Distribution, Sources, and Risk Assessment of Polycyclic Aromatic Hydrocarbons in the Estuary of Hongze Lake, China
Abstract
:1. Introduction
2. Material and Methods
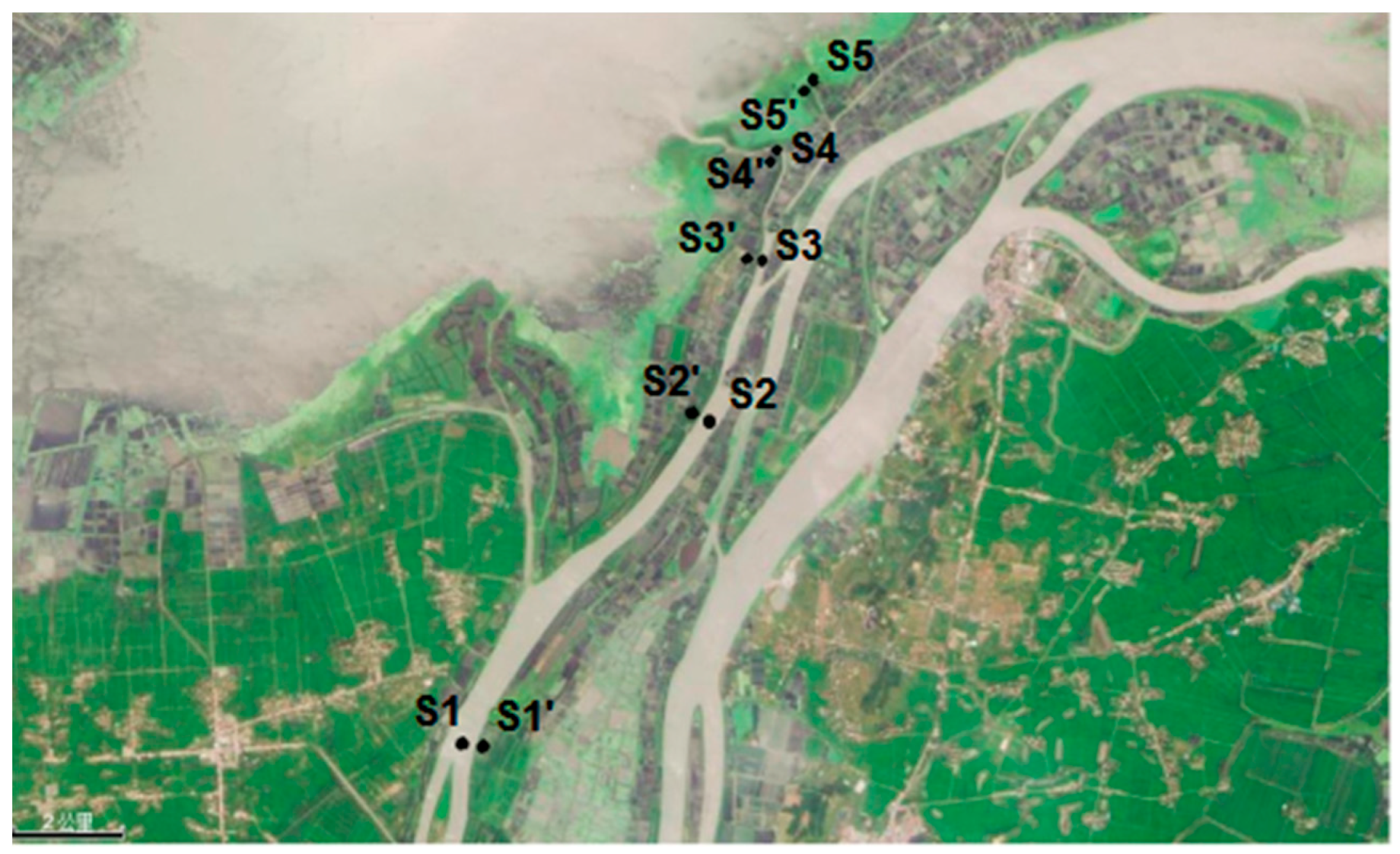
2.1. Study Area and Sampling
2.2. PAHs Extraction and Analyses
2.3. Data Statistical Analysis
3. Results and Discussion
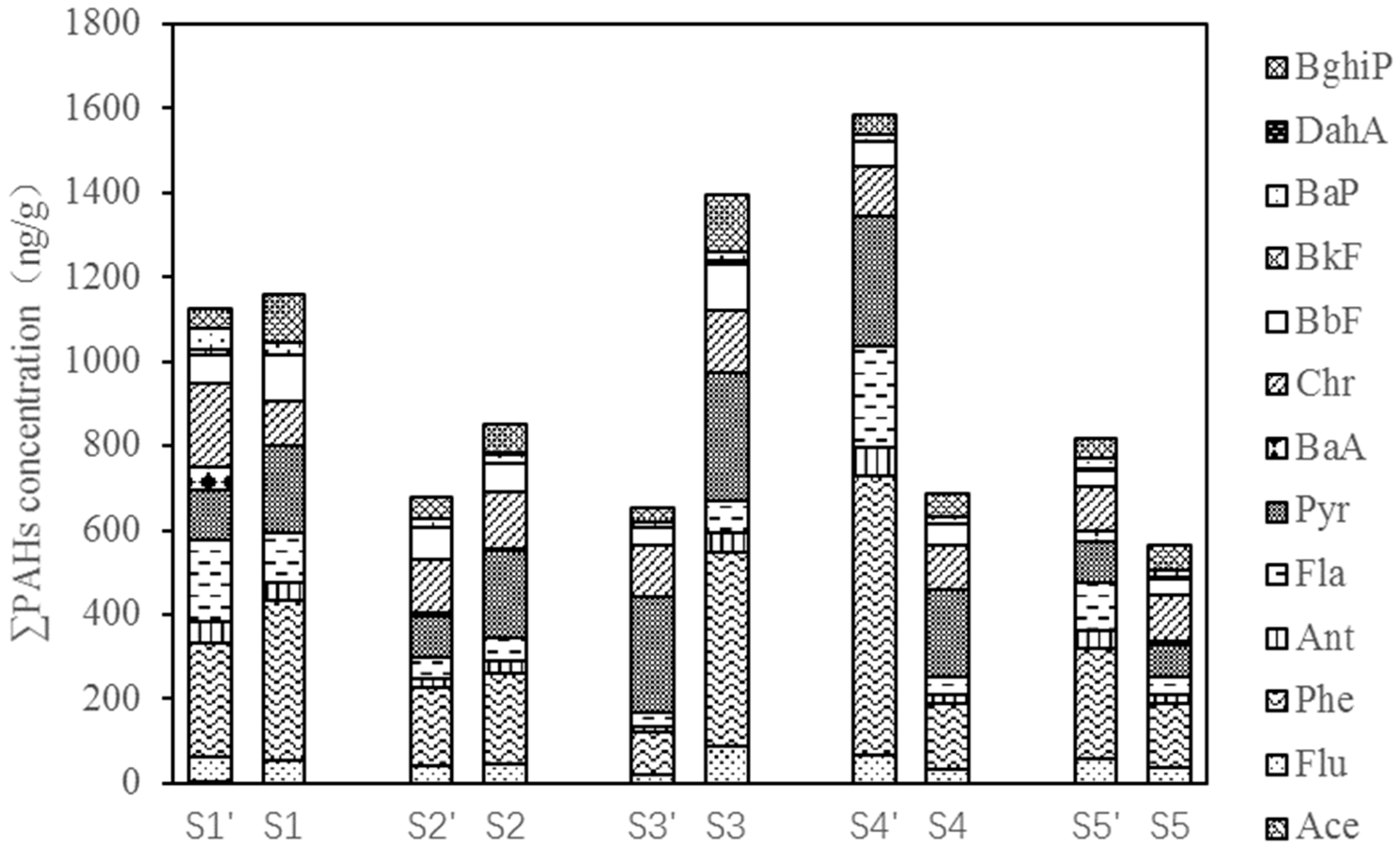
3.1. Presence of PAHs
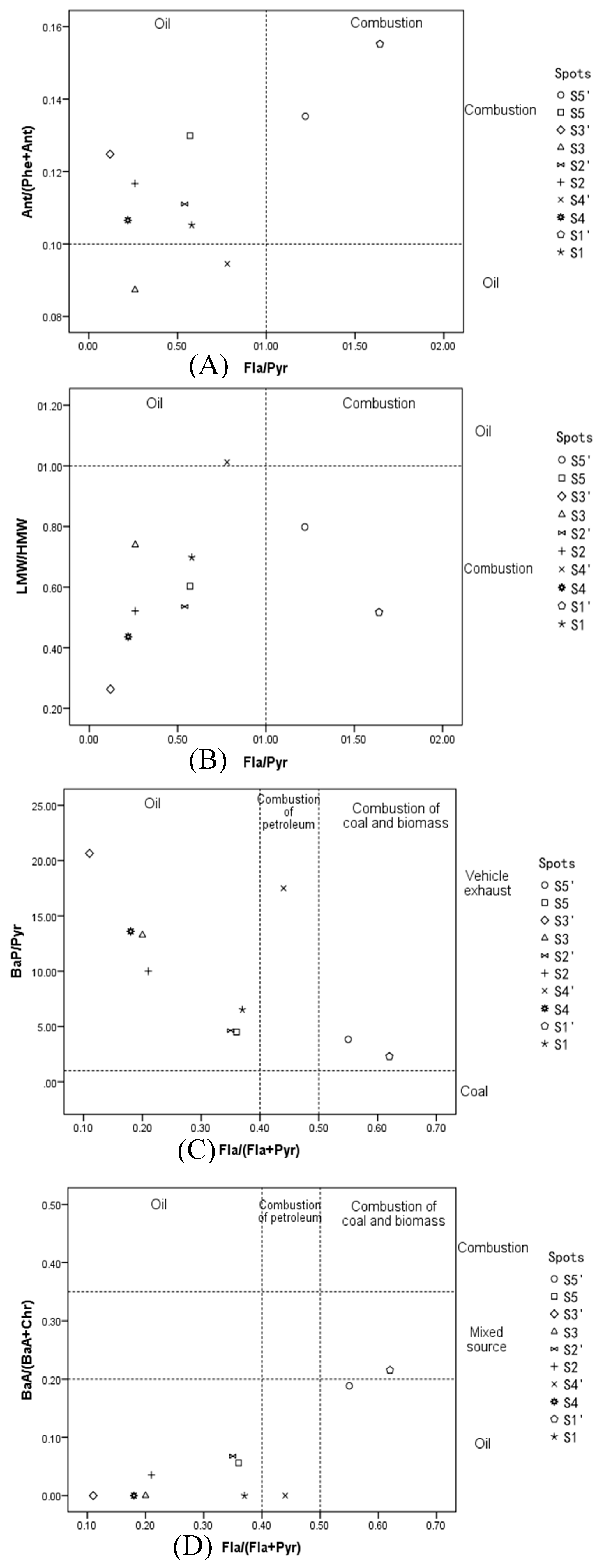
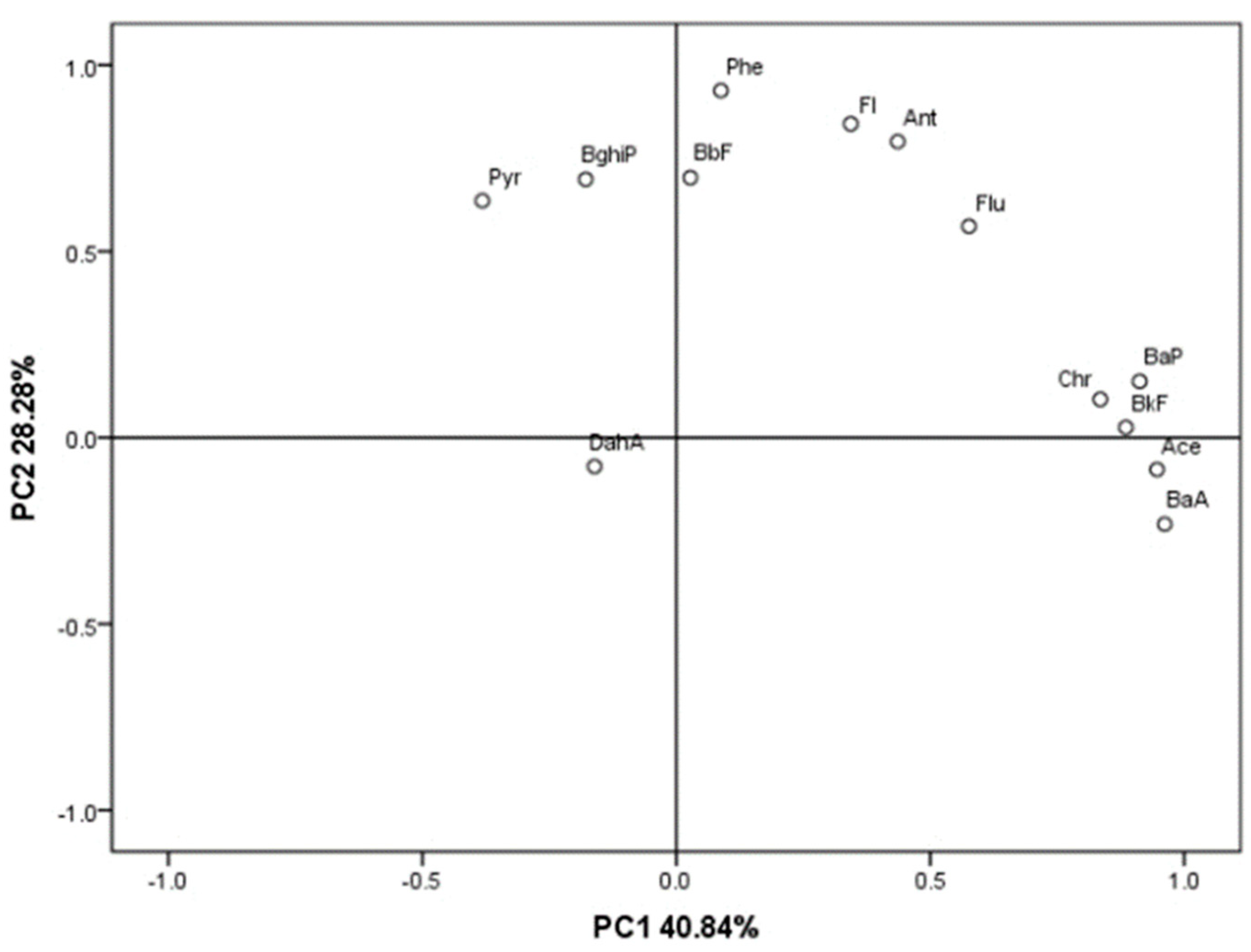
3.2. Sources of PAHs
3.3. Risk Assessment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| PAHs | Molecular Formula | Solubility (S) | Ring Numbers | Boiling Point | Carcinogenic * |
|---|---|---|---|---|---|
| Nap | C10H8 | 31.7 | 2 | 218 | |
| Acy | C12H8 | 16.1 | 3 | 279 | |
| Ace | C12H10 | 3.8 | 3 | 275 | |
| Flu | C13H8 | 1.9 | 3 | 298 | |
| Phe | C14H10 | 1.1 | 3 | 340 | |
| Ant | C14H10 | 0.045 | 3 | 341 | |
| Pyr | C16H10 | 0.132 | 4 | 384 | |
| Fla | C16H10 | 0.26 | 4 | 384 | |
| Chr | C18H12 | 0.0015 | 4 | 448 | * |
| BaA | C18H12 | 0.011 | 4 | 438 | * |
| BkF | C20H12 | 0.0008 | 5 | 481 | * |
| BbF | C20H12 | 0.0015 | 5 | 481 | * |
| BaP | C20H12 | 0.0038 | 5 | 500 | * |
| InP | C22H12 | 0.00019 | 6 | N/A | * |
| DBA | C22H14 | 0.0006 | 5 | N/A | * |
| BghiP | C22H12 | 0.00026 | 6 | 542 | * |
Appendix B
| Time (min) | Excitation Wavelength (λem/nm) | Emission Wavelength (λem/nm) |
|---|---|---|
| 2 | 280 | 330 |
| 9.2 | 260 | 380 |
| 14 | 280 | 450 |
| 16.5 | 260 | 380 |
| 28 | 290 | 410 |
| 35.5 | 290 | 500 |
Appendix C
