Assessment of Performance of Two Rapid Methods for On-Site Control of Microbial and Biofilm Contamination
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Cultures and Growth Conditions
2.2. Biofilm Growth
- Clean and sterile stainless steel surfaces where monospecies and mixed biofilms are grown.
- Clean and sterile stainless steel surfaces, without microbial contamination, used as control
- Clean and sterile stainless steel surfaces where planktonic cells are inoculated to show the presence of biofilms without biofilm formation.
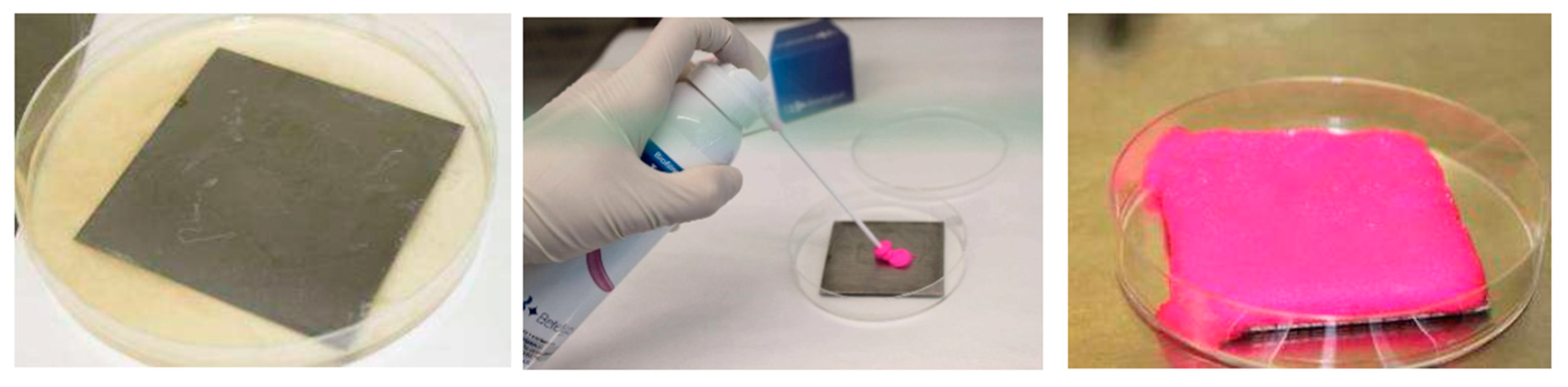
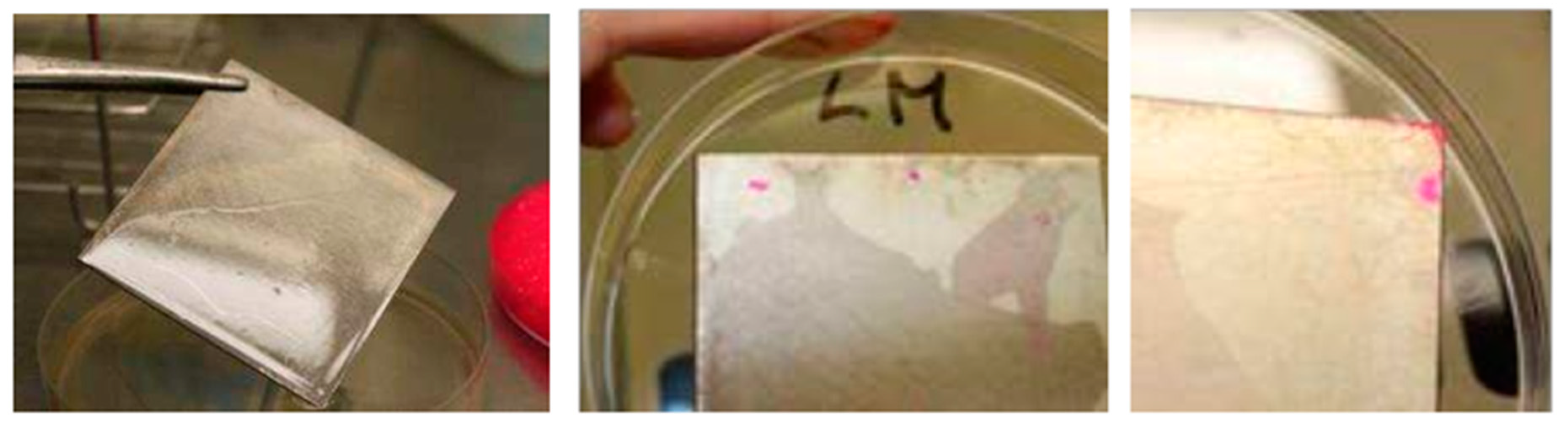
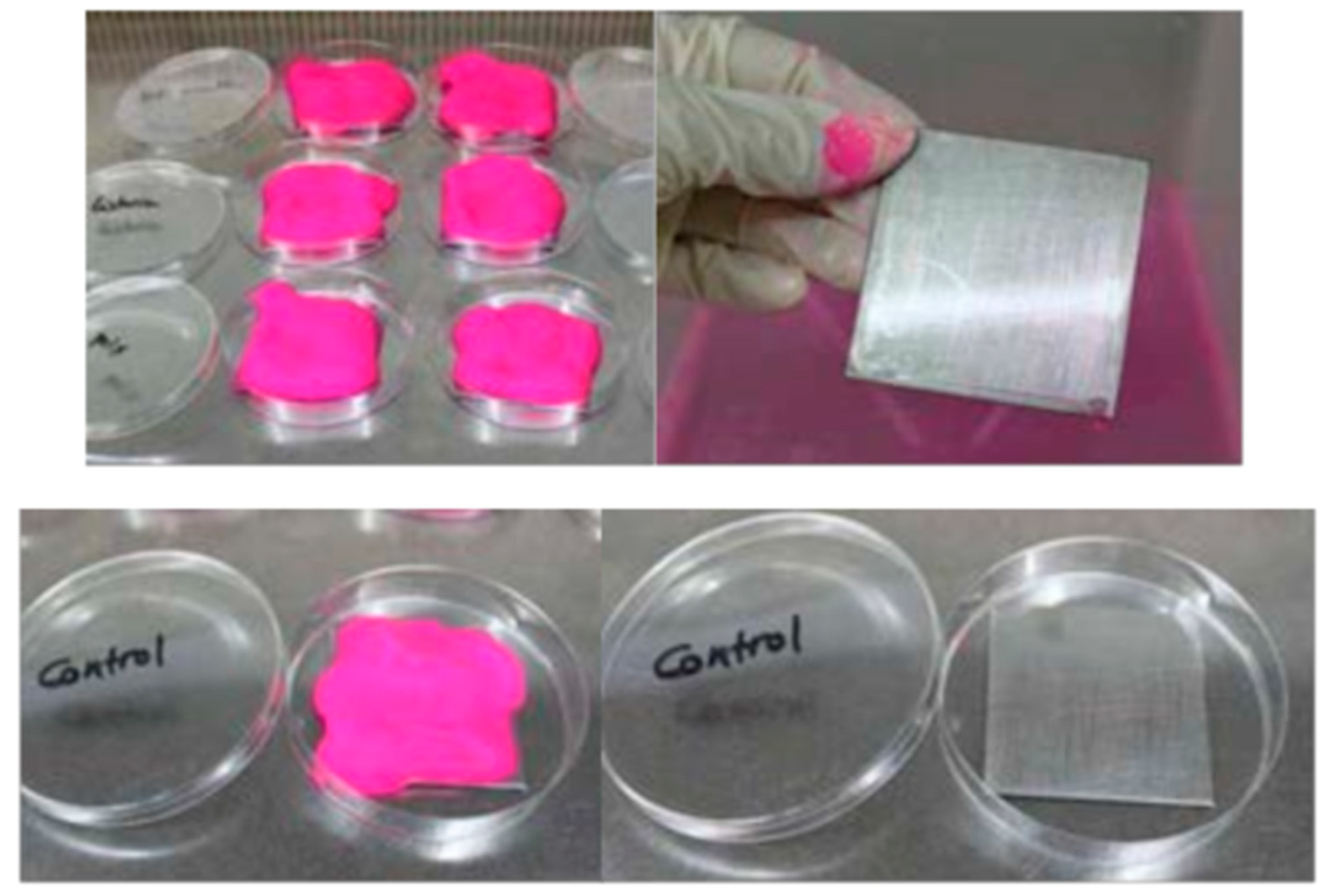
2.3. Staining of Biofilms
2.4. Bacterial Counts and Determination of Biomass FreshCheck Studies
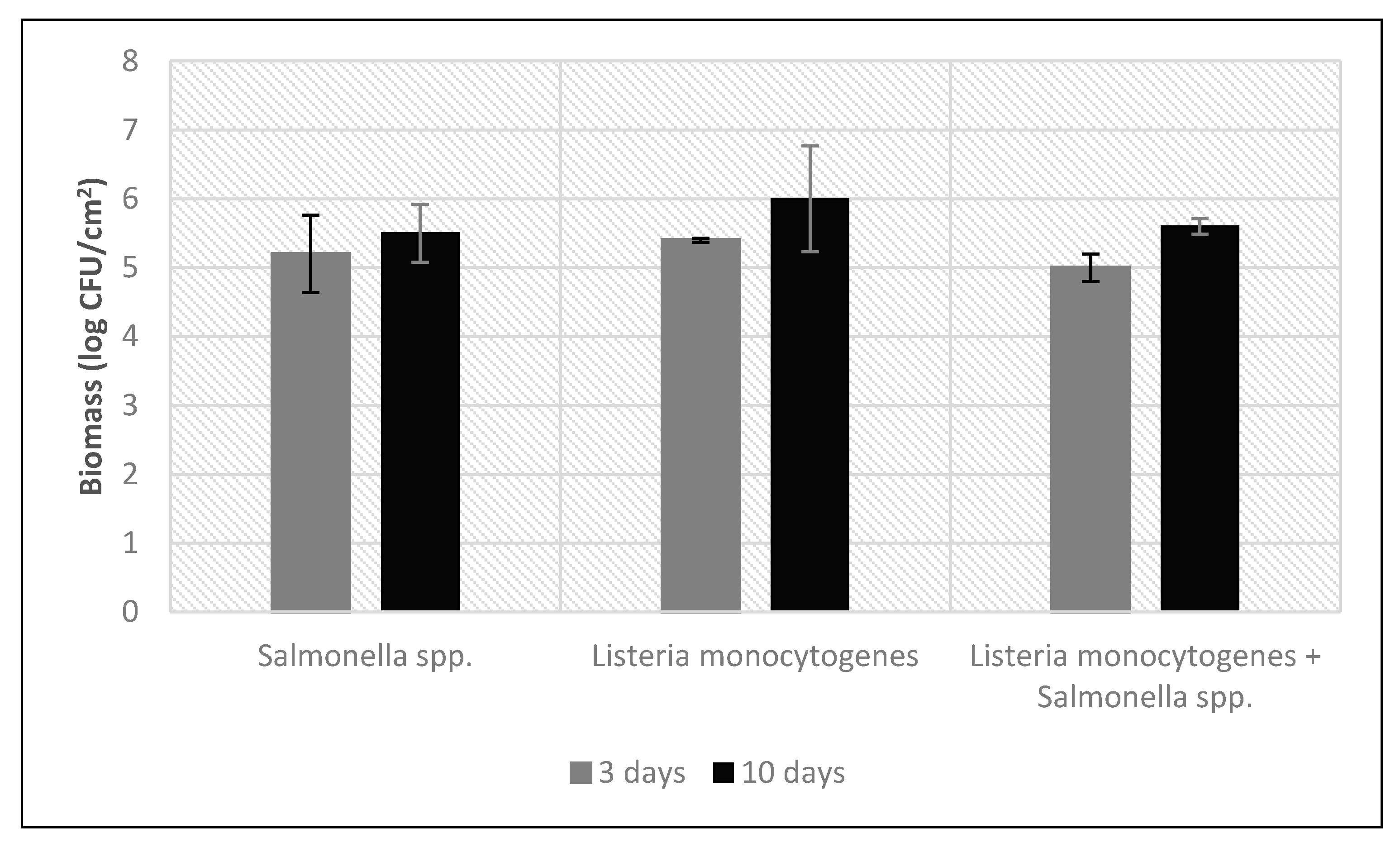
3. Results
3.1. Biofilm Staining
3.2. Microbial Detection Using FreshCheck
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Newell, D.G.; Koopmans, M.; Verhoef, L.; Duizer, E.; Aidara-Kane, A.; Sprong, H.; Opsteegh, M.; Langelaar, M.; Threfall, J.; Scheutz, F.; et al. Food-borne diseases—The challenges of 20 years ago still persist while new ones continue to emerge. Int. J. Food Microbiol. 2010, 139, S3–S15. [Google Scholar] [CrossRef] [PubMed]
- Orihuel, E.; Bertó, R.; Canet, J.J.; Lorenzo, F.; Milvaques, A.; Sanz-Puig, M.; Sabater, N. Listeria Monocytogenes in the Meat Industry; Betelgeux, S.L., Ed.; Christeyns Food Hygiene: Ador, Spain, 2019; ISBN 978-84-942180-4-0. [Google Scholar]
- EFSA; ECDC. The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 262. [Google Scholar]
