Ceramic Materials Containing Volcanic Ash and Characterized by Photoluminescent Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- A commercial filtered and degassed clay, AFD-000055-Argilla Tornio, was purchased from the Colorobbia Italia S.P.A., Vinci, Italy. It is the clay commonly used by the potters for the manufacturing of their marketed products.
- Hydrochloric acid (ACS reagent 37%) and sodium hydroxide (reagent grade, ≥98%, pellets (anhydrous)) were purchased from Sigma Aldrich, Milano, Italy.
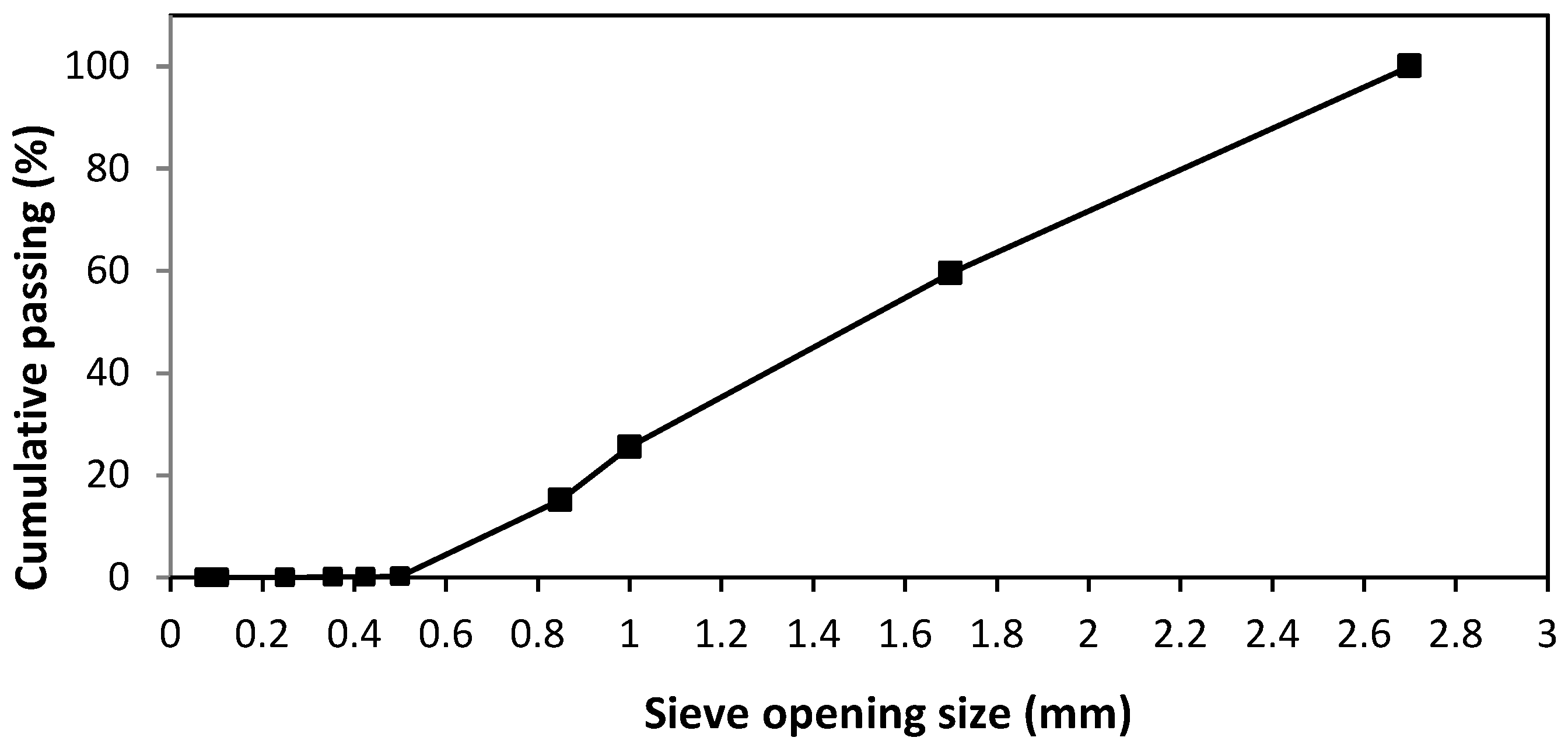
- The volcanic ash (VA) was collected from the slopes of the Etna volcano (Sicily, Italy) after the eruption in 2020.
- The commercial photoluminescent powder used in the present investigation (ZYYINI- MYX-RAG-JM04576-03-FBA) was purchased from ZYYINI, Shenzhen, China
