PEG-b-PLGA Nanoparticles Loaded with Geraniin from Phyllanthus Watsonii Extract as a Phytochemical Delivery Model
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
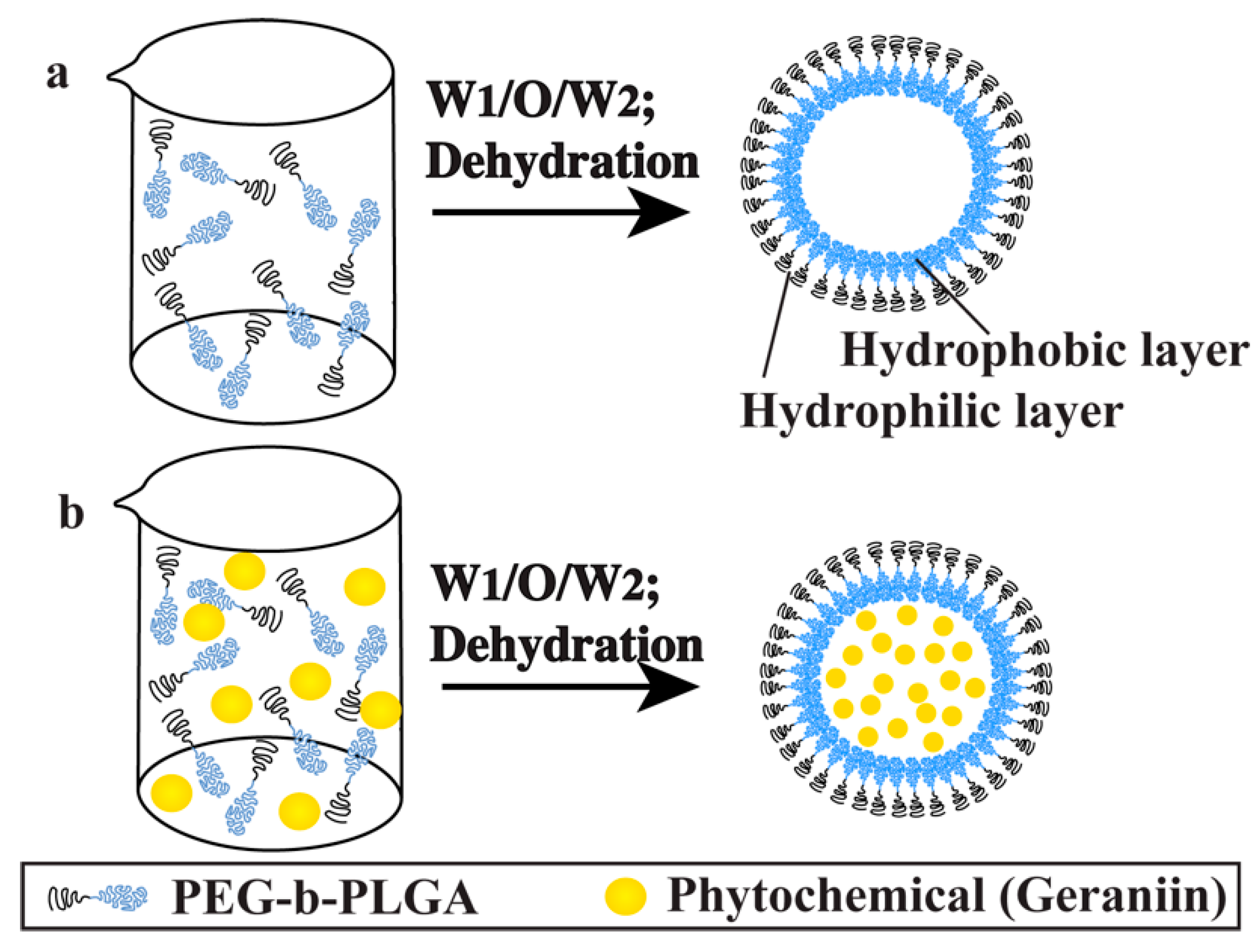
2.1. Synthesis of poly(ethylene glycol)-block-poly(lactic-co-glycolic acid) (PEG-b-PLGA) Nanoparticles (NPs)
2.2. Encapsulation of Geraniin into PEG-b-PLGA Nanoparticles (NPs)
2.3. Characterization of the Nanoparticles (NPs)
2.3.1. Particle Size and Zeta Potential
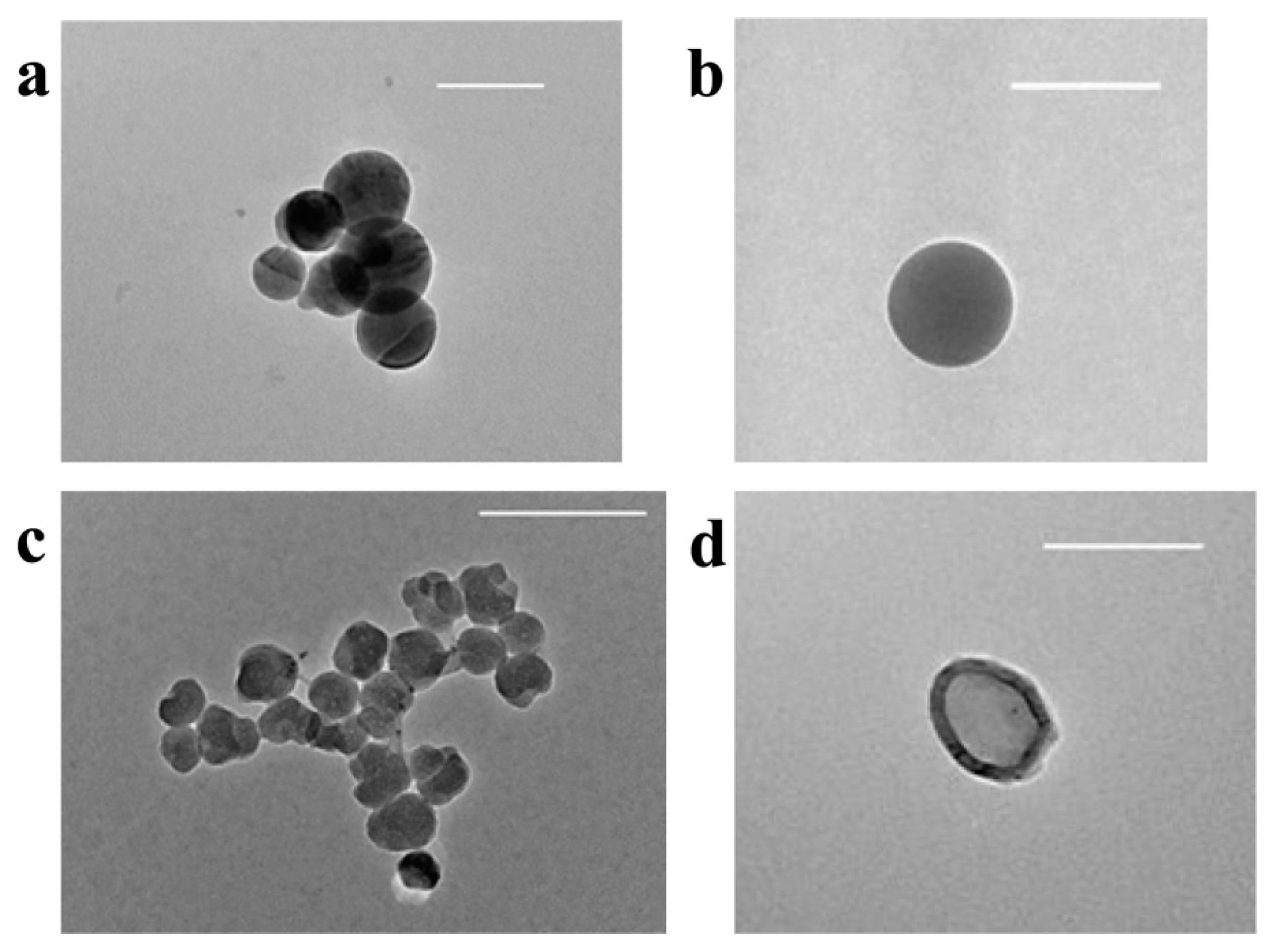
2.3.2. Transmission Electron Microscopy (TEM)
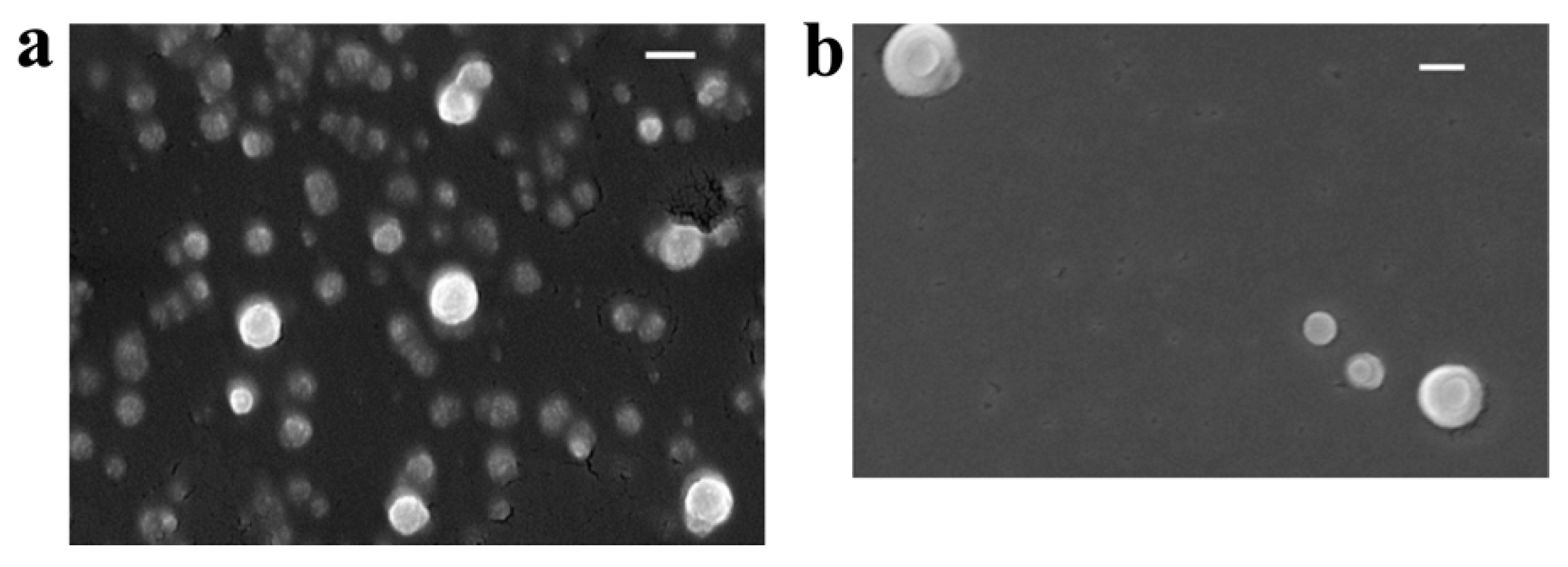
2.3.3. Field Emission Scanning Electron Microscopy (FESEM)
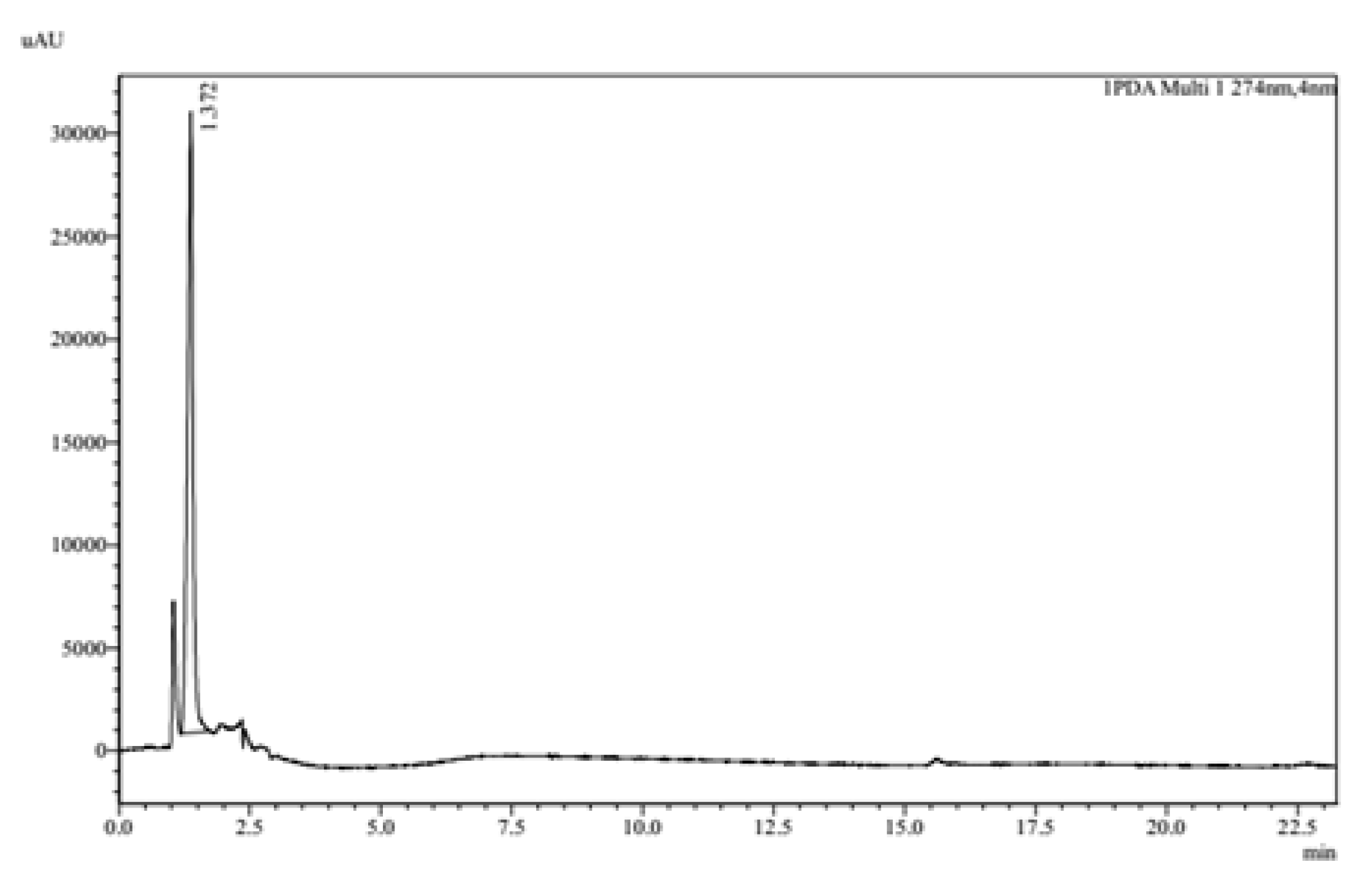
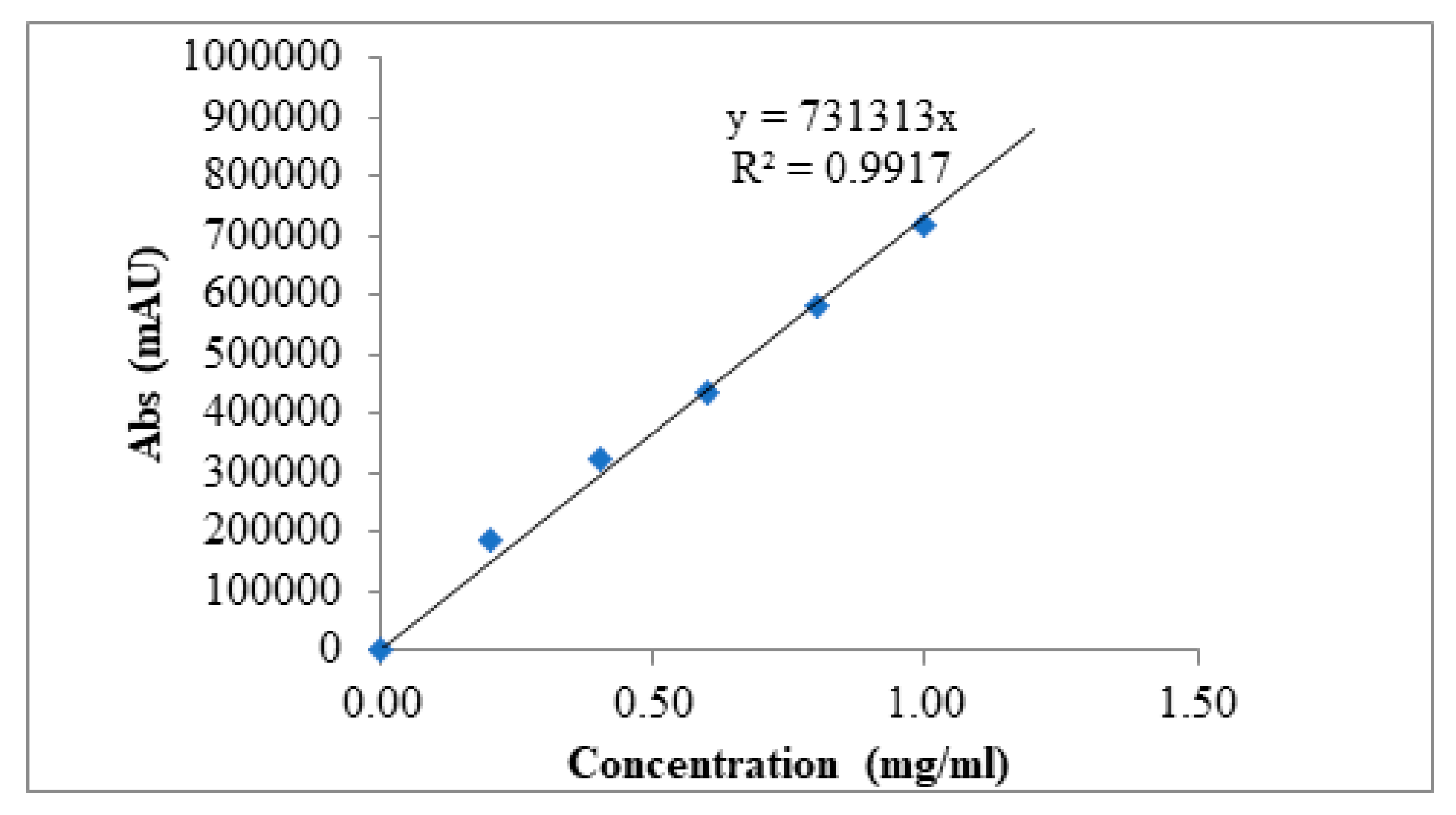
2.4. High-Performance Liquid Chromatography (HPLC)
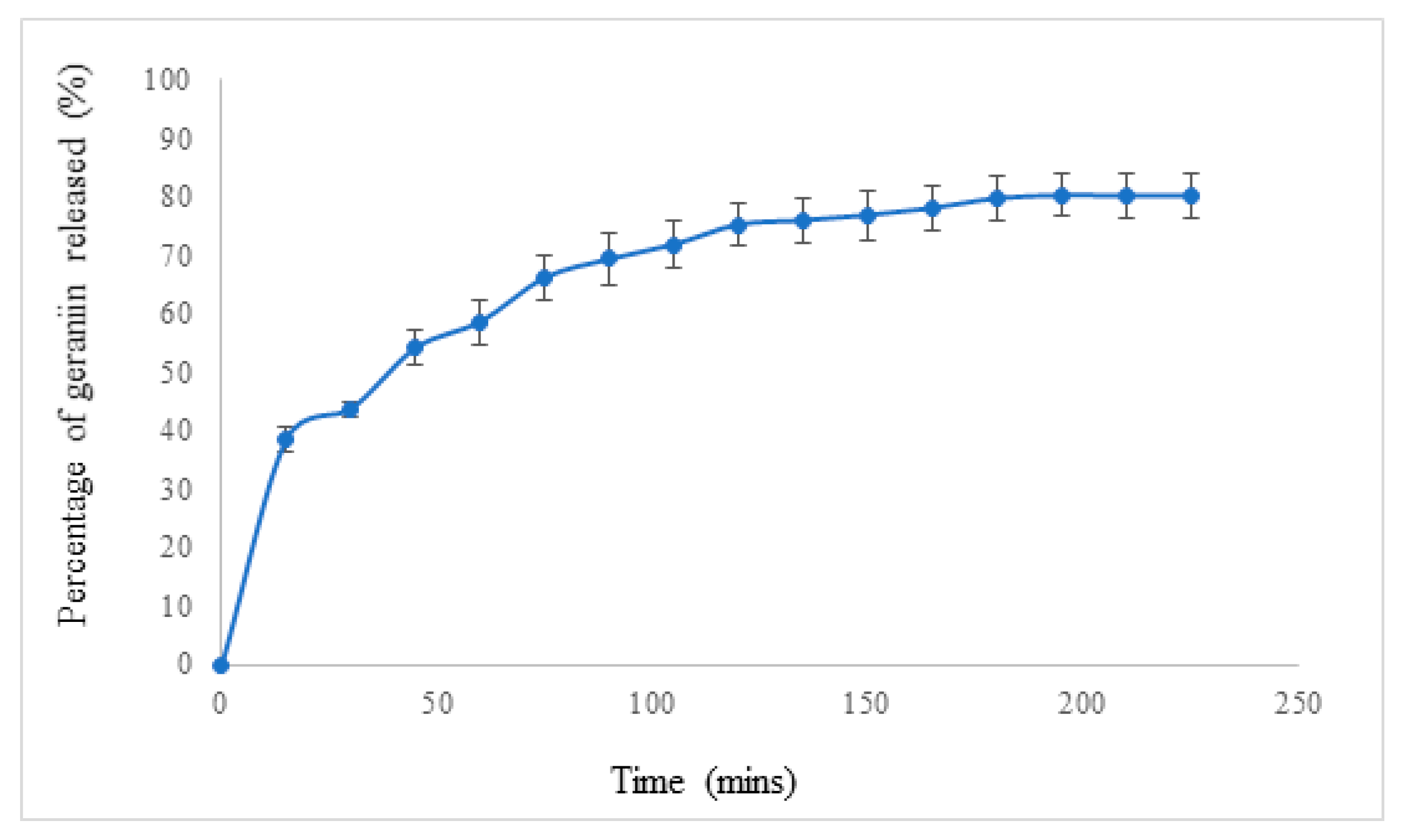
2.5. In Vitro Drug Release of poly(ethylene glycol)-block-poly(lactic-co-glycolic acid) (PEG-b-PLGA) Nanoparticles (NPs) Encapsulated with Geraniin
2.6. Cell Culture of CCD 841 CoN
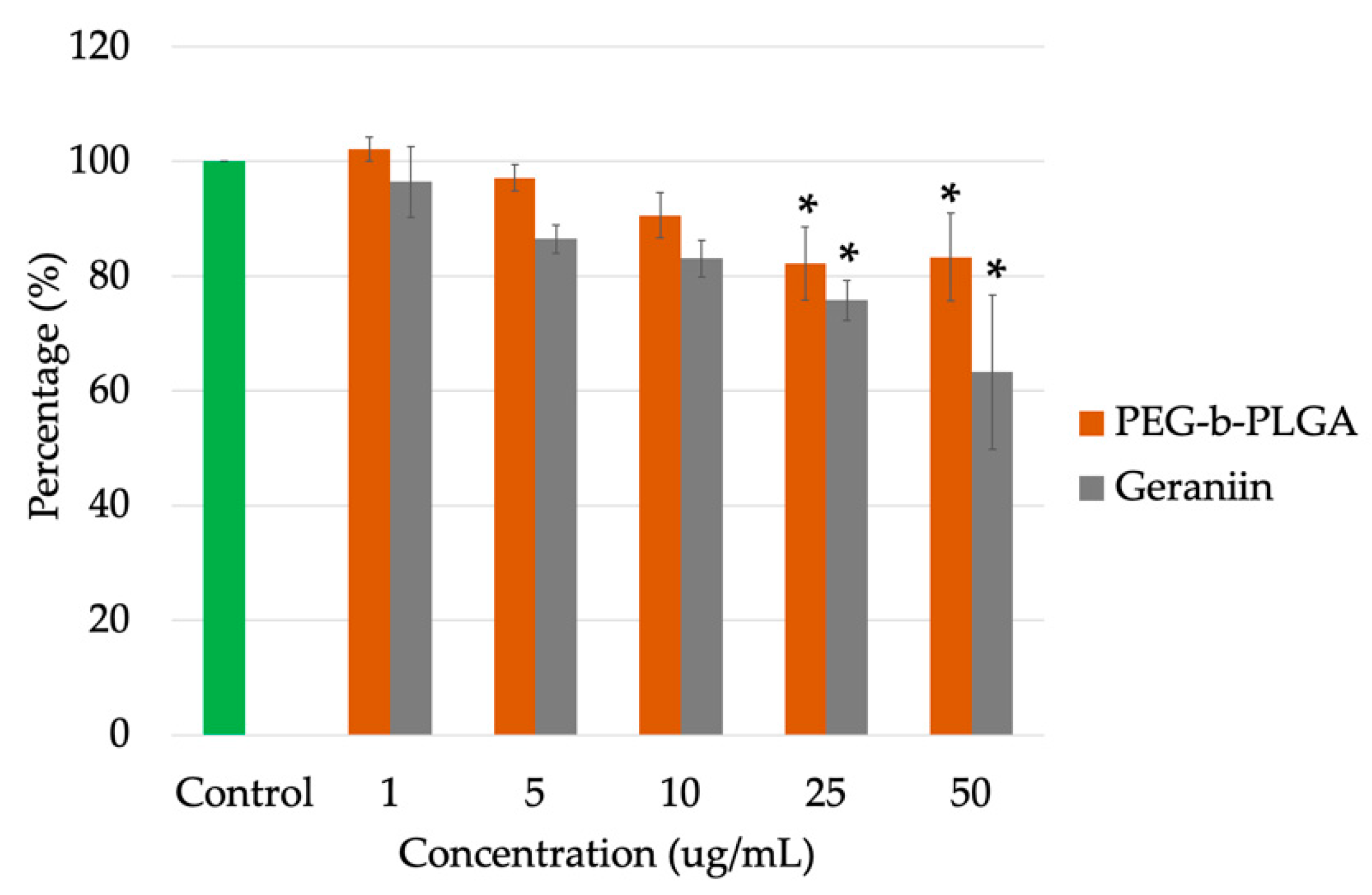
2.7. Cell Viability Assay
2.8. Statistical Analysis
3. Results
3.1. Synthesis of poly(ethylene glycol)-block-poly(lactic-co-glycolic acid) (PEG-b-PLGA) Nanoparticles (NPs) Encapsulated with and without Geraniin
3.2. Characterization of the poly(ethylene glycol)-block-poly(lactic-co-glycolic acid) (PEG-b-PLGA) Nanoparticles (NPs)
3.2.1. Particle Size Analysis
3.2.2. Zeta Potential Analysis
3.2.3. Transmission Electron Microscopy (TEM)
3.2.4. Field Emission Scanning Electron Microscopy (FESEM)
3.2.5. High Performance Liquid Chromatography (HPLC)
3.3. In Vitro Drug Release Study
3.4. In Vitro Cytotoxicity Study