- Lelieveld, H.L.M.; Mostert, M.A.; White, B.; Holah, J.T. Hygiene in Food Processing: Principles and Practice, 2nd ed.; Woodhead Publishing Limited: Cambridge, UK, 2003; ISBN 9781855734661. [Google Scholar]
- Holah, J.T.; Lavaud, A.; Peters, W.; Dye, K.A. Future techniques for disinfectant efficacy testing. Int. Biodeterior. Biodegrad. 1998, 41, 273–279. [Google Scholar] [CrossRef]
- Bessems, E. The effect of practical conditions on the efficacy of disinfectants. Int. Biodeterior. Biodegrad. 1998, 41, 177–183. [Google Scholar] [CrossRef]
- Magalhães, R.; Ferreira, V.; Brandão, T.R.S.; Palencia, R.C.; Almeida, G.; Teixeira, P. Persistent and non-persistent strains of Listeria monocytogenes: A focus on growth kinetics under different temperature, salt, and pH conditions and their sensitivity to sanitizers. Food Microbiol. 2016, 57, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S. Do Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Puga, C.H.; Sanjose, C.; Orgaz, B. Biofilm development at low temperatures enhances Listeria monocytogenes resistance to chitosan. Food Control 2016, 65, 143–151. [Google Scholar] [CrossRef]
- Melero, B.; Stessl, B.; Manso, B.; Wagner, M.; Esteban-Carbonero, Ó.J.; Hernández, M.; Rovira, J.; Rodriguez-Lázaro, D. Listeria monocytogenes colonization in a newly established dairy processing facility. Int. J. Food Microbiol. 2019, 289, 64–71. [Google Scholar] [CrossRef]
- Gibson, H.; Taylor, J.H.; Hall, K.E.; Holah, J.T. Effectiveness of cleaning techniques used in the food industry in terms of the removal of bacterial biofilms. J. Appl. Microbiol. 1999, 87, 41–48. [Google Scholar] [CrossRef]