| Sampling Points | Culture Pond (S1’) | Culture Pond Sediment (S1) | Farmland (S2’) | Farmland Sediment (S2) | Reed Land (S3’) | Reed Land Sediment (S3) | Poplar Forest (S4’) | Poplar Forest Sediment (S4) | Withy Grove (S5’) | Withy Grove Sediment (S5) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PAHs | |||||||||||
| NaP | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Acy | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Ace | 3.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Flu | 59.2 | 53.2 | 40.1 | 44.7 | 19.6 | 87.4 | 68.7 | 34.9 | 60.5 | 37.6 | |
| Phe | 271.1 | 378.4 | 185.0 | 218 | 101.7 | 461.8 | 660.1 | 155.1 | 260.9 | 151.4 | |
| Ant | 49.8 | 44.5 | 23.1 | 28.8 | 14.5 | 44.2 | 68.9 | 18.5 | 40.8 | 22.6 | |
| Fla | 193.3 | 118.1 | 52.4 | 54.0 | 32.8 | 77.2 | 239.0 | 44.2 | 115.2 | 42.7 | |
| Pyr | 118.0 | 204.2 | 96.8 | 207.0 | 272.7 | 302.6 | 308.0 | 205.4 | 94.1 | 74.4 | |
| BaA a | 54.8 | 0 | 8.9 | 4.9 | 0 | 0 | 0 | 0 | 25.2 | 6.7 | |
| Chr a | 199.6 | 108.6 | 122.5 | 134.5 | 124.1 | 146.8 | 119.7 | 106.2 | 108.5 | 112.5 | |
| BbF a | 68.5 | 106.7 | 75.9 | 68.1 | 39.7 | 111.5 | 56.6 | 52.3 | 34.9 | 38.5 | |
| BkF a | 11.5 | 0 | 0 | 0 | 0 | 5.8 | 0 | 0 | 6.5 | 3.3 | |
| BaP a | 51.6 | 31.3 | 20.9 | 20.7 | 13.2 | 22.8 | 17.6 | 15.1 | 24.5 | 16.5 | |
| DBA a | 0 | 0 | 0 | 3.1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| BghiP | 44.0 | 113.0 | 50.9 | 67.0 | 33.0 | 135.6 | 47.2 | 54.7 | 44.8 | 56.2 | |
| InP a | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 1124 | 1158 | 676 | 851 | 651 | 1396 | 1586 | 686 | 816 | 562 | |
References