- A ceramic glaze purchased from the Colorobbia Italia S.P.A group was used to fix the photoluminescent powder onto the surface of the specimens. Glaze generates, after firing, a transparent, colorless and waterproofing thin glass coating on the surface of the ceramic material.
2.2. Methods
3. Results and Discussion
4. Conclusions
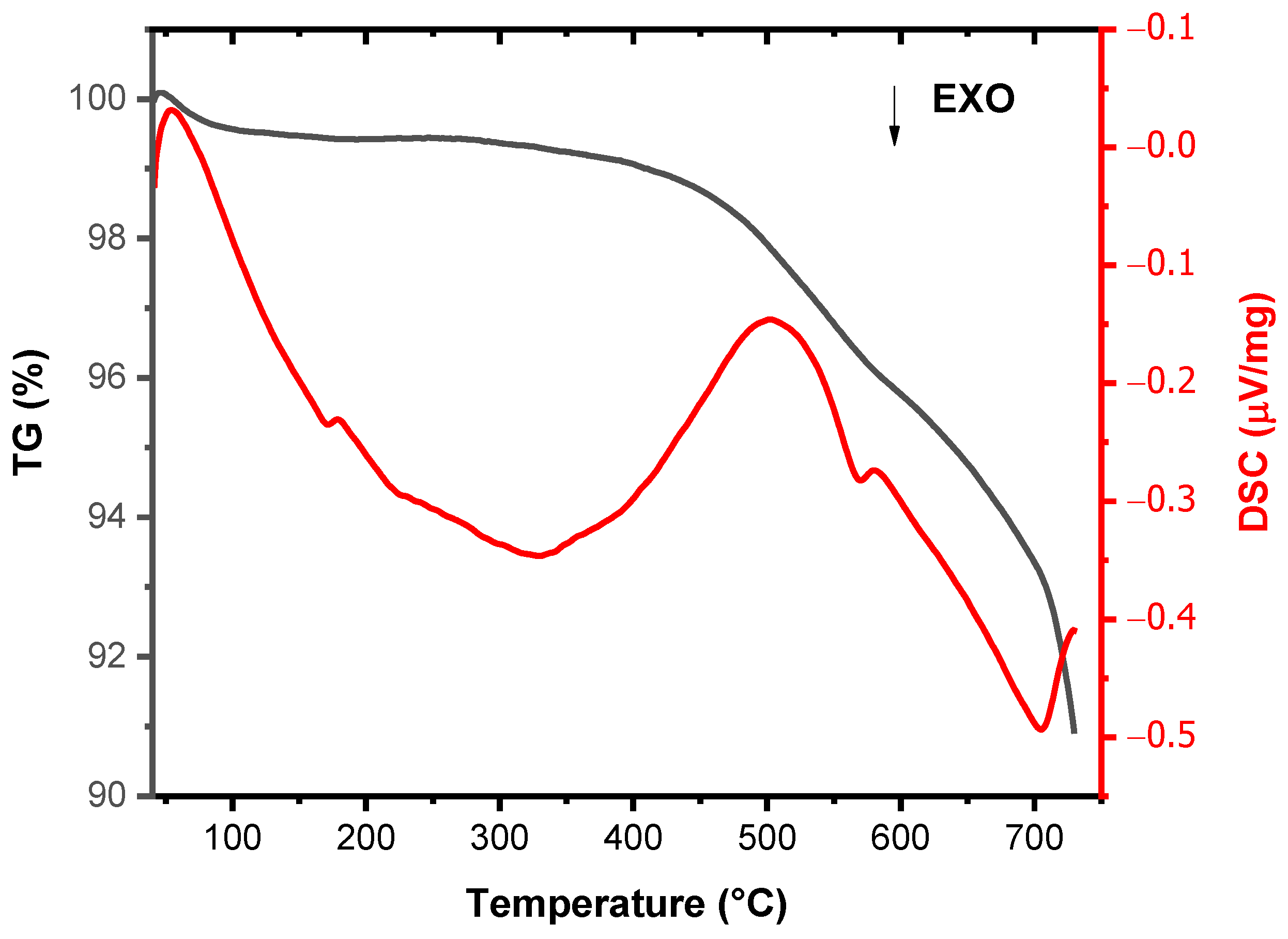
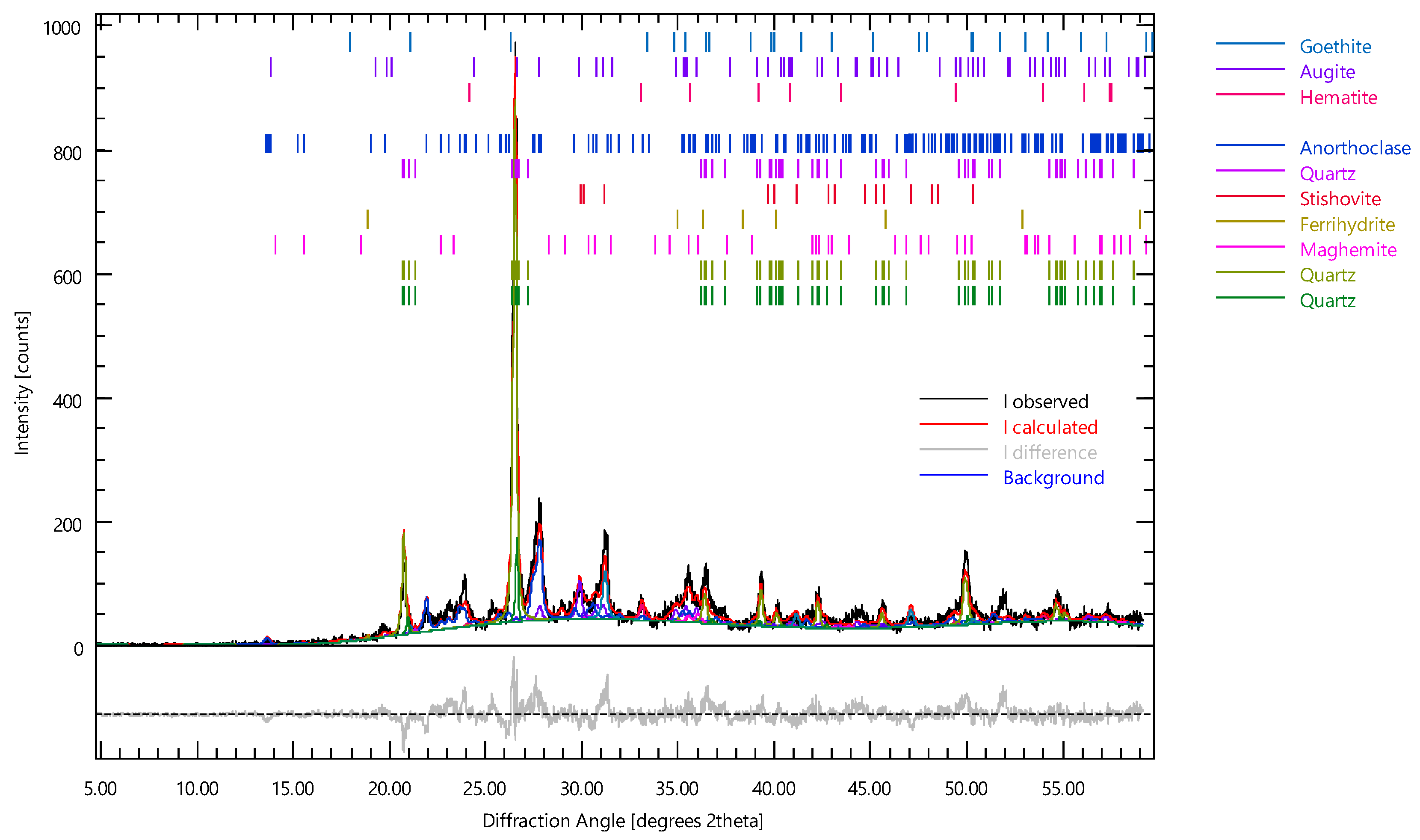
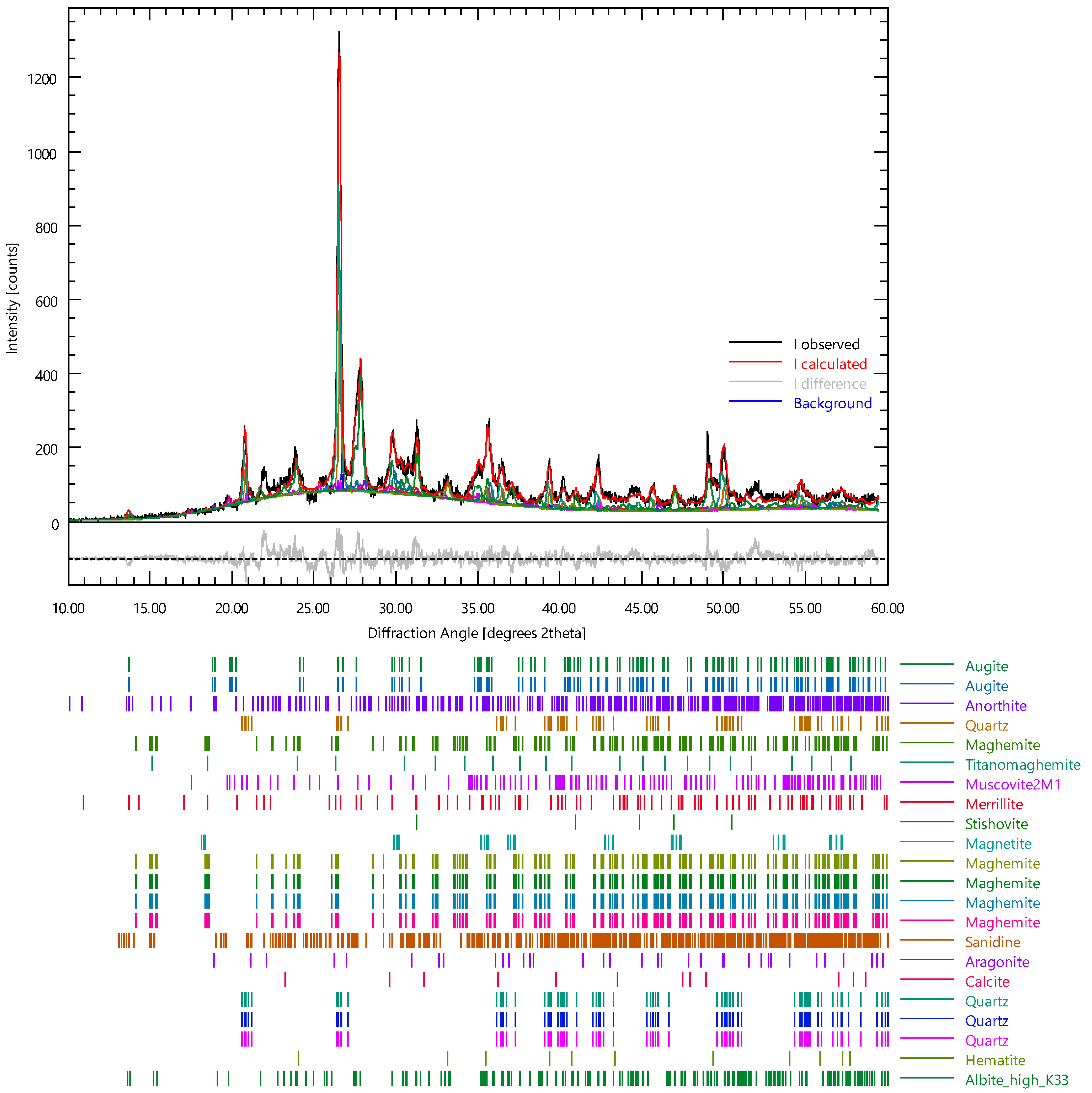
- The Etna volcanic ash contains inorganic glasses, albite, stishovite, augite–albite, and anorthite. The high content of iron excludes their use in the production of porcelain Upon firing at 950 °C, an increase in the intensity and number of peaks of the crystalline phases was observed and ascribed to minerals formed during the thermal treatment through recrystallisation and sintering.
- Workable and moldable mixtures were prepared by adding up to 30 wt% of VA. The mix with 50 wt% of volcanic ash failed to provide satisfactory fresh properties.
