Tetraethylammonium Acetate and Tetraethylammonium Bromide-Based Deep Eutectic Solvents as Thermodynamic CO2 Gas Hydrate Inhibitors
Abstract
:1. Introduction
2. Methodology
2.1. Materials
2.2. Experimental Procedure
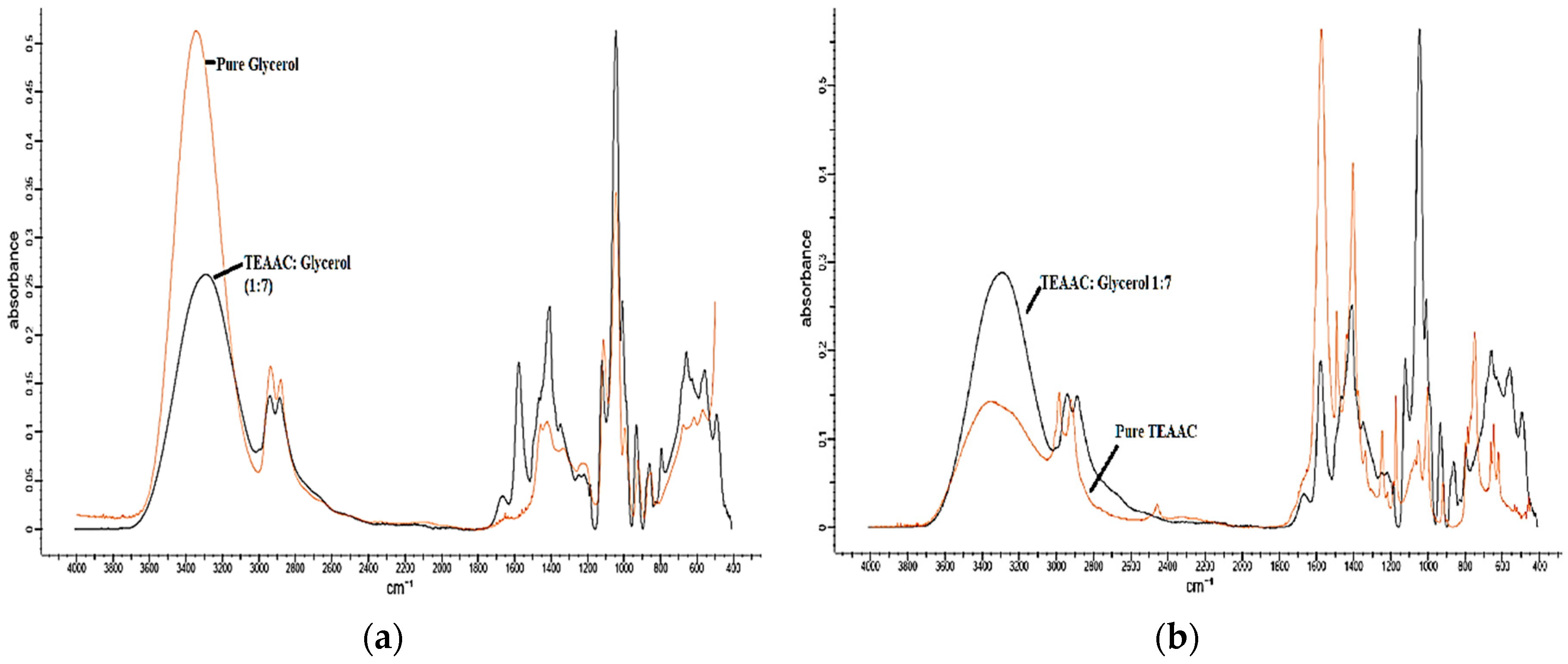
2.2.1. Characterization Using Fourier Transform Infrared Spectroscopy (FTIR)
2.2.2. Gas Hydrate Inhibition Study
2.3. Average Suppression Temperature (Ŧ) and Dissociation Enthalpies (ΔHdiss)
3. Results
3.1. Materials Characterization
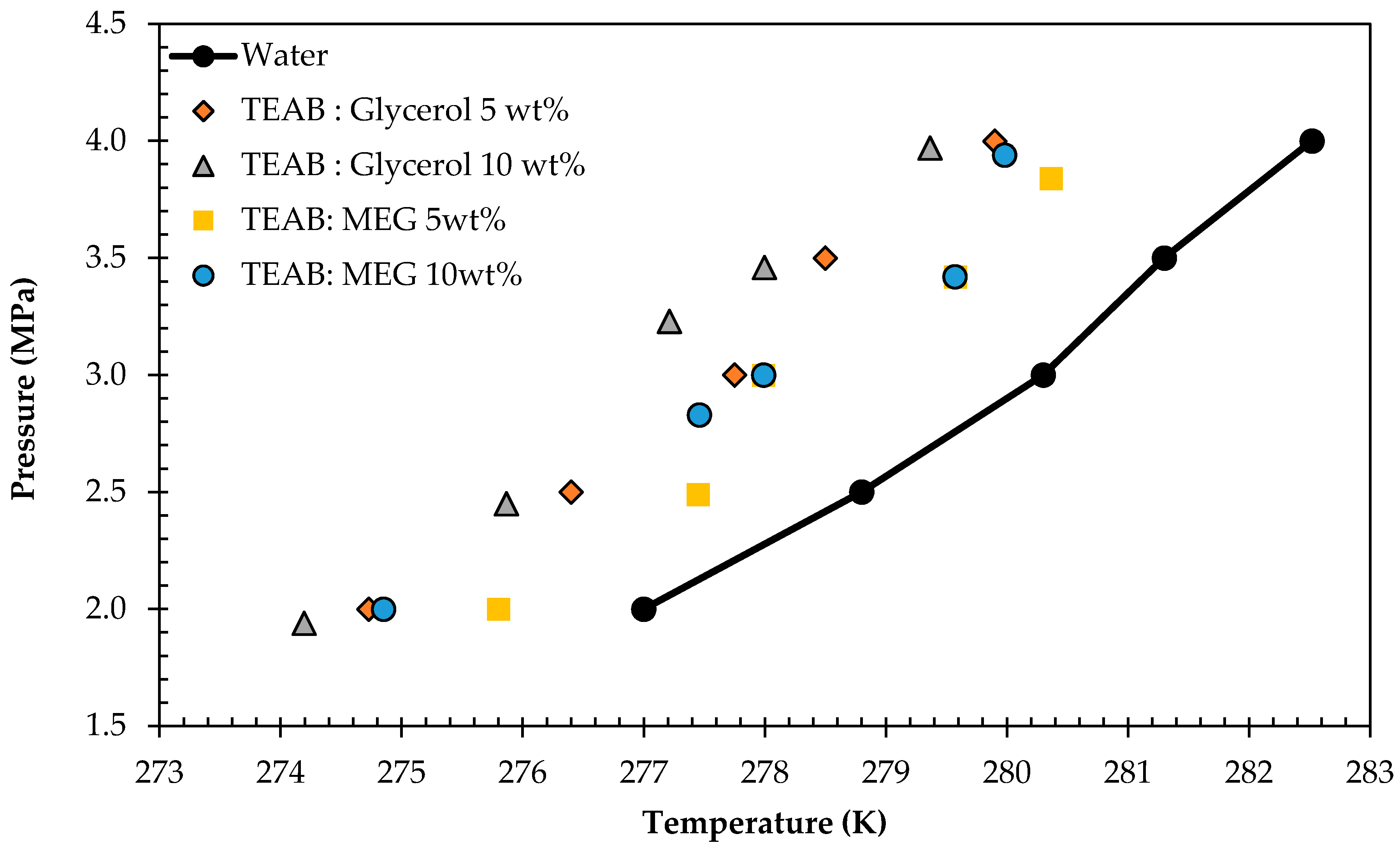
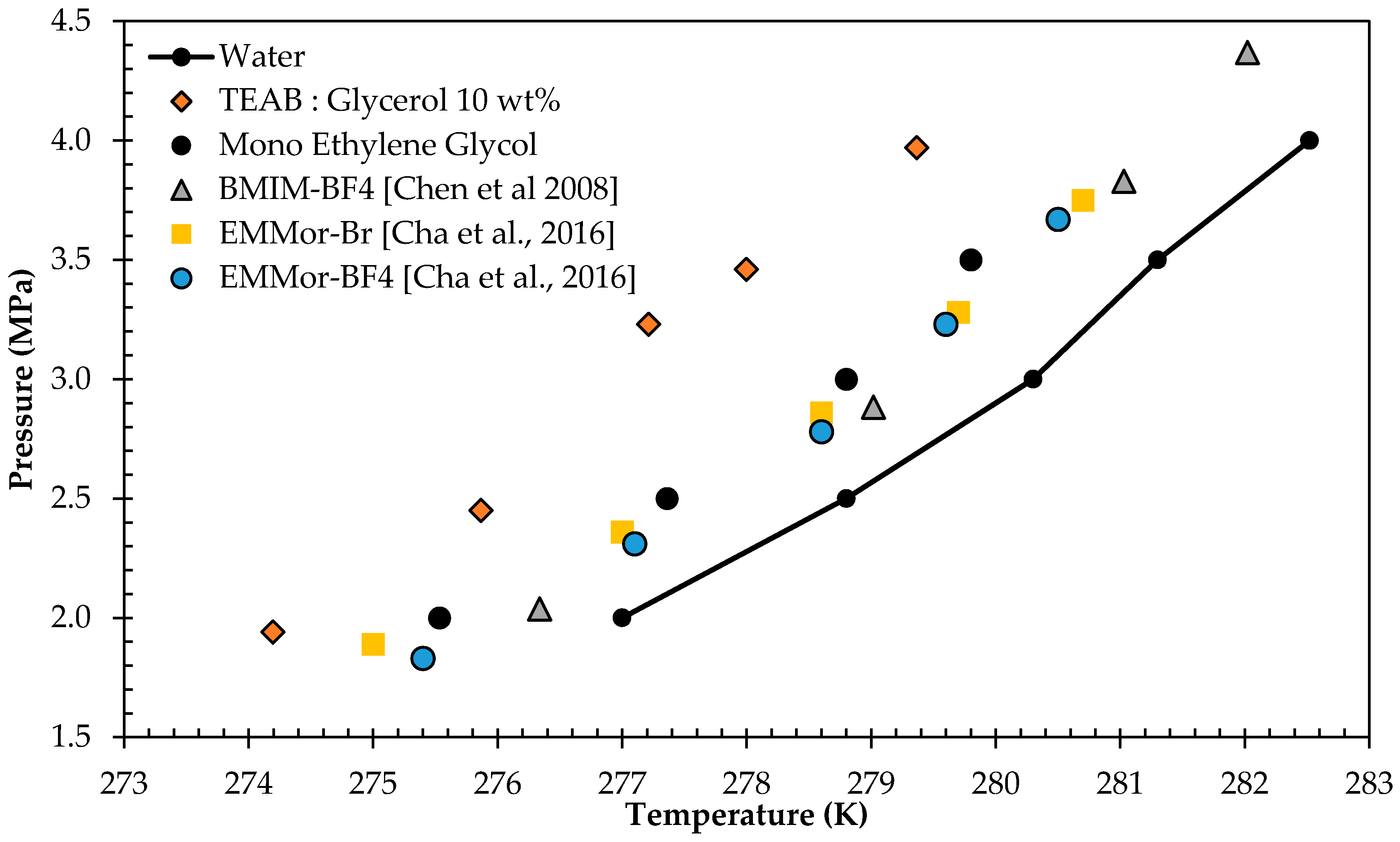
3.2. Gas Hydrate Inhibition Study
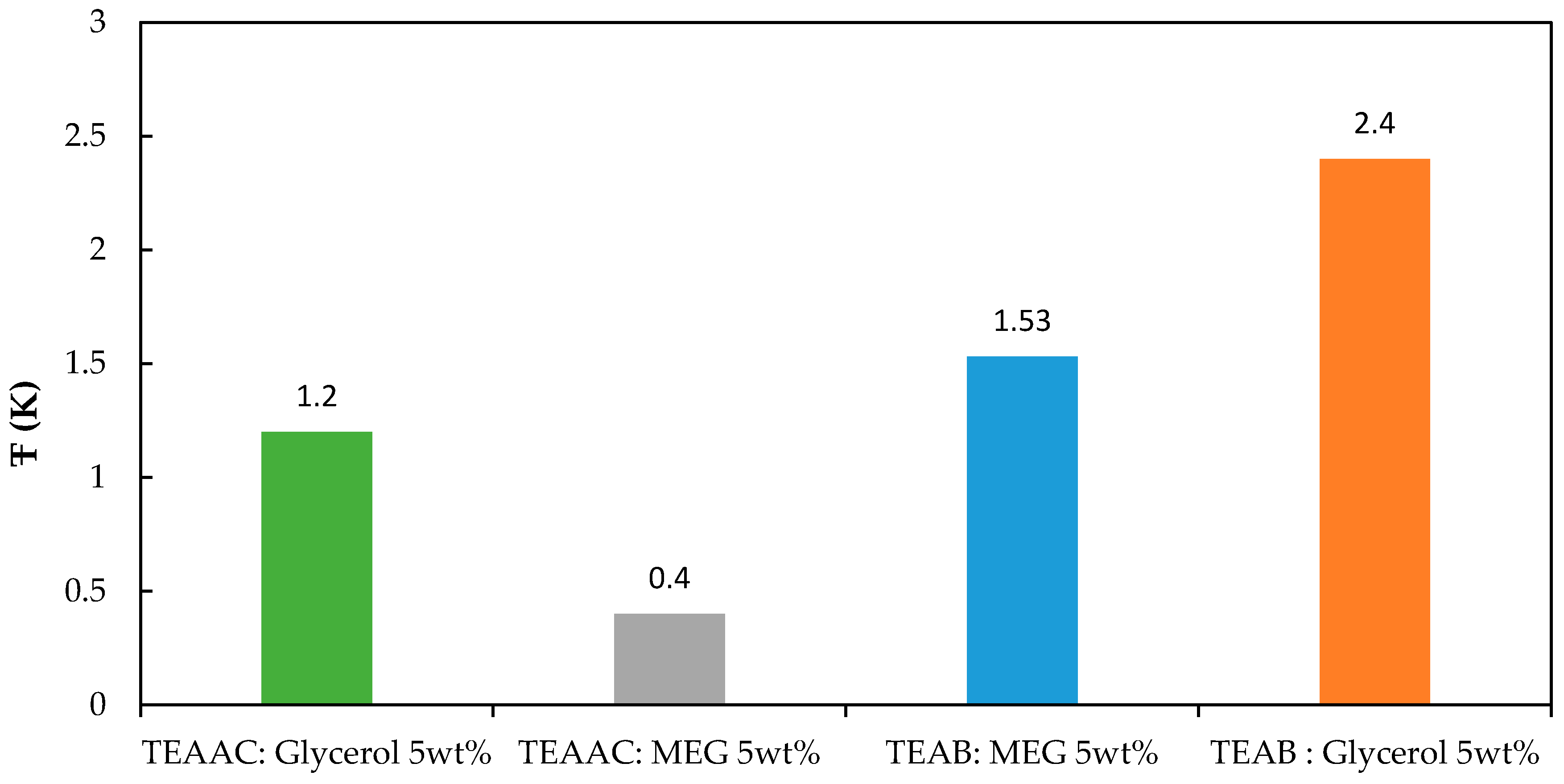
3.3. Average Suppression Temperature (Ŧ)
3.4. Gas Hydrate Dissociation Enthalpies (ΔHdiss)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, M.S.; Lal, B.; Keong, L.K.; Sabil, K.M. Experimental evaluation and thermodynamic modelling of AILs alkyl chain elongation on methane riched gas hydrate system. Fluid Phase Equilib. 2018, 473, 300–309. [Google Scholar] [CrossRef]
- Partoon, B.; Nashed, O.; Kassim, Z.; Sabil, K.M.; Sangwai, J.; Lal, B. Gas Hydrate Equilibrium Measurement of Methane + Carbon Dioxide + Tetrahydrofuran+ Water System at High CO2 Concentrations. Procedia Eng. 2016, 148, 1220–1224. [Google Scholar] [CrossRef] [Green Version]
- Bavoh, C.B.; Nashed, O.; Khan, M.S.; Partoon, B.; Lal, B.; Sharif, A.M. The impact of amino acids on methane hydrate phase boundary and formation kinetics. J. Chem. Thermodyn. 2018, 117, 48–53. [Google Scholar] [CrossRef]
- Khan, M.S.; Partoon, B.; Bavoh, C.B.; Lal, B.; Mellon, N.B. Influence of tetramethylammonium hydroxide on methane and carbon dioxide gas hydrate phase equilibrium conditions. Fluid Phase Equilib. 2017, 440, 1–8. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Bavoh, C.B.; Keong, L.K.; Bustam, A.; Mellon, N.B. Influence of Ammonium based Compounds for Gas Hydrate Mitigation: A Short Review. Indian J. Sci. Technol. 2017, 10, 1–6. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Lal, B.; Khan, M.S.; Osei, H.; Ayuob, M. Combined Inhibition Effect of 1-Ethyl-3-methy-limidazolium Chloride + Glycine on Methane Hydrate. J. Phys. Conf. Ser. 2018, 1123, 012060. [Google Scholar] [CrossRef]
