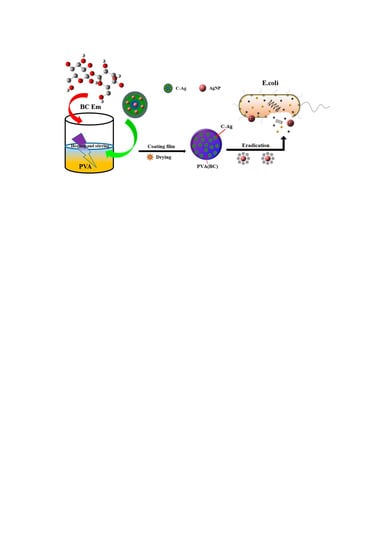
Preparation of Polyvinyl Alcohol/Bacterial-Cellulose-Coated Biochar–Nanosilver Antibacterial Composite Membranes
Abstract
:Featured Application
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Composite Membranes
2.2.1. Preparation of Bacterial Cellulose (BC) and BC Emulsion
2.2.2. Preparation of the C-Ag Composites
2.2.3. Preparation of PVA/BC/C-Ag Composite Membranes
2.3. Characterization of PVA/BC/C-Ag Composite Membranes
2.4. Mechanical Properties of PVA/BC/C-Ag Composite Membranes
2.5. Swelling Performance of PVA/BC/C-Ag Composite Membranes
2.6. Antibacterial Activity of PVA/BC/C-Ag and PVA/BC Composite Membranes
2.7. Silver Loss from PVA/BC/C-Ag Composite Membranes
2.8. Antibacterial Persistence of PVA/BC/C-Ag Composite Membranes
2.9. Antibacterial Activity of PVA/BC/C-Ag Composite Membranes in Actual Water
3. Results and Discussion
3.1. Characterization of PVA/BC/C-Ag Composite Membranes
3.1.1. Fourier-Transform Infrared (FT-IR) Spectrometry and X-ray Diffraction Analysis
3.1.2. SEM Analysis
3.1.3. TG and DSC Analysis
3.2. Mechanical Properties and Swelling Properties of PVA/BC/C-Ag Composite Membranes
3.3. Antibacterial Activity of PVA/BC/C-Ag Composite Membranes
3.4. Silver Loss from PVA/BC/C-Ag Composite Membranes
3.5. Antibacterial Persistence and Reusability of PVA/BC/C-Ag Composite Membranes
3.6. Antibacterial Activity of PVA/BC/C-Ag Composite Membranes in Actual Water
3.7. Synergistic and Antibacterial Mechanisms of PVA/BC/C-Ag Composite Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Haramoto, E.; Kitajima, M.; Hata, A.; Torrey, J.R.; Masago, Y.; Sano, D.; Katayama, H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018, 135, 168–186. [Google Scholar] [CrossRef]
- Dhadge, V.L.; Medhi, C.R.; Changmai, M.; Purkait, M.K. House hold unit for the treatment of fluoride, iron, arsenic and microorganism contaminated drinking water. Chemosphere 2018, 199, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Pan, X.; Ben, W.; Wang, J.; Hou, P.; Qiang, Z. Adsorptive removal of antibiotics from water using magnetic ion exchange resin. J. Environ. Sci. 2017, 52, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Abo Markeb, A.; Alonso, A.; Sanchez, A.; Font, X. Adsorption process of fluoride from drinking water with magnetic core-shell Ce-Ti@Fe3O4 and Ce-Ti oxide nanoparticles. Sci. Total Environ. 2017, 598, 949–958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsen, H.T. Membrane Filtration in Water Treatment—Removal of Micropollutants. Chem. Adv. Environ. Purif. Process. Water 2014. [Google Scholar] [CrossRef]
- Jiang, B.; Tian, C.; Song, G.; Pan, Q.; Wang, Z.; Shi, L.; Qiao, Y.; Fu, H. A green route to synthesize novel Ag/C antibacterial agent. Mater. Res. Bull. 2012, 47, 458–463. [Google Scholar] [CrossRef]
- Khandanlou, R.; Ahmad, M.B.; Shameli, K.; Saki, E.; Kalantari, K. Studies on properties of rice straw/polymer nanocomposites based on polycaprolactone and Fe3O4 nanoparticles and evaluation of antibacterial activity. Int. J. Mol. Sci. 2014, 15, 18466–18483. [Google Scholar] [CrossRef] [Green Version]
- Song, X.J.; Wang, Y.C.; Wang, C.Z.; Huang, M.H.; Gul, S.; Jiang, H.Q. Solar-Intensified Ultrafiltration System Based on Porous Photothermal Membrane for Efficient Water Treatment. ACS Sustain. Chem. Eng. 2019, 7, 4889–4896. [Google Scholar] [CrossRef]
- Gholami Derami, H.; Jiang, Q.; Ghim, D.; Cao, S.; Chandar, Y.J.; Morrissey, J.J.; Jun, Y.-S.; Singamaneni, S. A Robust and Scalable Polydopamine/Bacterial Nanocellulose Hybrid Membrane for Efficient Wastewater Treatment. ACS Appl. Nano Mater. 2019, 2, 1092–1101. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, X.; Tian, H.; Zhong, L.; Ma, C.; Zhou, Y.; Chen, S.; Li, D. Synthesis of antibacterial film CTS/PVP/TiO2/Ag for drinking water system. Carbohydr. Polym. 2012, 89, 1060–1066. [Google Scholar] [CrossRef]