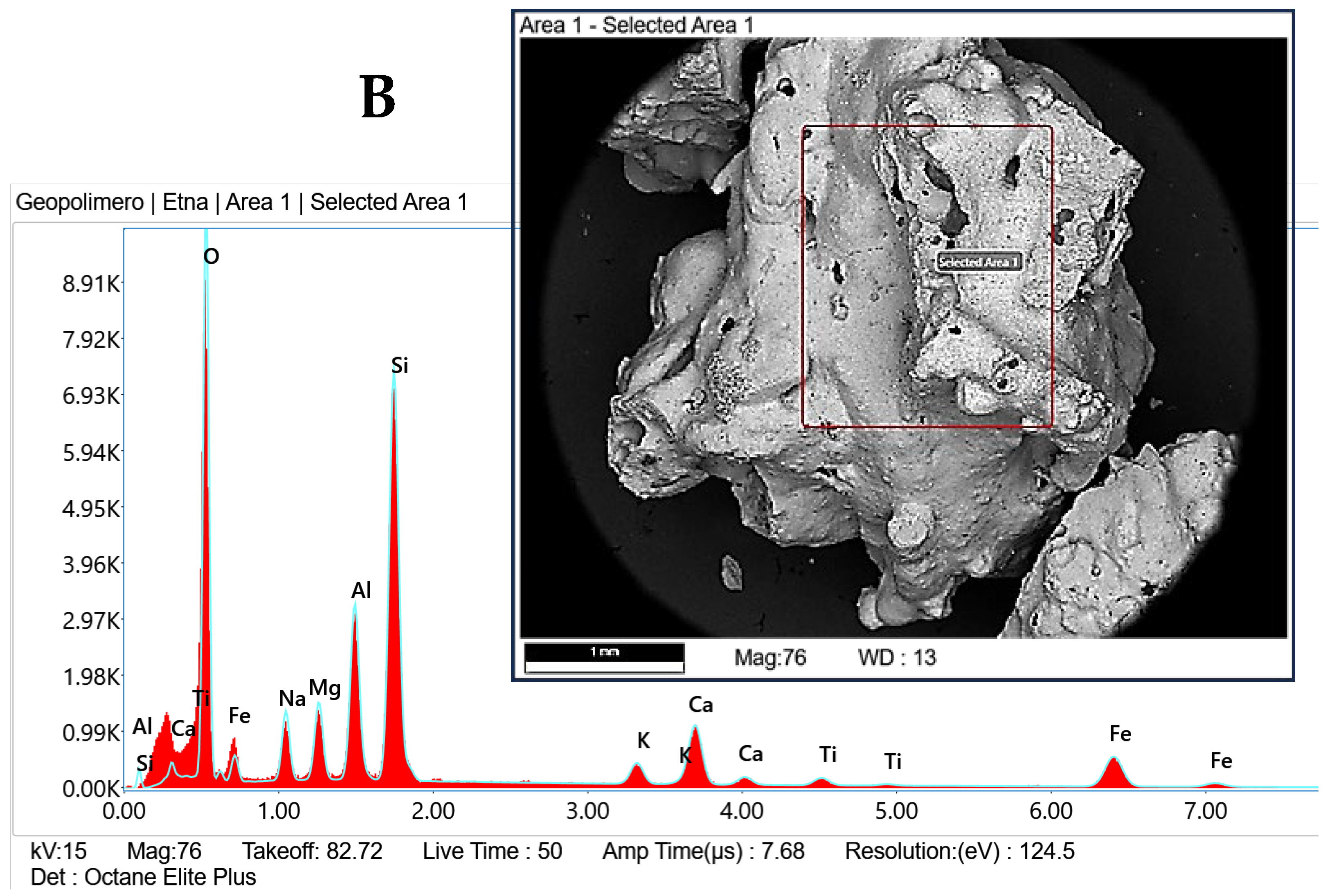
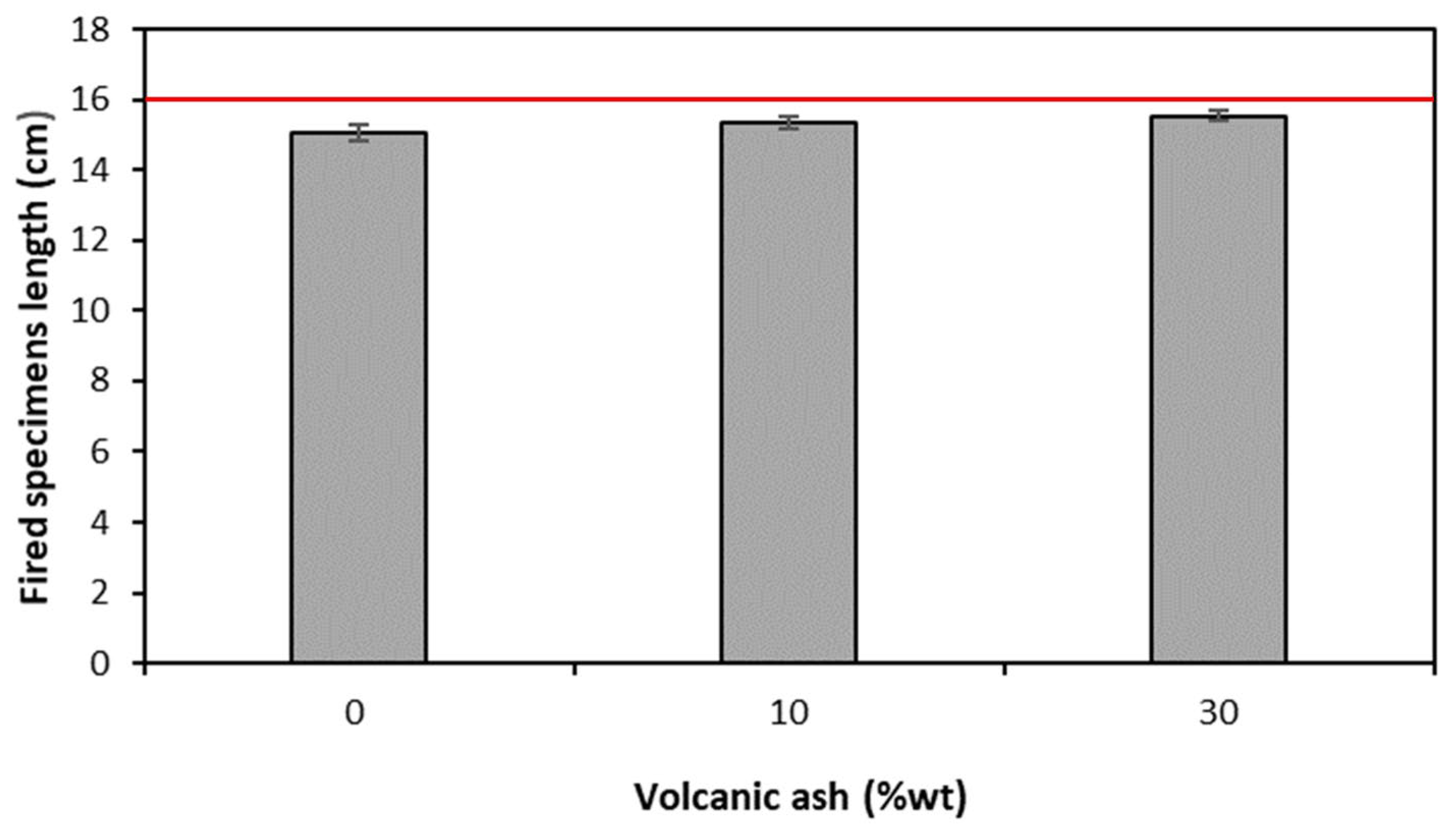
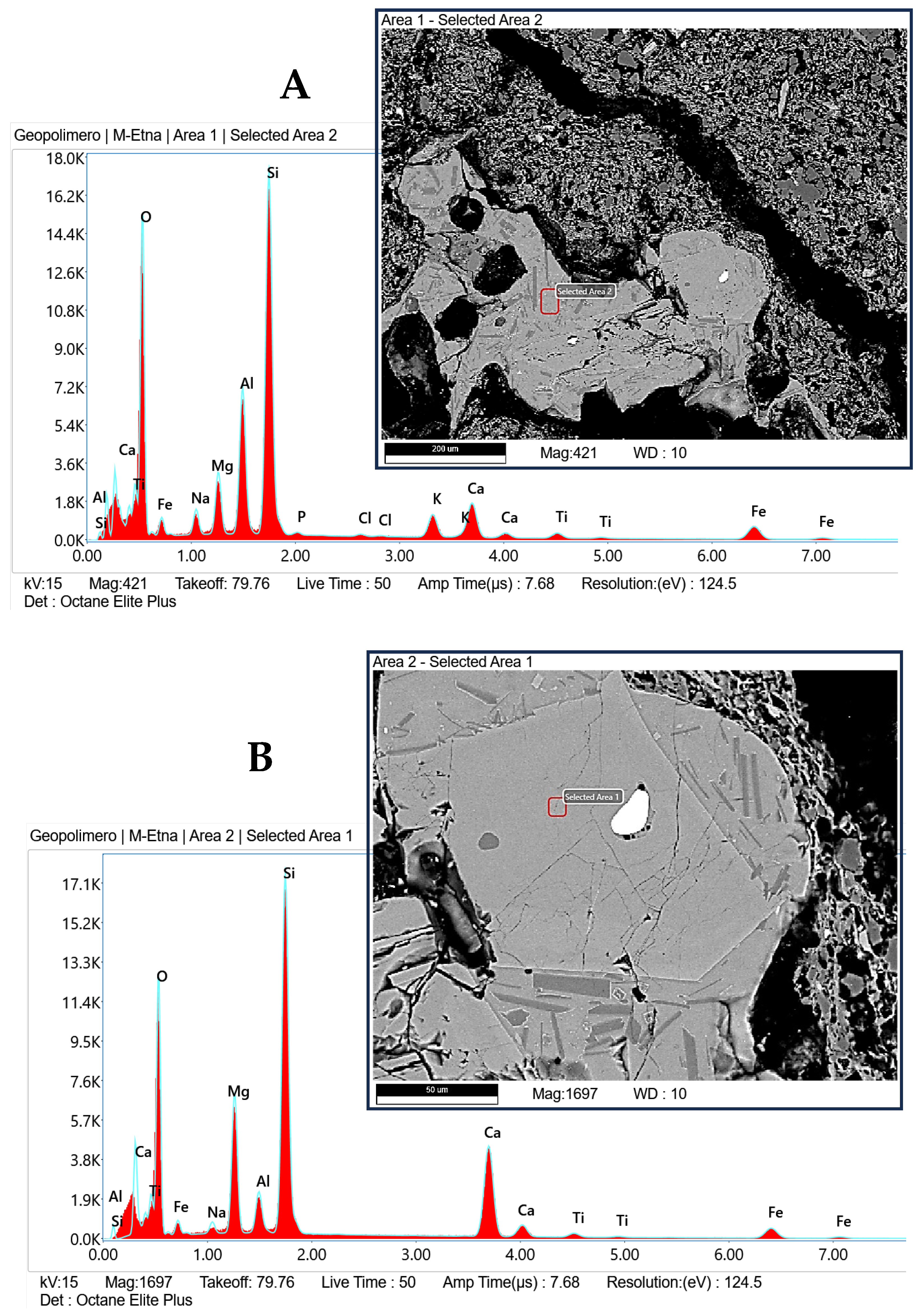
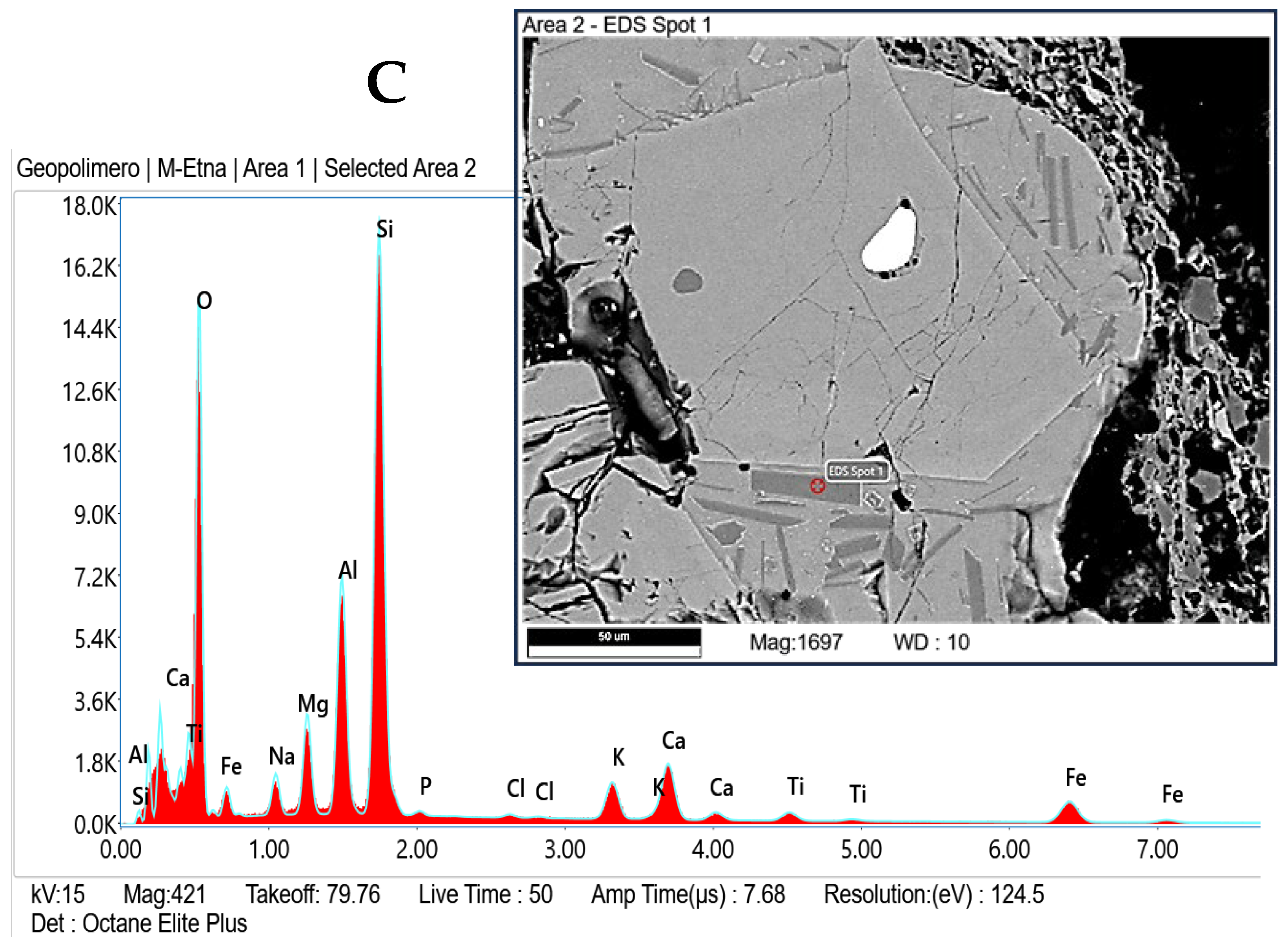
- Weight loss after firing decreases with the increasing of the percentage of clay replacement with volcanic ash, whereas dimensional stability increases. Embedded volcanic ash particles are clearly visible in the microstructure of bricks. It can be assumed that, at the used firing temperature, VA mainly plays the role of nonplastics or temper, providing support for the body.