- Khan, M.S.; Bavoh, C.B.; Partoon, B.; Nashed, O.; Lal, B.; Mellon, N.B. Impacts of ammonium based ionic liquids alkyl chain on thermodynamic hydrate inhibition for carbon dioxide rich binary gas. J. Mol. Liq. 2018, 261, 283–290. [Google Scholar] [CrossRef]
- Jai Krishna Sahith, S.; Pedapati, S.R.; Lal, B. Application of artificial neural networks on measurement of gas hydrates in pipelines. Test Eng. Manag. 2019, 81, 5769–5774. [Google Scholar]
- Qasim, A.; Khan, M.S.; Lal, B.; Shariff, A.M.; Saad, M.; Lal, B.; Mohammad, A. A perspective on dual purpose gas hydrate and corrosion inhibitors for flow assurance. J. Pet. Sci. Eng. 2019, 183, 106418. [Google Scholar] [CrossRef]
- Yaqub, S.; Lal, B.; Partoon, B.; Mellon, N.B. Investigation of the task oriented dual function inhibitors in gas hydrate inhibition: A review. Fluid Phase Equilib. 2018, 477, 40–57. [Google Scholar] [CrossRef]
- Sivabalan, V.; Hassan, N.A.; Qasim, A.; Lal, B.; Bustam, M.A. Density measurement of aqueous tetraethylammonium bromide and tetraethylammonium iodide solutions at different temperatures and concentrations. S. Afr. J. Chem. Eng. 2020, 32, 62–67. [Google Scholar] [CrossRef]
- Vinayagam, S.; Belkhir, W.; Yoann, M.; Ali, Q.; Bhajan, L. Corrosion inhibition study on glycerol as simultaneous gas hydrate and corrosion inhibitor in gas pipelines. Malays. J. Anal. Sci. 2020, 24, 62–69. [Google Scholar]
- Nallakukkala, S.; Sivabalan, V.; Lal, B.; Mokhtar Che Ismail, N.D.N. Nonionic Surfactants as Corrosion Inhibitors for Carbon Steel in Hydrochloric acid Medium. TEST Eng. Manag. 2019, 81, 5830–5835. [Google Scholar]
- Lal, B.; Nashed, O. Chemical Additives for Gas Hydrates; Green Energy and Technology; Springer International Publishing: Cham, Switzerland, 2020; ISBN 978-3-030-30749-3. [Google Scholar]