- Elie, M.; Clausen, C.; Yestrebsky, C. Reductive degradation of oxygenated polycyclic aromatic hydrocarbons using an activated magnesium/co-solvent system. Chemosphere 2013, 91, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liu, L.; Zhang, J.; Tan, F.; Zhao, X.; Wang, X.; Zheng, B.; Ma, J.; Liu, Z.; Meng, W. Distribution characteristics and ecological risk assessment of PAHs in the sediments of Huaihe River. J. Environ. Health 2012, 29, 555–560. [Google Scholar]
- Cui, H.; Wang, Y.; Wang, X.; Li, C. Research on pollution situation of PAHs remediation. Green Sci. 2012, 6, 134–136. [Google Scholar]
- Sany, T.; Rezayi, H.; Rahman, S.; Sasekumar, S. Human health risk of polycyclic aromatic hydrocarbons from consumption of blood cockle and exposure to contaminated sediments and water along the Klang Strait, Malaysia. Mar. Pollut. Bull. 2014, 84, 268–279. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Zheng, B.; Zhang, L.; Jia, N.; Qin, Y.; Zhao, X.; Jia, J. Heavy metal pollution and potential ecological risk assessment of sediments in Bohai Bay estuary. Chin. Environ. Sci. 2010, 30, 666–670. [Google Scholar]
- Eatherall, A.; Naden, P.S.; Cooper, D.M. Simulating carbon flux to the estuary: The first step. Sci. Environ. 1998, 210, 519–533. [Google Scholar] [CrossRef]
- Qiu, H. Migration mechanism of organic pollutants in national water-body sediments. J. Geogr. Geol. 2011, 3, 239. [Google Scholar] [CrossRef]
- Xiao, Q.; Liang, B.; Liu, X.; Liu, X.; Wang, J.; Liu, F.; Cui, B. Distribution, sources, and ecological risk assessment of polycyclic aromatic hydrocarbons in surface sediments from the Haihe River, a typical polluted urban river in Northern China. Environ. Sci. Pollut. Res. 2017, 24, 17153–17165. [Google Scholar]