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Espanol, L.; Larrea, A.; Andreu, V.; Mendoza, G.; Arruebo, M.; Sebastian, V.; Auroraprado, M.S.; Kedorhackmann, E.R.M.; Santoro, M.I.R.M.; Santamaria, J. Dual encapsulation of hydrophobic and hydrophilic drugs in PLGA nanoparticles by a single-step method: Drug delivery and cytotoxicity assays. RSC Adv. 2016, 6, 111060–111069. [Google Scholar] [CrossRef] [Green Version]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG-PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control. Release 2014. [Google Scholar] [CrossRef]
- Rafiei, P.; Haddadi, A. Docetaxel-loaded PLGA and PLGA-PEG nanoparticles for intravenous application: Pharmacokinetics and biodistribution profile. Int. J. Nanomed. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef]
- Odeh, F.; Al-Jaber, H.; Khater, D. Nanoflora—How Nanotechnology Enhanced the Use of Active Phytochemicals. In Application of Nanotechnology in Drug Delivery; Sezer, A.D., Ed.; IntechOpen: London, UK, 2014. [Google Scholar]
- Tang, Y.Q.; Jaganath, I.B.; Sekaran, S.D. Phyllanthus spp. induces selective growth inhibition of PC-3 and mewo human cancer cells through modulation of cell cycle and induction of apoptosis. PLoS ONE 2010. [Google Scholar] [CrossRef]
- Bala, I.; Bhardwaj, V.; Hariharan, S.; Kumar, M.N.V.R. Analytical methods for assay of ellagic acid and its solubility studies. J. Pharm. Biomed. Anal. 2006. [Google Scholar] [CrossRef]
- Cheng, J.T.; Chang, S.S.; Hsu, F.L. Antihypertensive Action of Geraniin in Rats. J. Pharm. Pharmacol. 1994. [Google Scholar] [CrossRef]
- Konishi, Y.; Hitomi, Y.; Yoshioka, E. Intestinal Absorption of p-Coumaric and Gallic Acids in Rats after Oral Administration. J. Agric. Food Chem. 2004. [Google Scholar] [CrossRef]
- Romeo, J. Chemical Ecology and Phytochemistry of Forest Ecosystems: Proceedings of the Phytochemical Society of North America; Elsevier Science: Oxford, UK, 2005. [Google Scholar]