- Qasim, A.; Khan, M.S.; Lal, B.; Shariff, A.M. Phase equilibrium measurement and modeling approach to quaternary ammonium salts with and without monoethylene glycol for carbon dioxide hydrates. J. Mol. Liq. 2019, 282, 106–114. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Lal, B.; Osei, H.; Sabil, K.M.; Mukhtar, H. A review on the role of amino acids in gas hydrate inhibition, CO2 capture and sequestration, and natural gas storage. J. Nat. Gas Sci. Eng. 2019, 64, 52–71. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Shariff, A.M.; Mukhtar, H. Ammonium hydroxide ILs as dual-functional gas hydrate inhibitors for binary mixed gas (carbon dioxide and methane) hydrates. J. Mol. Liq. 2019, 274, 33–44. [Google Scholar] [CrossRef]
- Khan, M.S.; Lal, B.; Partoon, B.; Keong, L.K.; Bustam, A.B.; Mellon, N.B. Experimental Evaluation of a Novel Thermodynamic Inhibitor for CH4 and CO2 Hydrates. Procedia Eng. 2016, 148, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Liew, C.S.; Kurnia, K.A.; Cornelius, B.; Lal, B. Application of COSMO-RS in Investigating Ionic Liquid as Thermodynamic Hydrate Inhibitor for Methane Hydrate. Procedia Eng. 2016, 148, 862–869. [Google Scholar] [CrossRef] [Green Version]
- Kartikawati, N.A.; Lal, B.; Mutalib, M.I.B.A.; Sufian, S.B.; Shariff, A.M. Density Measurement of Aqueous solution of Tetrapropylammonium Hydroxide at different Temperatures and Concentrations: Data and Correlation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 458. [Google Scholar] [CrossRef]
- Nashed, O.; Sabil, K.M.; Lal, B.; Ismail, L.; Jaafar, A.J. Study of 1-(2-Hydroxyethyle) 3-methylimidazolium Halide as Thermodynamic Inhibitors. Appl. Mech. Mater. 2014, 625, 337–340. [Google Scholar] [CrossRef]
- Sabil, K.M.; Nashed, O.; Lal, B.; Ismail, L.; Japper-Jaafar, A. Experimental investigation on the dissociation conditions of methane hydrate in the presence of imidazolium-based ionic liquids. J. Chem. Thermodyn. 2015, 84, 7–13. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Al-Murshedi, A.Y.M. Deep eutectic Solvent-Water Mixtures. Ph.D. Thesis, University of Leicester, Leicester, UK, 2018. [Google Scholar]