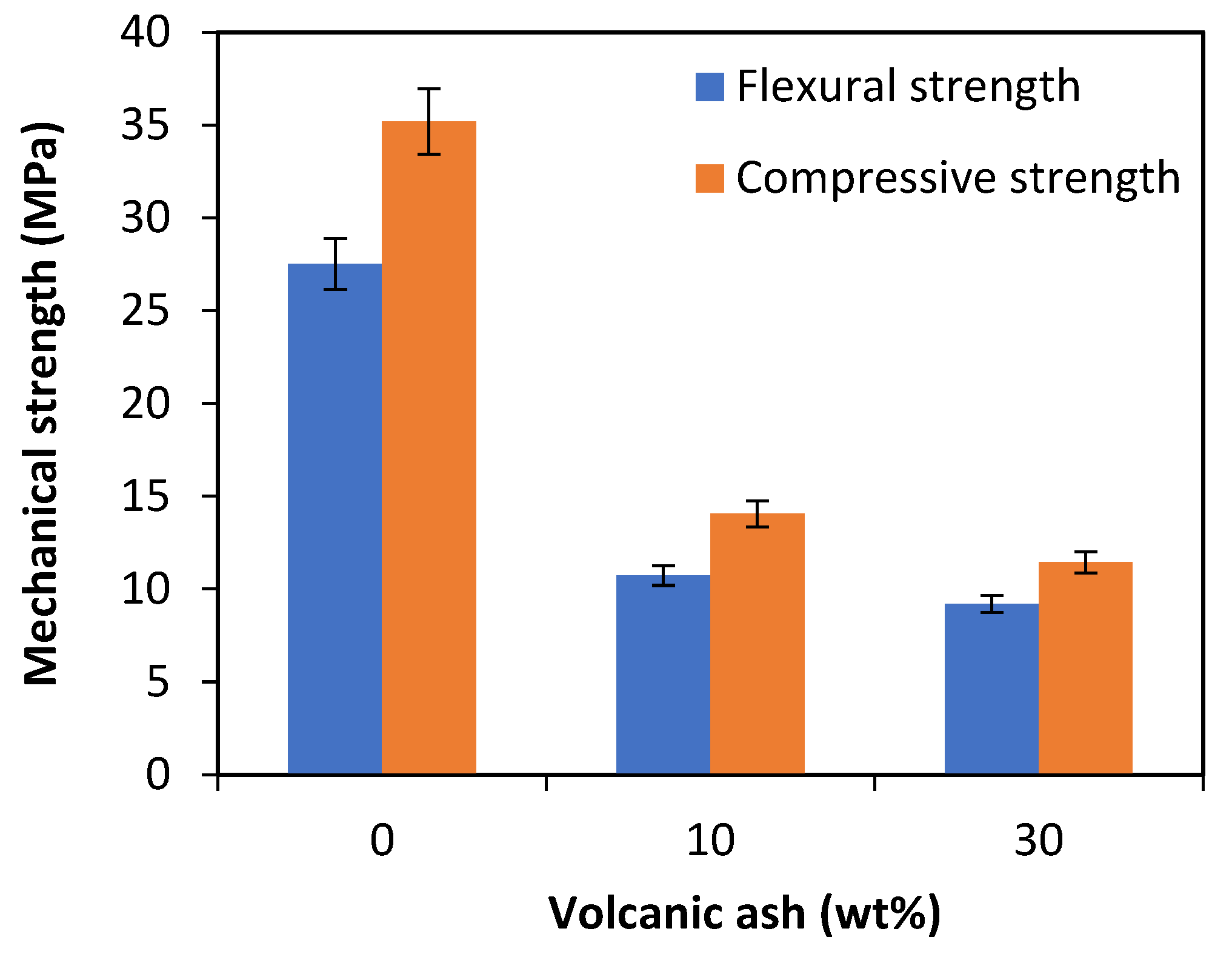
- Increasing the percentage of VA results in a significant decrease in the mechanical properties up to 66% at the highest replacement percentage. This is due to the presence of volcanic ash particles only partially melted and not firmly joined to the clay matrix, as also confirmed by values of the dynamic elastic modulus and SEM analysis. However, measured the values are higher than those required by the European standard EN 771-2 [43], British Standard BS 3921:1985 [44] and ASTM C62-13 [45] for bricks that can be used in normal weathering.
- The addition of volcanic ash lowers the sorptivity values by up to 10 wt% and hinders the absorption of water of bricks. The produced bricks with VA meet the second-class brick water absorption criteria according to ASTM C20-00 [56].
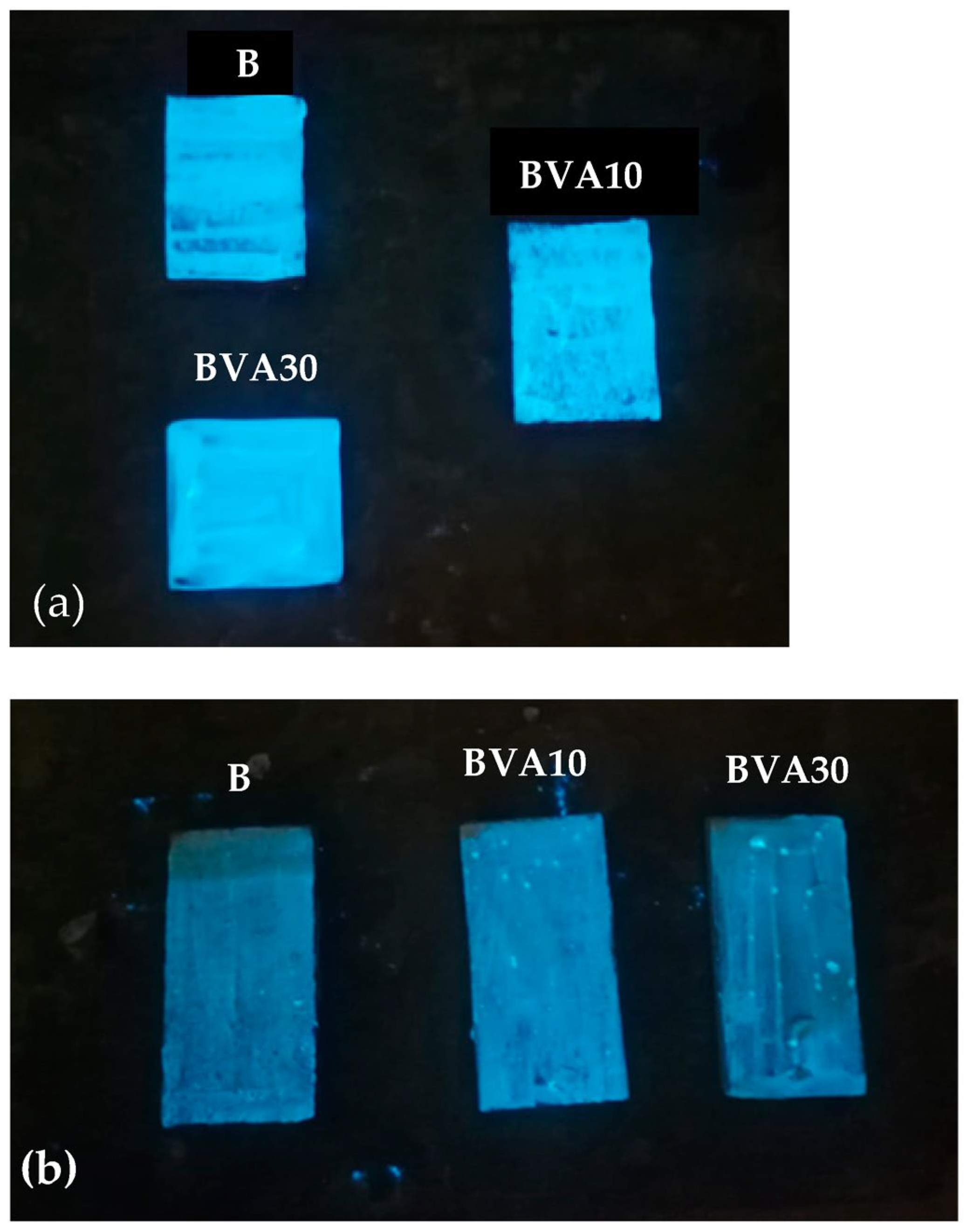
- A procedure to provide surface of bricks with intense photoluminescent properties, ensuring uniform brightness, was identified.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barone, G.; De Giudici, G.; Gimeno, D.; Lanzafame, G.; Podda, F.; Cannas, C.; Giuffrida, A.; Barchitta, M.; Agodi, A.; Mazzoleni, P. Surface reactivity of Etna volcanic ash and evaluation of health risks. Sci. Total Environ. 2021, 761, 143248. [Google Scholar] [CrossRef] [PubMed]
- Tesone, A.I.; Lasagni Vitar, R.M.; Tau, J.; Maglione, G.A.; Llesuy, S.; Tasat, D.R.; Berra, A. Volcanic ash from Puyehue-Cord on Caulle volcanic complex and Calbuco promote a differential response of pro-inflammatory and oxidative stress mediator son human conjunctival epithelial cells. Environ. Res. 2018, 167, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Barsotti, S.; Andronico, D.; Neri, A.; Del Carlo, P.; Baxter, P.J.; Aspinall, W.P.; Hincks, T. Quantitative assessment of volcanic ash hazards for heath and infrastructure at Mt. Etna (Italy) by numerical simulation. J. Volcanol. Geoth. Res. 2010, 192, 85–96. [Google Scholar] [CrossRef]
- Horwell, C.J.; Baxter, P.J. The respiratory health hazards of volcanic ash: A review for volcanic risk mitigation. Bull. Volcanol. 2006, 69, 1–24. [Google Scholar] [CrossRef]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Belviso, C.; Cavalcante, F.; Lettino, A.; Vagliasindi, F.G.A.; Roccaro, P. Removal of ammonium from wastewater by zeolite synthetized from volcanic ash: Batch and column tests. J. Environ. Chem. Eng. 2022, 10, 107539. [Google Scholar] [CrossRef]
- Milios, L. Advancing to a Circular Economy: Three essential ingredients for a comprehensive policy mix. Sustain. Sci. 2017, 13, 861–878. [Google Scholar] [CrossRef] [PubMed]
- Candamano, S.; Tassone, F.; Iacobini, I.; Crea, F.; De Fazio, P. The Properties and Durability of Self-Leveling and Thixotropic Mortars with Recycled Sand. Appl. Sci. 2022, 12, 2732. [Google Scholar] [CrossRef]
- Djon Li Ndjock, B.I.; Baenla, J.; Mbah, J.B.B.; Elimbi, A.; Cyr, M. Amorphous phase of volcanic ash and microstructure of cement product obtained from phosphoric acid activation. SN Appl. Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Latif, D.O.; Rifa’i, A.; Suryolelono, K.B. Chemical characteristics of volcanic ash in Indonesia for soil stabilization: Morphology and mineral content. GEOMATE J. 2016, 11, 2606–2610. [Google Scholar] [CrossRef]
- Serra, M.F.; Conconi, M.S.; Suarez, G.; Aglietti, E.F.; Rendtorff, N.M. Volcanic ash as flux in clay based triaxial ceramic materials, effect of the firing temperature in phases and mechanical properties. Ceram. Int. 2015, 41, 6169–6177. [Google Scholar] [CrossRef]
- Alemayehu, E.; Lennartz, B. Virgin volcanic rocks: Kinetics and equilibrium studies for the adsorption of cadmium from water. J. Hazard. Mater. 2009, 169, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Siddique, R. Effect of volcanic ash on the properties of cement paste and mortar. Resources. Conserv. Recycl. 2011, 56, 66–70. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Wang, K.; Tang, Q.; Nzeukou, A.N.; Billong, N.; Melo, U.C.; Cui, X. Review on the use of volcanic ashes for engineering applications. Res. Conserv. Recy. 2018, 137, 177–190. [Google Scholar] [CrossRef]
- Djon Li Ndjock, B.I.; Elimbi, A.; Cyr, M. Rational utilization of volcanic ashes based on factors affecting their alkaline activation. J. Non-Cryst. Solids 2017, 463, 31–39. [Google Scholar] [CrossRef]