- Lee, J.; Tsai, C.; Kao, J.; Kao, M.; Tsai, S.; Chang, C.; Huang, L.; Kuo, S.; Lin, J.; Way, T. Geraniin-mediated apoptosis by cleavage of focal adhesion kinase through up-regulation of Fas ligand expression in human melanoma cells. Mol. Nutr. Food Res. 2008. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Cheng, H.Y.; Lin, T.C.; Chiang, L.C.; Lin, C.C. The in vitro activity of geraniin and 1,3,4,6-tetra-O-galloyl-β-d-glucose isolated from Phyllanthus urinaria against herpes simplex virus type 1 and type 2 infection. J. Ethnopharmacol. 2007. [Google Scholar] [CrossRef] [PubMed]
- Londhe, J.S.; Devasagayam, T.P.A.; Foo, L.Y.; Shastry, P.; Ghaskadbi, S.S. Geraniin and amariin, ellagitannins from Phyllanthus amarus, protect liver cells against ethanol induced cytotoxicity. Fitoterapia 2012. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, U.D.; Ling, L.T.; Manaharan, T.; Appleton, D. Rapid isolation of geraniin from Nephelium lappaceum rind waste and its anti-hyperglycemic activity. Food Chem. 2011. [Google Scholar] [CrossRef]
- Chung, A.P.Y.S.; Ton, S.H.; Gurtu, S.; Palanisamy, U.D. Ellagitannin geraniin supplementation ameliorates metabolic risks in high-fat diet-induced obese Sprague Dawley rats. J. Funct. Foods 2014. [Google Scholar] [CrossRef]
- Tobío, M.; Gref, R.; Sánchez, A.; Langer, R.; Alonso, M.J. Stealth PLA-PEG nanoparticles as protein carriers for nasal administration. Pharm. Res. 1998. [Google Scholar] [CrossRef]
- Weissenboeck, A.; Bogner, E.; Wirth, M.; Gabor, F. Binding and uptake of wheat germ agglutinin-grafted PLGA-nanospheres by Caco-2 monolayers. Pharm. Res. 2004. [Google Scholar] [CrossRef]
- McCall, R.L.; Sirianni, R.W. PLGA nanoparticles formed by single- or double-emulsion with vitamin E-TPGS. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.P.; Li, X.Q.; Zhang, W.X.; Wang, H.P. A method for the preparation of stable dispersion of zero-valent iron nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2007. [Google Scholar] [CrossRef]