- Potdar, M.; Kelso, G.; Schwarz, L.; Zhang, C.; Hearn, M. Recent Developments in Chemical Synthesis with Biocatalysts in Ionic Liquids. Molecules 2015, 20, 16788–16816. [Google Scholar] [CrossRef] [PubMed]
- Kar, M.; Plechkova, N.V.; Seddon, K.R.; Pringle, J.M.; MacFarlane, D.R. Ionic Liquids–Further Progress on the Fundamental Issues. Aust. J. Chem. 2019, 72, 3. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Mondal, A.; Balasubramanian, S. Recent advances in modeling green solvents. Curr. Opin. Green Sustain. Chem. 2017, 5, 37–43. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep eutectic solvents: Physicochemical properties and gas separation applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Tang, B.; Row, K.H. Recent developments in deep eutectic solvents in chemical sciences. Mon. Chem. Chem. Mon. 2013, 144, 1427–1454. [Google Scholar] [CrossRef]
- Lee, D.; Go, W.; Oh, J.; Lee, J.; Jo, I.; Kim, K.-S.; Seo, Y. Thermodynamic inhibition effects of an ionic liquid (choline chloride), a naturally derived substance (urea), and their mixture (deep eutectic solvent) on CH4 hydrates. Chem. Eng. J. 2020, 399, 125830. [Google Scholar] [CrossRef]
- Ghaedi, H.; Lal, B.; Ayoub, M.; Shariff, A.M.; Sufian, S. Measurement and correlation of physicochemical properties of phosphonium-based deep eutectic solvents at several temperatures (293.15 K–343.15 K) for CO2 capture. J. Chem. Thermodyn. 2017, 113, 41–51. [Google Scholar] [CrossRef]
- Ghaedi, H.; Ayoub, M.; Sufian, S.; Shariff, A.M.; Lal, B.; Wilfred, C.D. Density and refractive index measurements of transition-temperature mixture (deep eutectic analogues) based on potassium carbonate with dual hydrogen bond donors for CO2 capture. J. Chem. Thermodyn. 2018, 118, 147–158. [Google Scholar] [CrossRef]