- Julinova, M.; Vanharova, L.; Jurca, M. Water-soluble polymeric xenobiotics—Polyvinyl alcohol and polyvinylpyrrolidon—And potential solutions to environmental issues: A brief review. J. Environ. Manag. 2018, 228, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.J. The Role of Polyvinyl Alcohol in Cartilage Repair of the Ankle and First Metatarsophalangeal Joint. Clin. Podiatr. Med. Surg. 2018, 35, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Sriplai, N.; Mongkolthanaruk, W.; Eichhorn, S.J.; Pinitsoontorn, S. Magnetically responsive and flexible bacterial cellulose membranes. Carbohydr. Polym. 2018, 192, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Zhao, F.; Peng, Q.; Zhou, Z.; Han, Y. Production and characterization of bacterial cellulose produced by Gluconacetobacter xylinus isolated from Chinese persimmon vinegar. Carbohydr. Polym. 2018, 194, 200–207. [Google Scholar] [CrossRef]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; de Souza, C.F.; Martin, A.A.; da Silva, R.; de Freitas, R.A. Bacterial cellulose in biomedical applications: A review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Quero, F.; Nogi, M.; Yano, H.; Abdulsalami, K.; Holmes, S.M.; Sakakini, B.H.; Eichhorn, S.J. Optimization of the mechanical performance of bacterial cellulose/poly(L-lactic) acid composites. ACS Appl. Mater. Interfaces 2010, 2, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Zhang, F.; Wang, F.; Wang, H. Catalytic reduction of NOx by biomass-derived activated carbon supported metals. Chin. J. Chem. Eng. 2018, 26, 2077–2083. [Google Scholar] [CrossRef]
- An, Y.-Z.; Wang, C.-H.; Miao, P.; Wang, X.-X.; Liang, J.-Y.; Liu, J. Improved decontamination performance of biofilm systems using carbon fibers as carriers for microorganisms. New Carbon Mater. 2018, 33, 188–192. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, L.; Zhong, L.; Zhou, Y.; Xue, J.; Li, Y. Preparation of an antibacterial chitosan-coated biochar-nanosilver composite for drinking water purification. Carbohydr. Polym. 2019, 219, 290–297. [Google Scholar] [CrossRef]
- Shao, L.S.; Li, J.J.; Guang, Y.; Zhang, Y.L.; Zhang, H.; Che, X.Y.; Wang, Y.H. PVA/polyethyleneimine-functionalized graphene composites with optimized properties. Mater. Des. 2016, 99, 235–242. [Google Scholar] [CrossRef]
- Xia, S.H.; Teng, S.H.; Wang, P. Synthesis of bioactive polyvinyl alcohol/silica hybrid fibers for bone regeneration. Mater. Lett. 2018, 213, 181–184. [Google Scholar] [CrossRef]
- Kashyap, S.; Pratihar, S.K.; Behera, S.K. Strong and ductile graphene oxide reinforced PVA nanocomposites. J. Alloy Compd. 2016, 684, 254–260. [Google Scholar] [CrossRef]
- Lv, X.D.; Li, G.H.; Pang, Z.Y.; Li, D.W.; Lei, L.; Lv, P.F.; Mushtaq, M.; Wei, Q.F. Fabricate BC/Fe3O4@PPy 3D nanofiber film as flexible electrode for supercapacitor application. J. Phys. Chem. Solids 2018, 116, 153–160. [Google Scholar] [CrossRef]
- Yang, C.-C.; Lee, Y.-J.; Chiu, S.-J.; Lee, K.-T.; Chien, W.-C.; Lin, C.-T.; Huang, C.-A. Preparation of a PVA/HAP composite polymer membrane for a direct ethanol fuel cell (DEFC). J. Appl. Electrochem. 2008, 38, 1329–1337. [Google Scholar] [CrossRef]
- Wang, J.; Gao, C.; Zhang, Y.; Wan, Y. Preparation and in vitro characterization of BC/PVA hydrogel composite for its potential use as artificial cornea biomaterial. Mater. Sci. Eng. 2010, 30, 214–218. [Google Scholar] [CrossRef]
- Sulaeva, I.; Henniges, U.; Rosenau, T.; Potthast, A. Bacterial cellulose as a material for wound treatment: Properties and modifications. A review. Biotechnol. Adv. 2015, 33, 1547–1571. [Google Scholar] [CrossRef]
- Foresti, M.L.; Vazquez, A.; Boury, B. Applications of bacterial cellulose as precursor of carbon and composites with metal oxide, metal sulfide and metal nanoparticles: A review of recent advances. Carbohydr. Polym. 2017, 157, 447–467. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zheng, S.; Hu, Z.; Zhong, L.; Wang, Y.; Zhang, X.; Xue, J. Preparation of Polyvinyl Alcohol/Bacterial-Cellulose-Coated Biochar–Nanosilver Antibacterial Composite Membranes. Appl. Sci. 2020, 10, 752. https://doi.org/10.3390/app10030752
Zhang L, Zheng S, Hu Z, Zhong L, Wang Y, Zhang X, Xue J. Preparation of Polyvinyl Alcohol/Bacterial-Cellulose-Coated Biochar–Nanosilver Antibacterial Composite Membranes. Applied Sciences. 2020; 10(3):752. https://doi.org/10.3390/app10030752
Chicago/Turabian StyleZhang, Liang, Sen Zheng, Zhihui Hu, Lvling Zhong, Yao Wang, Xiaomin Zhang, and Juanqin Xue. 2020. "Preparation of Polyvinyl Alcohol/Bacterial-Cellulose-Coated Biochar–Nanosilver Antibacterial Composite Membranes" Applied Sciences 10, no. 3: 752. https://doi.org/10.3390/app10030752