- Feng, S.S.; Mu, L.; Win, K.Y.; Huang, G. Nanoparticles of biodegradable polymers for clinical administration of paclitaxel. Curr. Med. Chem. 2004, 11, 413–424. [Google Scholar] [CrossRef]
- Lee, S.H.; Tang, Y.; Rathkrishnan, A.; Wang, S.M.; Ong, K.C.; Manikam, R.; Payne, B.J.; Jaganath, I.B.; Sekaran, S.D. Effects of cocktail of four local Malaysian medicinal plants (Phyllanthus spp.) against dengue virus 2. BMC Complement. Altern. Med. 2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Kröger, M.; Liu, W.K. Shape effect in cellular uptake of PEGylated nanoparticles: Comparison between sphere, rod, cube and disk. Nanoscale 2015. [Google Scholar] [CrossRef] [PubMed]
- Devasvaran, K.; Tan, J.J.; Ng, C.T.; Fong, L.Y.; Yong, Y.K. Malaysian Tualang Honey Inhibits Hydrogen Peroxide-Induced Endothelial Hyperpermeability. Oxid. Med. Cell. Longev. 2019. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, K.; Meka, S.R.K.; Bagchi, A.; Krishna, N.S.; Ramachandra, S.G.; Madras, G.; Chatterjee, K. Polyester derived from recycled poly(ethylene terephthalate) waste for regenerative medicine. RSC Adv. 2014. [Google Scholar] [CrossRef]
- Win, K.Y.; Feng, S.S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005. [Google Scholar] [CrossRef]
- Mahobia, S.; Bajpai, J.; Bajpai, A.K. An in-vitro investigation of swelling controlled delivery of insulin from egg albumin nanocarriers. Iran. J. Pharm. Res. 2016. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008. [Google Scholar] [CrossRef] [Green Version]
- Paka, G.D.; Ramassamy, C. Optimization of Curcumin-Loaded PEG-PLGA Nanoparticles by GSH Functionalization: Investigation of the Internalization Pathway in Neuronal Cells. Mol. Pharm. 2017. [Google Scholar] [CrossRef]