- Lelieveld, H.; Mostert, T.; Holah, J. Handbook of Hygiene Control in the Food Industry; Woodhead Publishing Limited: Cambridge, UK, 2005; ISBN 9781845690533. [Google Scholar]
- Davidson, C.A.; Griffith, C.J.; Peters, A.C.; Fielding, L.M. Evaluation of two methods for monitoring surface cleanliness—ATP bioluminescence and traditional hygiene swabbing. Luminescence 1999, 14, 33–38. [Google Scholar] [CrossRef]
- Bajerski, F.; Stock, J.; Hanf, B.; Darienko, T.; Heine-Dobbernack, E.; Lorenz, M.; Naujox, L.; Keller, E.R.J.; Schumacher, H.M.; Friedl, T.; et al. ATP content and cell viability as indicators for cryostress across the diversity of life. Front. Physiol. 2018, 9, 921. [Google Scholar] [CrossRef] [PubMed]
- Jasson, V.; Jacxsens, L.; Luning, P.; Rajkovic, A.; Uyttendaele, M. Alternative microbial methods: An overview and selection criteria. Food Microbiol. 2010, 27, 710–730. [Google Scholar] [CrossRef] [PubMed]
- Simões, M.; Simões, L.V.M. A review of current and emergent biofilm control strategies. LWT Food Sci. Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef]
- Cadena, M.; Kelman, T.; Marco, M.L.; Pitesky, M. Understanding Antimicrobial Resistance (AMR) Profiles of Salmonella Biofilm and Planktonic Bacteria Challenged with Disinfectants Commonly Used During Poultry Processing. Foods 2019, 8, 275. [Google Scholar] [CrossRef]
- Orihuel, E.; Bertó, R.; Lorenzo, F.; López, C.; San Jose, C.; Orgaz, B. Biofilm-marking composition and method for detection of same on surfaces. European Patent EP2634260A1, 4 November 2013. [Google Scholar]