- Jia, H.; Huang, P.; Wang, Q.; Han, Y.; Wang, S.; Zhang, F.; Pan, W.; Lv, K. Investigation of inhibition mechanism of three deep eutectic solvents as potential shale inhibitors in water-based drilling fluids. Fuel 2019, 244, 403–411. [Google Scholar] [CrossRef]
- Aissaoui, T.; AlNashef, I.M.; Benguerba, Y. Dehydration of natural gas using choline chloride based deep eutectic solvents: COSMO-RS prediction. J. Nat. Gas Sci. Eng. 2016, 30, 571–577. [Google Scholar] [CrossRef]
- Nkuku, C.A.; LeSuer, R.J. Electrochemistry in Deep Eutectic Solvents. J. Phys. Chem. B 2007, 111, 13271–13277. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2006; pp. 1–23. ISBN 9780470027318. [Google Scholar]
- Sundramoorthy, J.D.; Sabil, K.M.; Lal, B.; Hammonds, P. Catastrophic Crystal Growth of Clathrate Hydrate with a Simulated Natural Gas System during a Pipeline Shut-In Condition. Cryst. Growth Des. 2015, 15, 1233–1241. [Google Scholar] [CrossRef]
- Sahith, S.J.K.; Pedapati, S.R.; Lal, B. Investigation on Gas Hydrates Formation and Dissociation in Multiphase Gas Dominant Transmission Pipelines. Appl. Sci. 2020, 10, 5052. [Google Scholar] [CrossRef]
- Akib, S.; Hashim, M.A.; Hayyan, A.; Alsaadi, M.A.; AlOmar, M.K.; Hayyan, M. Glycerol-based deep eutectic solvents: Physical properties. J. Mol. Liq. 2015, 215, 98–103. [Google Scholar] [CrossRef]
- Khan, M.S.; Yaqub, S.; Manner, N.; Karthwathi, N.A.; Qasim, A.; Mellon, N.B.; Lal, B. Experimental Equipment Validation for Methane (CH4) and Carbon Dioxide (CO2) Hydrates. IOP Conf. Ser. Mater. Sci. Eng. 2018, 344, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Sloan, D.; Koh, C.; Sum, A.K.; Ballard, A.L.; Creek, J.; Eaton, M.; Lachance, J.; cMullen, N.; Palermo, T.; Shoup, G.; et al. Natural Gas Hydrates in Flow Assurance; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 9781856179454. [Google Scholar]
- Abbasi, A.; Hashim, F.M. Development of a hydrate formation prediction model for sub-sea pipeline. Pet. Sci. Technol. 2017, 35, 443–450. [Google Scholar] [CrossRef]