- Liu, Y.; Taylor, L.A. Characterization of lunar dust and a synopsis of available lunar simulants. Planet. Space Sci. 2011, 59, 1769–1783. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Papynov, E.K.; Nepomnyushchaya, V.A.; Ivanets, A.I.; Belov, A.A.; Dran’kov, A.N.; Yarusova, S.B.; Buravlev, I.Y.; Tarabanova, A.E.; Fedorets, A.N.; et al. Hydrothermal synthesis and spark plasma sintering of NaY zeolite as solid-state matrices for cesium-137 immobilization. J. Eur. Ceram. Soc. 2022, 42, 3004–3014. [Google Scholar] [CrossRef]
- Yarusova, S.B.; Shichalin, O.O.; Belov, A.A.; Azon, S.A.; Buravlev, I.Y.; Golub, A.V.; Mayorov, V.Y.; Gerasimenko, A.V.; Papynov, E.K.; Ivanets, A.I.; et al. Synthesis of amorphous KAlSi3O8 for cesium radionuclide immobilization into solid matrices using spark plasma sintering technique. Ceram. Int. 2022, 48, 3808–3817. [Google Scholar] [CrossRef]
- Průša, D.; Šuhajda, K.; Žajdlík, T.; Svobodová, K.; Šťastník, S.; Hobzova, K.; Venkrbec, V. Effect of Microwave Radiation on the Compressive Strength of Solid Ceramic Brick. Buildings 2023, 13, 1018. [Google Scholar] [CrossRef]
- Stepien, A.; Kostrzewa, P. The impact of basalt components on the structure of bricks formed as a result of hydrothermal treatment. Buildings 2019, 9, 192. [Google Scholar] [CrossRef]
- Panasenko, A.E.; Shichalin, O.O.; Yarusova, S.B.; Ivanets, A.I.; Belov, A.A.; Dran’kov, A.N.; Azon, S.A.; Fedorets, A.N.; Buravlev, I.Y.; Shlyk, D.K.; et al. A novel approach for rice straw agricultural waste utilization: Synthesis of solid aluminosilicate matrices for cesium immobilization. Nucl. Eng. Technol. 2022, 54, 3250–3259. [Google Scholar] [CrossRef]
- Guo, C.; Kong, J.; Wang, Z.; Meng, X.; Zhao, Y.; Wu, W.; Quan, H. Study on Preparation and Properties of Sintered Brick from Multi-Source Solid Waste. Appl. Sci. 2022, 12, 10181. [Google Scholar] [CrossRef]
- Stepien, A.; Potrzeszcz-Sut, B.; Prentice, D.P.; Oey, T.J.; Balonis, M. The role of glass compounds in autoclaved bricks. Buildings 2020, 10, 41. [Google Scholar] [CrossRef]
- Belfiore, C.M.; Amato, C.; Pezzino, A.; Viccaro, M. An end of waste alternative for volcanic ash: A resource in the manufacture of ceramic tiles. Constr. Build. Mater. 2020, 263, 120118. [Google Scholar] [CrossRef]
- Cultrone, G. The use of Mount Etna volcanic ash in the production of bricks with good physical-mechanical performance: Converting a problematic waste product into a resource for the construction industry. Ceram. Int. 2022, 48, 5724–5736. [Google Scholar] [CrossRef]
- De Luca, P.; De Luca, P.; Candamano, S.; Crea, F.; Nagy, J.B. Preparation and characterization of plasters with photodegradative action. Buildings 2018, 8, 122. [Google Scholar] [CrossRef]
- Danfá, S.; Martins, R.C.; Quina, M.J.; Gomes, J. Supported TiO2 in Ceramic Materials for the Photocatalytic Degradation of Contaminants of Emerging Concern in Liquid Effluents: A Review. Molecules 2021, 26, 5363. [Google Scholar] [CrossRef]
- Candamano, S.; Sgambitterra, E.; Lamuta, C.; Pagnotta, L.; Chakraborty, S.; Crea, F. Graphene nanoplatelets in geopolymeric systems: A new dimension of nanocomposites. Mater. Lett. 2019, 236, 550–553. [Google Scholar] [CrossRef]
- Lamuta, C.; Bruno, L.; Candamano, S.; Pagnotta, L. Piezoresistive characterization of graphene/metakaolin based geopolymeric mortar composites. MRS Adv. 2017, 2, 3773–3779. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, H.; Pan, A.; Yang, B.; He, L.; Wu, Y. Rare Earth-Free Luminescent Materials for WLEDs: Re-cent Progress and Perspectives. Adv. Mater. Technol. 2021, 6, 1–26. [Google Scholar] [CrossRef]
- Gao, Y.; Shen, H.; Li, D.; Yang, D. Efficient sensitized photoluminescence of Er silicate in silicon oxide films embedded with amorphous silicon clusters, part I: Fabrication. Opt. Mater. Express 2019, 9, 4329–4338. [Google Scholar] [CrossRef]
- Döbelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Žibret, L.; Wisniewski, W.; Horvat, B.; Božič, M.; Gregorc, B.; Ducman, V. Clay rich river sediments calcined into precursors for alkali activated materials. Appl. Clay Sci. 2023, 234, 106848. [Google Scholar] [CrossRef]
- AFNOR XP P18-594; Granulats–Méthodes D’essai de Réactivité aux Alcalis (Aggregates–Test Methods on Reactivity to Alkalis). Association Française de Normalisation: Paris, France, 2004.