- Parveen, S.; Misra, R.; Sahoo, S.K. Nanoparticles: A boon to drug delivery, therapeutics, diagnostics and imaging. Nanomed. Nanotechnol. Biol. Med. 2012. [Google Scholar] [CrossRef]
- Stiufiuc, R.; Iacovita, C.; Nicoara, R.; Stiufiuc, G.; Florea, A.; Achim, M.; Lucaciu, C.M. One-step synthesis of PEGylated gold nanoparticles with tunable surface charge. J. Nanomater. 2013. [Google Scholar] [CrossRef]
- Elendran, S.; Wang, L.W.; Prankerd, R.; Palanisamy, U.D. The physicochemical properties of geraniin, a potential antihyperglycemic agent. Pharm. Biol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Sahoo, S.K. Polymeric nanoparticles for cancer therapy. J. Drug Target. 2008. [Google Scholar] [CrossRef]
- Singh, R.; Lillard, J.W. Nanoparticle-based targeted drug delivery. Exp. Mol. Pathol. 2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, N.; Park, J.H. Endocytosis and exocytosis of nanoparticles in mammalian cells. Int. J. Nanomed. 2014. [Google Scholar] [CrossRef] [Green Version]
- Clarke, S. Development of Hierarchical Magnetic Nanocomposite Materials for Biomedical Applications. Ph.D. Thesis, Dublin City University, Dublin, Ireland, 2013. [Google Scholar]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010. [Google Scholar] [CrossRef] [PubMed]
- Carpineti, M.; Ferri, F.; Giglio, M.; Paganini, E.; Perini, U. Salt-induced fast aggregation of polystyrene latex. Phys. Rev. A 1990. [Google Scholar] [CrossRef]
- Florez, L.V.; Herrmann, C.; Cramer, J.M.; Hauser, C.P.; Koynov, K.; Landfester, K.; Crespy, D.; Mailander, V. How shape influences uptake: Interactions of anisotropic polymer nanoparticles and human mesenchymal stem cells. Small 2012. [Google Scholar] [CrossRef]