- Garapati, N.; Anderson, B.J. Statistical thermodynamics model and empirical correlations for predicting mixed hydrate phase equilibria. Fluid Phase Equilib. 2014, 373, 20–28. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, Y.; Zeng, P.; Yang, W.; Liang, Q.; Peng, X.; Liu, Y.; Hu, Y. Effect of 1-butyl-3-methylimidazolium tetrafluoroborate on the formation rate of CO2 hydrate. J. Nat. Gas Chem. 2008, 17, 264–267. [Google Scholar] [CrossRef]
- Cha, J.H.; Ha, C.; Kang, S.P.; Kang, J.W.; Kim, K.S. Thermodynamic inhibition of CO2 hydrate in the presence of morpholinium and piperidinium ionic liquids. Fluid Phase Equilib. 2016, 413, 75–79. [Google Scholar] [CrossRef]
- Nasir, Q.; Lau, K.K.; Lal, B.; Sabil, K.M. Hydrate dissociation condition measurement of CO2-rich mixed gas in the presence of methanol/ethylene glycol and mixed methanol/ethylene glycol + electrolyte aqueous solution. J. Chem. Eng. Data 2014. [Google Scholar] [CrossRef]
- Partoon, B.; Sabil, K.M.; Roslan, H.; Lal, B.; Keong, L.K. Impact of acetone on phase boundary of methane and carbon dioxide mixed hydrates. Fluid Phase Equilib. 2016, 412, 51–56. [Google Scholar] [CrossRef]
- Bavoh, C.B.; Lal, B.; Keong, L.K.; Binti Jasamai, M.; Binti Idress, M. Synergic Kinetic Inhibition Effect of EMIM-Cl + PVP on CO2 Hydrate Formation. Procedia Eng. 2016, 148, 1232–1238. [Google Scholar] [CrossRef] [Green Version]
- Garg, S.; Shariff, A.M.; Shaikh, M.S.; Lal, B.; Suleman, H.; Faiqa, N. Experimental data, thermodynamic and neural network modeling of CO2 solubility in aqueous sodium salt of l-phenylalanine. J. CO2 Util. 2017, 19, 146–156. [Google Scholar] [CrossRef]
- Ullah, R.; Atilhan, M.; Anaya, B.; Khraisheh, M.; García, G.; ElKhattat, A.; Tariq, M.; Aparicio, S. A detailed study of cholinium chloride and levulinic acid deep eutectic solvent system for CO2 capture via experimental and molecular simulation approaches. Phys. Chem. Chem. Phys. 2015, 17, 20941–20960. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Chemical Name | Chemical Formula | Chemical Structure |