- Gao, X.X.; Cyr, M.; Multon, S.; Sellier, A. A comparison of methods for chemical assessment of reactive silica in concrete aggregates by selective dissolution. Cem. Concr. Compos. 2013, 37, 82–94. [Google Scholar] [CrossRef]
- Karaman, S.; Ersahin, S.; Gunal, H. Firing temperature and firing time influence on mechanical and physical properties of clay bricks. J. Sci. Ind. Res. 2006, 65, 153–159. [Google Scholar]
- Kuzmickas, L.; Andrade, F.R.D.D.; Szabó, G.A.J.; Motta, J.F.M.; Cabral, M., Jr. Influence of diopside: Feldspar ratio in ceramic reactions assessed by quantitative phase analysis (X-ray diffraction-Rietveld method). Cerâmica 2013, 59, 345–350. [Google Scholar] [CrossRef]
- Coppola, L.; Coffetti, D.; Crotti, E.; Candamano, S.; Crea, F.; Gazzaniga, G.; Pastore, T. The combined use of admixtures for shrinkage reduction in one-part alkali activated slag-based mortars and pastes. Constr. Build. Mater. 2020, 248, 118682. [Google Scholar] [CrossRef]
- UNI EN 1015-18:2002; Methods of Test for Mortar for Masonry Determination of Water Absorption Coefficient Due to Capillary Action of Hardened Mortar. UNI: Milan, Italy, 2002.
- Candamano, S.; Crea, F.; Iorfida, A. Mechanical characterization of basalt fabric-reinforced alkali-activated matrix composite: A preliminary investigation. Appl. Sci. 2020, 10, 2865. [Google Scholar] [CrossRef]
- ASTM C67/C67M-21; Standard Test Methods for Sampling and Testing Brick and Structural Clay Tile. ASTM International: West Conshohocken, PA, USA, 2023.
- Shahreza, S.K.; Niklewski, J.; Molnár, M. Experimental investigation of water absorption and penetration in clay brick masonry under simulated uniform water spray exposure. J. Build. Eng. 2021, 43, 102583. [Google Scholar] [CrossRef]
- UNI-EN 196–1; Methods of Testing Cement-Part 1: Determination of Strength. UNI: Milan, Italy, 2016.
- UNI EN 771-2; Specification for Masonry Units–Part 2: Calcium Silicate Masonry Units. UNI: Milan, Italy, 2015.
- BS3921: 1985; Specifications for Clay Brick, United Kingdom. British Standards Institution: London, UK, 1985.
- ASTM C62-13a; Standard Specification for Building Brick (Solid Masonry Units Made from Clay or Shale. ASTM International: West Conshohocken, PA, USA, 2013.
- Wilson, T.; Stewart, C. Volcanic Ash. In Encyclopedia of Natural Hazards; Encyclopedia of Earth Sciences Series; Bobrowsky, P.T., Ed.; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Song, W.; Lavallee, Y.; Wadsworth, F.B.; Hess, K.U.; Dingwell, D.B. Wetting and spreading of molten volcanic ash in jet engines. J. Phys. Chem. Lett. 2017, 8, 1878–1884. [Google Scholar] [CrossRef]