- Rani, S.A.; Pitts, B.; Beyenal, H.; Veluchamy, R.A.; Lewandowski, Z.; Davison, W.M.; Buckingham-Meyer, K.; Stewart, P.S. Spatial patterns of DNA replication, protein synthesis, and oxygen concentration within bacterial biofilms reveal diverse physiological states. J. Bacteriol. 2007, 189, 4223–4233. [Google Scholar] [CrossRef]
- Lorenzo, F.; Orihuel, E.; Bertó, R.; López, C. Control de la presencia de biofilms en las industrias alimentarias. Aliment. Equipos Tecnol. 2011, 264, 43–47. [Google Scholar]
- Lorenzo, F.; Orihuel, E.; López, C.; Catalá, M.; Bertó, R.; Orihuel, E.; San Jose, C.; Orgaz, B. A rapid, easy technique for routine control of biofilms on surfaces. In Proceedings of the 2nd Workshop on Food Safety: Technologies and Innovations Applied to Food Safety, Valencia, Spain, 5–6 July 2012. [Google Scholar]
- Bond, A.; Simpson, J.; Peach, R. Colour changing compositions. International Patent Application WO 2018/185486 A1, 11 October 2018. [Google Scholar]
- Milagres, A.M.F.; Machuca, A.; Napoleão, D. Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J. Microbiol. Methods 1999, 37, 1–6. [Google Scholar] [CrossRef]
- Campden BRI. Rapid Methods for Hygiene Determination; Campden BRI: Chipping Campden, UK, 2018. [Google Scholar]
- Stiefel, P.; Rosenberg, U.; Schneider, J.; Mauerhofer, S.; Maniura-Weber, K.; Ren, Q. Is biofilm removal properly assessed? Comparison of different quantification methods in a 96-well plate system. Appl. Microbiol. Biotechnol. 2016, 100, 4135–4145. [Google Scholar] [CrossRef] [PubMed]
- Gamble, G.R.; Lawrence, K.C.; Park, B.; Yoon, S.; Heitschmidt, G.W. Food Grade Dye for Assessment of Biofilm Removal from Stainless Steel by Cleaning and Sanitizing Agents. Food Prot. Trends 2019, 39, 442–448. [Google Scholar]