|---|---|---|
| Tetraethylammonium Acetate Tetra-Hydrate | (C2H5)4N(OCOCH3) · 4H2O | 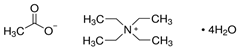 |
| Tetraethylammonium Bromide | (C2H5)4N(Br) | 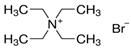 |
| Glycerol | C3H8O3 | 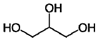 |
| Mono-Ethylene Glycol | (CH2OH)2 |  |
| Carbon Dioxide | CO2 |  |
| The Wavelength Range for Different Purposes of Testing | ||
|---|---|---|
| No | Purpose | Range (cm−1) |
| 1 | Testing for organics and hydrocarbons | 3200–2700 |
| 2 | Testing for hydroxyl or amino groups | 3650–3250 |
| 3 | Testing for carbonyl compounds | 1850–1650 |
| 4 | Testing for unsaturation | 1670–1620 |
| 5 | Testing for aromatics | 1615–1495 |
| 6 | Testing for multiple bonds | 2300–1990 |
| Water | TEAAC:Glycerol 5 wt % | TEAAC:MEG 5 wt % | TEAB:MEG 5 wt % | TEAB:Glycerol 5 wt % | |||||
|---|---|---|---|---|---|---|---|---|---|
| T/K | P/MPa | T/K | P/MPa | T/K | P/MPa | T/K | P/MPa | T/K | P/MPa |
| 277.00 | 2.00 | 276.34 | 2.30 | 275.14 | 2.00 | 275.80 | 2.00 | 274.73 | 2.00 |
| 278.80 | 2.50 | 278.30 | 2.70 | 278.50 | 2.50 | 277.45 | 2.49 | 276.40 | 2.50 |
| 280.30 | 3.00 | 279.17 | 3.00 | 280.15 | 3.00 | 277.99 | 3.00 | 277.75 | 3.00 |
| 281.43 | 3.50 | 280.53 | 3.50 | 281.02 | 3.50 | 279.57 | 3.42 | 278.50 | 3.50 |
| 282.52 | 4.00 | 281.42 | 3.80 | 282.39 | 4.00 | 280.36 | 3.84 | 279.90 | 4.00 |
| Regression Statistics | TEAAC:Glycerol 5 wt % | TEAAC:MEG 5 wt % | TEAB:MEG 5 wt % | TEAB:Glycerol 5 wt % |
|---|---|---|---|---|
| Multiple R | 0.978026 | 0.972272 | 0.972556 | 0.991241 |
| R square | 0.956535 | 0.945313 | 0.945866 | 0.982558 |
| Adjusted R square | 0.942047 | 0.927083 | 0.927822 | 0.976744 |
| Standard error | 0.510676 | 0.483046 | 0.480595 | 0.316228 |
| Significance F | 0.003897 | 0.005520 | 0.005435 | 0.000983 |
| Observations | 5 | 5 | 5 | 5 |
| Water | TEAB:Glycerol 10 wt % | TEAB:MEG 10 wt % | |||