- Feng, J.; Hu, P.; Zhang, F.; Wu, Y.; Liu, S.; Sun, J. Ecological risk assessment of polycyclic aromatic hydrocarbons in surface sediments from the middle and lower reaches of the Yellow River, China. Hum. Ecol. Risk Assess. Int. J. 2015, 1, 22–33. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, J.; Wu, Y. Distribution and source analysis of polycyclic aromatic hydrocarbons (PAHs) in surface sediments of the Yangtze River Basin. J. Ecotoxicol. 2016, 11, 566–572. [Google Scholar]
- Feng, J.; Zhai, M.; Sun, J.; Liu, Q. Distribution and sources of polycyclic aromatic hydrocarbons (PAHs) in sediment from the upper reach of Huaihe River, East China. Environ. Sci. Pollut. Res. 2012, 19, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, W. Polycyclic aromatic hydrocarbons in the water-SPM-sediment system from the middle reaches of Huai River, China: Distribution, partitioning, origin tracing and ecological risk assessment. Environ. Pollut. 2017, 230, 61–71. [Google Scholar]
- Liu, H.; Liu, D.; Chen, S. The Source and Ecological Risk of PAHs in Surface Sediments of Dongping Lake. Environ. Eng. 2014, 32, 126–129. [Google Scholar]
- Shi, S.; Yang, Y.; Shi, L. The Distributions and Sources of Polycyclic Aromatic Hydrocarbons in Surface Sediments of the Nansi Lake. J. Qingdao Univ. 2005, 20, 95–99. [Google Scholar]
- Zheng, X.; Yao, J.; Jiang, H.; Hao, B. Distribution and Risk Assessment of Polycyclic Aromatic Hydrocarbons in Surface Sediments from Luoma Lake. J. Anhui Agric. 2010, 38, 10858–10861. [Google Scholar]
- He, X.R.; Pang, Y.; Song, X.J.; Chen, B.L.; Feng, Z.H.; Ma, Y.Q. Distribution, sources and ecological risk assessment of PAHs in surface sediments from Guan River estuary, China. Mar. Pollut. Bull. 2014, 80, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Long, E.; Macdonald, R.; Smith, D.; Calder, D. Incidence of adverse biological effects within ranges of chemical concentrations in marine and estuarine sediments. Environ. Manag. 1995, 19, 81–97. [Google Scholar] [CrossRef]