- Chmielewski, R.A.N.; Frank, J.F. Biofilm Formation and Control in Food Processing Facilities. Compr. Rev. Food Sci. Food Saf. 2003, 2, 22–32. [Google Scholar] [CrossRef]




| Technique | Target for Detection | Cost per Sample * | Ease of Use † | Specificity ‡ |
|---|---|---|---|---|
| ATP bioluminescence | ATP molecules contained in living cells | Moderate | High | Moderate |
| Contact plating | Viable microorganisms | Low | High | High |
| Swabbing | Viable microorganisms | High | Low | High |
| Test strips | Residues of detergents or disinfectants | Low | High | High |
| Visual inspection | Visible residual soiling | Low | High | Low |
| Product Number | Product Name | Main Biocide Substance |
|---|---|---|
| 1 | Dectocide A30 | Alkylamine |
| 2 | Dectocide SB9 | Glutaraldehyde |
| 3 | Dexacide B10 | Benzalkonium chloride |
| 4 | Quacide PQ60 EC | Alkylamine + PHMB |
| 5 | Quacide DA80 | Lactic acid |
| 3 Days Biofilm | ||||
|---|---|---|---|---|
| Microorganism | Biomass (CFU/surface) n = 3 | Biomass (CFU/cm2) n = 3 | Biomass (log CFU/surface) n = 3 | Biomass (log CFU/cm2) n = 3 |
| Salmonella spp. | 2.8 × 106 | 1.6 × 105 | 6.45 ± 0.70 | 5.2 ± 0.56 |
| L. monocytogenes | 4.2 × 106 | 2.3 × 105 | 6.62 ± 0.04 | 5.4 ± 0.03 |
| Salmonella spp. + L. monocytogenes | 1.6 × 106 | 8.9 × 104 | 6.21 ± 0.25 | 5.0 ± 0.20 |
| 10 Days Biofilm | ||||
|---|---|---|---|---|
| Microorganism | Biomass (CFU/surface) n = 3 | Biomass (CFU/cm2) n = 3 | Biomass (log CFU/surface) n = 3 | Biomass (log CFU/cm2) n = 3 |
| Salmonella spp. | 6.2 × 106 | 3.5 × 105 | 6.79 ± 0.51 | 5.5 ± 0.42 |
| L. monocytogenes | 1.8 × 107 | 9.7 × 105 | 7.24 ± 0.93 | 6.0 ± 0.77 |