|---|---|---|---|---|---|
| T/K | P/MPa | T/K | P/MPa | T/K | P/MPa |
| 277 | 2.0 | 274 | 1.9 | 274.9 | 2.0 |
| 279 | 2.5 | 276 | 2.5 | 277.5 | 2.8 |
| 280 | 3.0 | 277 | 3.2 | 278.0 | 3.0 |
| 281 | 3.5 | 278 | 3.5 | 279.6 | 3.4 |
| 283 | 4.0 | 279 | 4.0 | 280.0 | 3.9 |
| TEAAC:Glycerol 5 wt % | TEAAC:MEG5 wt % | TEAB:MEG5 wt % | TEAB:Glycerol 5 wt % | |
|---|---|---|---|---|
| P/MPa | ΔT/K | ΔT/K | ΔT/K | ΔT/K |
| 2.0 | 2.00 | 1.35 | 0.70 | 1.80 |
| 2.5 | 0.83 | 0.03 | 1.13 | 2.13 |
| 3.0 | 1.60 | 0.10 | 2.30 | 2.55 |
| 3.5 | 0.90 | 0.40 | 1.70 | 2.90 |
| 4.0 | 0.60 | 0.10 | 1.80 | 2.60 |
| Ŧ (K) | 1.2 | 0.4 | 1.53 | 2.40 |
| Pressure (MPa) | Pure Water | TEAAC:Glycerol 5 wt % | TEAAC:MEG 5 wt % | TEAB:MEG 5 wt % | TEAB:Glycerol 5 wt % |
|---|---|---|---|---|---|
| 2.0 | 69.60 | 66.95 | 70.02 | 77.37 | 74.47 |
| 2.5 | 66.45 | 64.42 | 67.01 | 73.71 | 70.88 |
| 3.0 | 62.96 | 62.34 | 63.64 | 69.57 | 67.04 |
| 3.5 | 59.22 | 58.68 | 59.83 | 65.44 | 62.71 |
| 4.0 | 55.58 | 56.38 | 55.86 | 60.56 | 58.18 |
| Average (ΔHdiss) (kj/mol) | 62.76 | 61.75 | 63.27 | 69.33 | 66.66 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sivabalan, V.; Hasnor, N.; Lal, B.; Kassim, Z.; Shah Maulud, A. Tetraethylammonium Acetate and Tetraethylammonium Bromide-Based Deep Eutectic Solvents as Thermodynamic CO2 Gas Hydrate Inhibitors. Appl. Sci. 2020, 10, 6794. https://doi.org/10.3390/app10196794
Sivabalan V, Hasnor N, Lal B, Kassim Z, Shah Maulud A. Tetraethylammonium Acetate and Tetraethylammonium Bromide-Based Deep Eutectic Solvents as Thermodynamic CO2 Gas Hydrate Inhibitors. Applied Sciences. 2020; 10(19):6794. https://doi.org/10.3390/app10196794
Chicago/Turabian StyleSivabalan, Vinayagam, Nurasyikin Hasnor, Bhajan Lal, Zamzila Kassim, and Abdulhalim Shah Maulud. 2020. "Tetraethylammonium Acetate and Tetraethylammonium Bromide-Based Deep Eutectic Solvents as Thermodynamic CO2 Gas Hydrate Inhibitors" Applied Sciences 10, no. 19: 6794. https://doi.org/10.3390/app10196794






