 0.994 | 360 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polanský, R.; Hahn, P.; Kadlec, P.; Moravcová, D.; Prosr, P. Quantifying the Effect of Catalysts on the Lifetime of Transformer Oil. Appl. Sci. 2020, 10, 1309. https://doi.org/10.3390/app10041309
Polanský R, Hahn P, Kadlec P, Moravcová D, Prosr P. Quantifying the Effect of Catalysts on the Lifetime of Transformer Oil. Applied Sciences. 2020; 10(4):1309. https://doi.org/10.3390/app10041309
Chicago/Turabian StylePolanský, Radek, Pavel Hahn, Petr Kadlec, Daniela Moravcová, and Pavel Prosr. 2020. "Quantifying the Effect of Catalysts on the Lifetime of Transformer Oil" Applied Sciences 10, no. 4: 1309. https://doi.org/10.3390/app10041309