| Salmonella spp. + L. monocytogenes | 7.3 × 106 | 4.0 × 105 | 6.86 ± 0.13 | 5.6 ± 0.11 |
| Biocide | Before Disinfection (Both Microorganisms) | After Disinfection (L. monocytogenes) | After Disinfection (S. enterica) |
|---|---|---|---|
| 1 | Green | Purple | Purple |
| 2 | Green | Purple | Purple |
| 3 | Green | Purple | Purple |
| 4 | Green | Purple | Purple |
| 5 | Green | Purple | Purple |
| Microbial Concentration (CFU/mL) | Contact Slide | FreshCheck |
|---|---|---|
| 106 | >300 | Green |
| 105 | >300 | Green |
| 104 | >300 | Green |
| 103 | 52 | Green |
| 102 | - | Green |
| 101 | 2 | Green |
| 0 (control) | - | Purple |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo, F.; Sanz-Puig, M.; Bertó, R.; Orihuel, E. Assessment of Performance of Two Rapid Methods for On-Site Control of Microbial and Biofilm Contamination. Appl. Sci. 2020, 10, 744. https://doi.org/10.3390/app10030744
Lorenzo F, Sanz-Puig M, Bertó R, Orihuel E. Assessment of Performance of Two Rapid Methods for On-Site Control of Microbial and Biofilm Contamination. Applied Sciences. 2020; 10(3):744. https://doi.org/10.3390/app10030744
Chicago/Turabian StyleLorenzo, Fernando, Maria Sanz-Puig, Ramón Bertó, and Enrique Orihuel. 2020. "Assessment of Performance of Two Rapid Methods for On-Site Control of Microbial and Biofilm Contamination" Applied Sciences 10, no. 3: 744. https://doi.org/10.3390/app10030744
APA StyleLorenzo, F., Sanz-Puig, M., Bertó, R., & Orihuel, E. (2020). Assessment of Performance of Two Rapid Methods for On-Site Control of Microbial and Biofilm Contamination. Applied Sciences, 10(3), 744. https://doi.org/10.3390/app10030744