- Parakhonskiy, B.; Zyuzin, M.V.; Yashchenok, A.M.; Carregalromero, S.; Rejman, J.; Mohwald, H.; Parak, W.J.; Skirtach, A.G. The influence of the size and aspect ratio of anisotropic, porous CaCO3 particles on their uptake by cells. J. Nanobiotechnol. 2015. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Xiao, S.; Zhu, H.; Wang, L.; Liang, H. Shape-dependent internalization kinetics of nanoparticles by membranes. Soft Matter 2016. [Google Scholar] [CrossRef]
- Ahmad, S.A.A.; Palanisamy, U.D.; Tejo, B.A.; Chew, M.F.; Tham, H.W.; Hassan, S.S. Geraniin extracted from the rind of Nephelium lappaceum binds to dengue virus type-2 envelope protein and inhibits early stage of virus replication. Virol. J. 2017, 14, 229. [Google Scholar] [CrossRef] [Green Version]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.G.M.; Ming, L.C.; Lee, K.S.; Yuen, K.H. Influence of the encapsulation efficiency and size of liposome on the oral bioavailability of griseofulvin-loaded liposomes. Pharmaceutics 2016, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable controlled-release polymers and polymeric nanoparticles: Mechanisms of controlling drug release. Chem. Rev. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von Burkersroda, F.; Schedl, L.; Göpferich, A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials 2002. [Google Scholar] [CrossRef]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An Overview of Poly(lactic-co-glycolic) Acid (PLGA)-Based Biomaterials for Bone Tissue Engineering. Int. J. Mol. Sci 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Amin, F.U.; Shah, S.A.; Badshah, H.; Khan, M.; Kim, M.O. Anthocyanins encapsulated by PLGA@PEG nanoparticles potentially improved its free radical scavenging capabilities via p38/JNK pathway against Aβ1-42-induced oxidative stress. J. Nanobiotechnol. 2017, 15. [Google Scholar] [CrossRef] [Green Version]