- Maliszewska-Kordybach, B. Polycyclic aromatic hydrocarbons in agricultural soils in Poland: Preliminary proposals for criteria to evaluate the level of soil contamination. Appl. Geochem. 1996, 11, 121–127. [Google Scholar] [CrossRef]
- Mei, W.; Ruan, H.; Wu, H.; Le, H.; Jiang, M. Distribution and risk assessment of polycyclic aromatic hydrocarbons in Dishui lake sediments. Chin. Environ. Sci. 2013, 33, 2069–2074. [Google Scholar]
- Yunker, M.B.; Macdonald, R.W.; Goyette, D.; Paton, D.; Fowler, B.R.; Sullivan, D.; Boyd, J. Natural and anthropogenic inputs of hydrocarbons to the Strait of Georgia. Sci. Total Environ. 1999, 225, 181–209. [Google Scholar] [CrossRef]
- Yunker, M.B.; Macdonald, R.W.; Vingarzan, R.; Mitchell, R.H.; Goyette, D.; Sylvestre, S. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org. Geochem. 2002, 33, 489–515. [Google Scholar] [CrossRef]
- Soclo, H.H.; Garrigues, P.; Ewald, M. Origin of Polycyclic Aromatic Hydrocarbons (PAHs) in Coastal Marine Sediments: Case Studies in Cotonou (Benin) and Aquitaine (France) Areas. Mar. Pollut. Bull. 2000, 40, 387–396. [Google Scholar] [CrossRef]
- Khalili, N.R.; Scheff, P.A.; Holsen, T.M. PAH source fingerprints for coke ovens, diesel and, gasoline engines, highway tunnels, and wood combustion emissions. Atmos. Environ. 1995, 29, 533–542. [Google Scholar] [CrossRef]
- Lim, L.H.; Harrison, R.M.; Harrad, S. The contribution of traffic to atmospheric concentrations of polycyclic aromatic hydrocarbons (Statistical Data Included). Environ. Sci. Technol. 1999, 33, 3538–3542. [Google Scholar] [CrossRef]
- Nielsen, T.; Jørgensen, H.; Larsen, J.; Poulsen, M. City air pollution of polycyclic aromatic hydrocarbons and other mutagens: Occurrence, sources and health effects. Sci. Total Environ. 1996, 189, 41–49. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Wan, C.; Yue, D.; Ye, Y.; Wang, X. Surface runoff of urban road and source analysis of PAHs in related media in Beijing. Environ. Sci. 2008, 29, 478–1483. [Google Scholar]
- Lee, J.H.; Gigliotti, C.L.; Offenberg, J.H.; Eisenreich, S.J.; Turpin, B.J. Sources of polycyclic aromatic hydrocarbons to the Hudson River Airshed. Atmos. Environ. 2004, 38, 5971–5981. [Google Scholar] [CrossRef]
- Tian, F.; Chen, J.; Qiao, X.; Wang, Z.; Yang, P.; Wang, D.; Ge, L. Sources and seasonal variation of atmospheric polycyclic aromatic hydrocarbons in Dalian, China: Factor analysis with non-negative constraints combined with local source fingerprints. Atmos. Environ. 2009, 43, 2747–2753. [Google Scholar] [CrossRef]

| Location | Total Concentration (ng/g) | Average (ng/g) | Number of PAHs | References |
|---|---|---|---|---|
| Haihe River | 171.4–9511.2 | 2125.4 | 16 | [8] |
| Yellow River | 4010–8010 | — | 8 | [9] |
| Yangze River, China | 10.3–1239 | 178.0 | 14 | [10] |
| Huai River (upper reach) | 95.2–877.5 | — | 16 | [11] |
| Huai River (middle reach) | 810–28228 | 7955 | 16 | [12] |
| Huai River, China | 5.4–1293 | 143.0 | 16 | [2] |
| Dongping Lake | 49.40–133.67 | — | 16 | [13] |
| Nansi Lake | 229.17–609.94 | — | 14 | [14] |