- Keles, H.; Naylor, A.; Clegg, F.; Sammon, C. Investigation of factors influencing the hydrolytic degradation of single PLGA microparticles. Polym. Degrad. Stab. 2015. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.P.; Coutinho, O.P. Free radicals in the regulation of damage and cell death-basic mechanisms and prevention. Drug Discov. Ther. 2010, 4, 144. [Google Scholar]
- Lampe, K.J.; Namba, R.M.; Silverman, T.R.; Bjugstad, K.B.; Mahoney, M.J. Impact of lactic acid on cell proliferation and free radical-induced cell death in monolayer cultures of neural precursor cells. Biotechnol. Bioeng. 2009. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Park, J.H.; Kim, D.H.; Won, Y.H.; Maibach, H.I. Increased in vivo collagen synthesis and in vitro cell proliferative effect of glycolic acid. Dermatol. Surg. 1998. [Google Scholar] [CrossRef]
- Alcantar, N.A.; Aydil, E.S.; Israelachvili, J.N. Polyethylene glycol-coated biocompatible surfaces. J. Biomed. Mater. Res. 2000. [Google Scholar] [CrossRef]
- Tong, R.; Gabrielson, N.P.; Fan, T.M.; Cheng, J. Polymeric nanomedicines based on poly(lactide) and poly(lactide-co- glycolide). Curr. Opin. Solid State Mater. Sci. 2012. [Google Scholar] [CrossRef] [Green Version]
- Song, H.Y.; Kim, Y.; Seok, S.J.; Gil, H.; Yang, J.O.; Lee, E.; Hong, S. Cellular toxicity of surfactants used as herbicide additives. J. Korean Med. Sci. 2012. [Google Scholar] [CrossRef] [Green Version]
- Webster, R.; Elliott, V.; Park, B.K.; Walker, D.; Hankin, M.; Taupin, P. PEG and PEG conjugates toxicity: Towards an understanding of the toxicity of PEG and its relevance to PEGylated biologicals. In PEGylated Protein Drugs: Basic Science and Clinical Applications; Veronese, F.M., Ed.; Berkhäuser: Basel, Switzerland, 2009. [Google Scholar]