| Taihu Lake, China | 262.1–1087 | 443.02 | 16 | [15] |
| The estuary of Hongze Lake | 562.4–1586 | 950.7 | 16 | Present research |
| Ratio | Oil Source | Combustion Source | Transportation Source | |
|---|---|---|---|---|
| Oil | Coal and Biomass | |||
| LMW/HMW | >1 | <1 | ||
| FIa/Pyr | <1 | >1 | ||
| Ant/(Phe + Ant) | <0.1 | >0.1 | ||
| Fla/(Fla + Pyr) | <0.4 | 0.4–0.5 | >0.5 | |
| BaA/(BaA + Chr) | <0.2 | >0.35 | ||
| BaP/Pyr | <1 | 1–6 | ||
| Pollution Source | Characteristic Compounds |
|---|---|
| Byproduct of coal tar | Nap |
| Oil source | Flu, Fla, Pyr, BkF, In(1,2,3-cd)P |
| Diesel source | Phe, Flu, Pyr, Ant |
| Stable combustion source | Ace, Acy, Chr, BkF |
| Motor vehicles with liquefied petroleum | Flu, Pyr, B(g, h, i)P |
| Lubricating oil and industrial sources | Flu, high-ring PAHs |
| PAHs | ERL | ERM | K | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S5 | S1’ | S2’ | S3’ | S4’ | S5’ | |||
| NaP | 160 | 2100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Acy | 44 | 640 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ace | 16 | 500 | 0 | 0 | 0 | 0 | 0 | 0.19 | 0 | 0 | 0 | 0 |
| Flu | 19 | 540 | 2.80 | 2.35 | 4.60 | 1.84 | 1.98 | 3.12 | 2.11 | 1.03 | 3.62 | 3.18 |
| Phe | 240 | 1500 | 1.58 | 0.91 | 1.92 | 0.65 | 0.63 | 1.13 | 0.77 | 0.42 | 2.75 | 1.09 |
| Ant | 85 | 1000 | 0.52 | 0.34 | 0.52 | 0.22 | 0.27 | 0.59 | 0.27 | 0.17 | 0.81 | 0.48 |
| Fla | 600 | 510 | 0.20 | 0.09 | 0.13 | 0.07 | 0.07 | 0.32 | 0.09 | 0.05 | 0.40 | 0.19 |
| Pyr | 665 | 2600 | 0.31 | 0.31 | 0.46 | 0.31 | 0.11 | 0.18 | 0.15 | 0.41 | 0.46 | 0.14 |
| BaA | 261 | 1600 | 0 | 0.02 | 0 | 0 | 0.03 | 0.21 | 0.03 | 0 | 0 | 0.10 |
| Chr | 384 | 2800 | 0.28 | 0.35 | 0.38 | 0.28 | 0.29 | 0.52 | 0.32 | 0.32 | 0.31 | 0.28 |
| BbF | 320 | 1880 | 0.33 | 0.21 | 0.35 | 0.16 | 0.12 | 0.21 | 0.24 | 0.12 | 0.18 | 0.11 |
| BkF | 280 | 1620 | 0 | 0 | 0.02 | 0 | 0.01 | 0.04 | 0 | 0 | 0 | 0.02 |
| BaP | 430 | 1600 | 0.07 | 0.05 | 0.05 | 0.04 | 0.04 | 0.12 | 0.05 | 0.03 | 0.04 | 0.06 |
| DBA | 63 | 260 | 0 | 0.05 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| BghiP | 430 | 1600 | 0.26 | 0.16 | 0.32 | 0.13 | 0.13 | 0.10 | 0.12 | 0.08 | 0.11 | 0.10 |
| InP | — | — | — | — | — | — | — | — | — | — | — | — |
| Total | 4022 | 44,792 | 0.29 | 0.17 | 0.16 | 0.39 | 0.20 | 0.28 | 0.21 | 0.35 | 0.17 | 0.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Rong, Q.; Zhu, C.; Han, J.; Li, P. Distribution, Sources, and Risk Assessment of Polycyclic Aromatic Hydrocarbons in the Estuary of Hongze Lake, China. Environments 2019, 6, 92. https://doi.org/10.3390/environments6080092
Li C, Rong Q, Zhu C, Han J, Li P. Distribution, Sources, and Risk Assessment of Polycyclic Aromatic Hydrocarbons in the Estuary of Hongze Lake, China. Environments. 2019; 6(8):92. https://doi.org/10.3390/environments6080092
Chicago/Turabian StyleLi, Chuan, Qiuyu Rong, Chenming Zhu, Jiangang Han, and Pingping Li. 2019. "Distribution, Sources, and Risk Assessment of Polycyclic Aromatic Hydrocarbons in the Estuary of Hongze Lake, China" Environments 6, no. 8: 92. https://doi.org/10.3390/environments6080092
APA StyleLi, C., Rong, Q., Zhu, C., Han, J., & Li, P. (2019). Distribution, Sources, and Risk Assessment of Polycyclic Aromatic Hydrocarbons in the Estuary of Hongze Lake, China. Environments, 6(8), 92. https://doi.org/10.3390/environments6080092