- Parnaud, G.; Corpet, D.E.; Gamet-Payrastre, L. Cytostatic effect of polyethylene glycol on human colonic adenocarcinoma cells. Int. J. Cancer 2001. [Google Scholar] [CrossRef] [Green Version]
- Romera-Castillo, C.; Jaffé, R. Free radical scavenging (antioxidant activity) of natural dissolved organic matter. Mar. Chem. 2015. [Google Scholar] [CrossRef] [Green Version]
- Özyurt, H.; Luna, C.; Estévez, M. Redox chemistry of the molecular interactions between tea catechins and human serum proteins under simulated hyperglycemic conditions. Food Funct. 2016. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Miguel, O.G.; Calixto, J.B.; Santos, A.R.S.; Messana, I.; Ferrari, F.; Filho, V.C.; Pizzolatti, M.G.; Yunes, R.A. Chemical and preliminary analgesic evaluation of geraniin and furosin isolated from Phyllanthus sellowianus. Planta Med. 1996. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devasvaran, K.; Jairaman, S.; Yahaya, N.A.; Jaganath, I.B.S.; Khung, Y.L.; Lim, V.; Ngalim, S.H. PEG-b-PLGA Nanoparticles Loaded with Geraniin from Phyllanthus Watsonii Extract as a Phytochemical Delivery Model. Appl. Sci. 2020, 10, 4891. https://doi.org/10.3390/app10144891
Devasvaran K, Jairaman S, Yahaya NA, Jaganath IBS, Khung YL, Lim V, Ngalim SH. PEG-b-PLGA Nanoparticles Loaded with Geraniin from Phyllanthus Watsonii Extract as a Phytochemical Delivery Model. Applied Sciences. 2020; 10(14):4891. https://doi.org/10.3390/app10144891
Chicago/Turabian StyleDevasvaran, Kogilavanee, Sreegayathri Jairaman, Nur Azirah Yahaya, Indu Bala S. Jaganath, Yit Lung Khung, Vuanghao Lim, and Siti Hawa Ngalim. 2020. "PEG-b-PLGA Nanoparticles Loaded with Geraniin from Phyllanthus Watsonii Extract as a Phytochemical Delivery Model" Applied Sciences 10, no. 14: 4891. https://doi.org/10.3390/app10144891