- Melamud, S.G.; Yur’ev, B.P. Oxidation of iron ore at moderate and high temperatures. Steel Transl. 2016, 46, 384–389. [Google Scholar] [CrossRef]
- Clifford, J.F. High Temperature Reactions and Colour Development in Brick Clays. Ph.D. Dissertation, University of Surrey, Surrey, UK, 1984. [Google Scholar]
- Leonelli, C.; Kamseu, E.; Boccaccini, D.N.; Melo, U.C.; Rizzuti, A.; Billong, N.; Miselli, P. Volcanic ash as alternative raw materials for traditional vitrified ceramic products. Adv. Appl. Ceram. 2007, 106, 135–141. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Mackinnon, I.D.R.; Allen, C.; Gu, Y.T.; Xi, Y.F. Thermal behaviors of clay minerals as key components and additives for fired brick properties: A review. J. Build. Eng. 2023, 66, 105802. [Google Scholar] [CrossRef]
- Labaied, I.; Douzane, O.; Lajili, M.; Promis, G. Bricks Using Clay Mixed with Powder and Ashes from Lignocellulosic Biomass: A Review. Appl. Sci. 2022, 12, 10669. [Google Scholar] [CrossRef]
- Wang, S.; Gainey, L.; Marinelli, J.; Deer, B.; Wang, X.D.; Mackinnon, I.D.R.; Xi, Y. Effects of vermiculite on in-situ thermal behaviour, microstructure, physical and mechanical properties of fired clay bricks. Constr. Build. Mater. 2022, 316, 125828. [Google Scholar] [CrossRef]
- Cultrone, G.; Carrillo-Rosúa, J. Growth of metastable phases during brick firing: Mineralogical and microtextural changes induced by the composition of the raw material and the presence of additives. Appl. Clay Sci. 2020, 185, 105419. [Google Scholar] [CrossRef]
- De Bonis, A.; Cultrone, G.; Grifa, C.; Langella, A.; Morra, V. Clays from the bay of Naples (Italy): New insight on ancient and traditional ceramics. J. Eur. Ceram. Soc. 2014, 34, 3229–3244. [Google Scholar] [CrossRef]
- ASTM C20-00; Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water. ASTM International: West Conshohocken, PA, USA, 2022.
- Leiva, C.; Arenas, C.; Alonso-Fariñas, B.; Vilches, L.F.; Peceño, B.; Rodriguez-Galán, M.; Baena, F. Characteristics of fired bricks with co-combustion fly ashes. J. Build. Eng. 2016, 5, 114–118. [Google Scholar] [CrossRef]
- Wang, L.; Shang, Z.; Shi, M.; Cao, P.; Yang, B.; Zou, J. Preparing and testing the reliability of long-afterglow SrAl2O4:Eu2+, Dy3+ phosphor flexible films for temperature sensing. RSC Adv. 2020, 10, 11418–11425. [Google Scholar] [CrossRef] [PubMed]


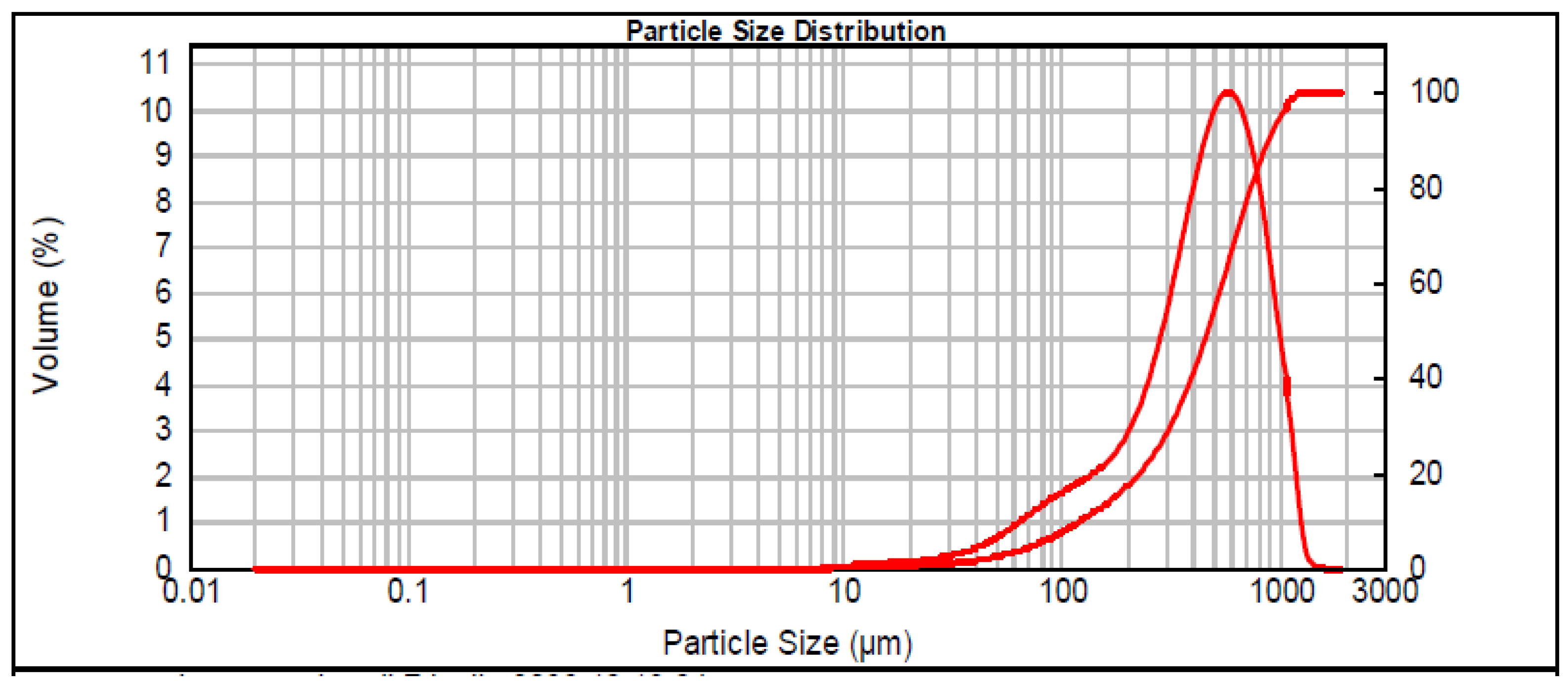
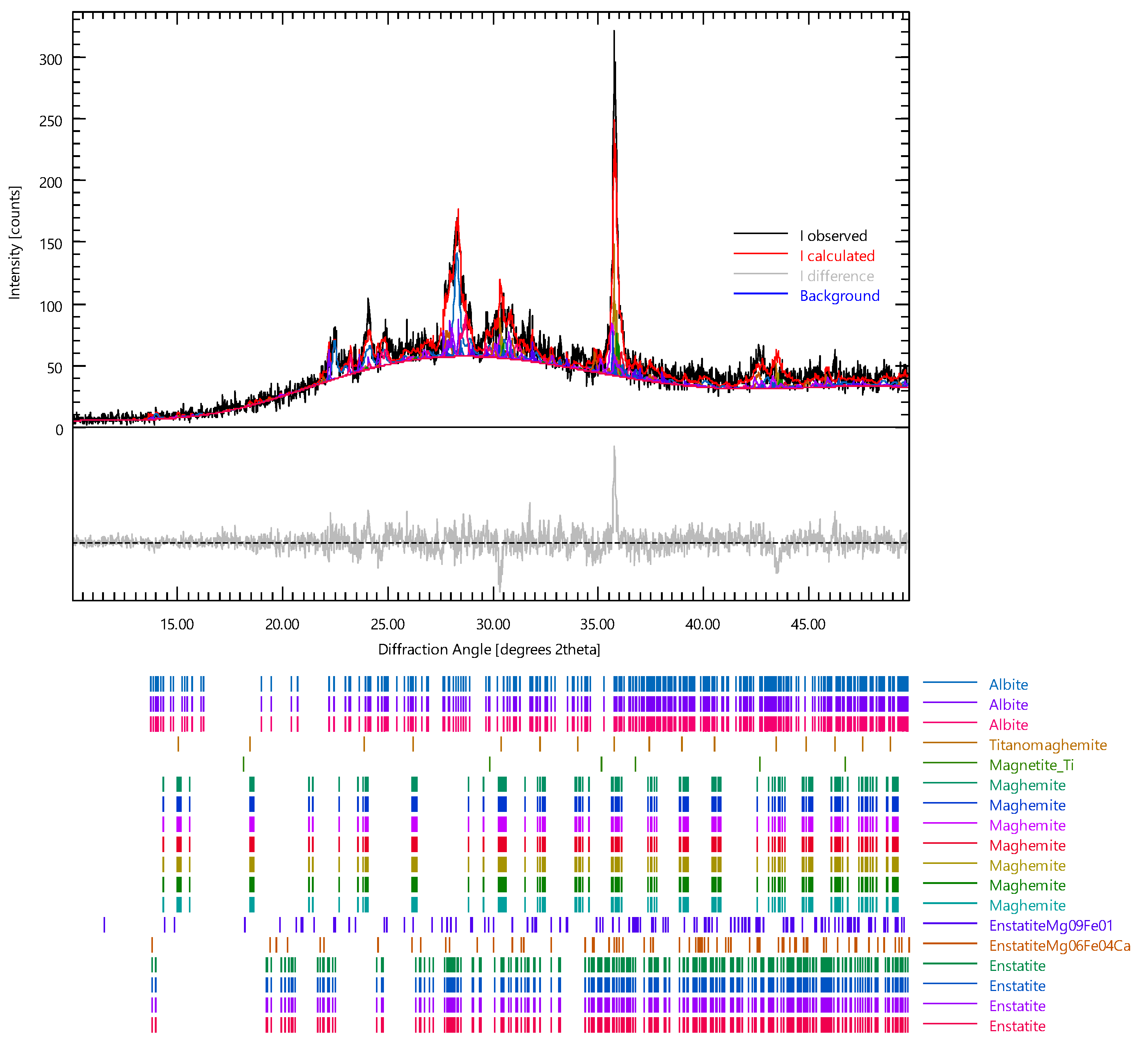
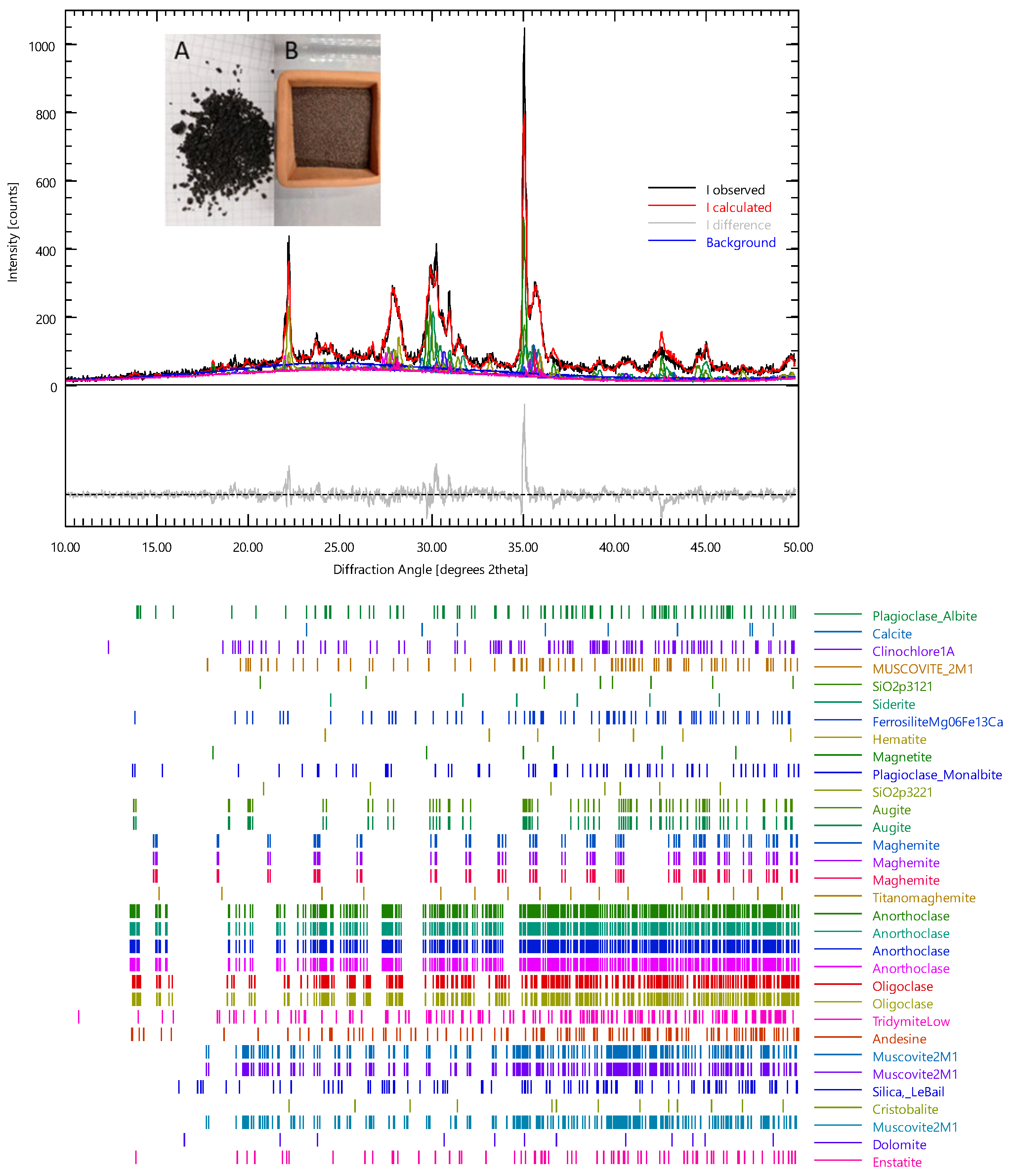






| Samples | Dried Clay (kg) | Volcanic Ash (kg) | Volcanic Ash (wt%) * | Water (wt%) * |
|---|---|---|---|---|
| B | 5 | 0.0 | 0.0 | 27 |
| BVA10 | 4.5 | 0.5 | 10 | 24 |
| BVA30 | 3.5 | 1.5 | 30 | 19 |
| BVA50 | 2.5 | 2.5 | 50 | - |
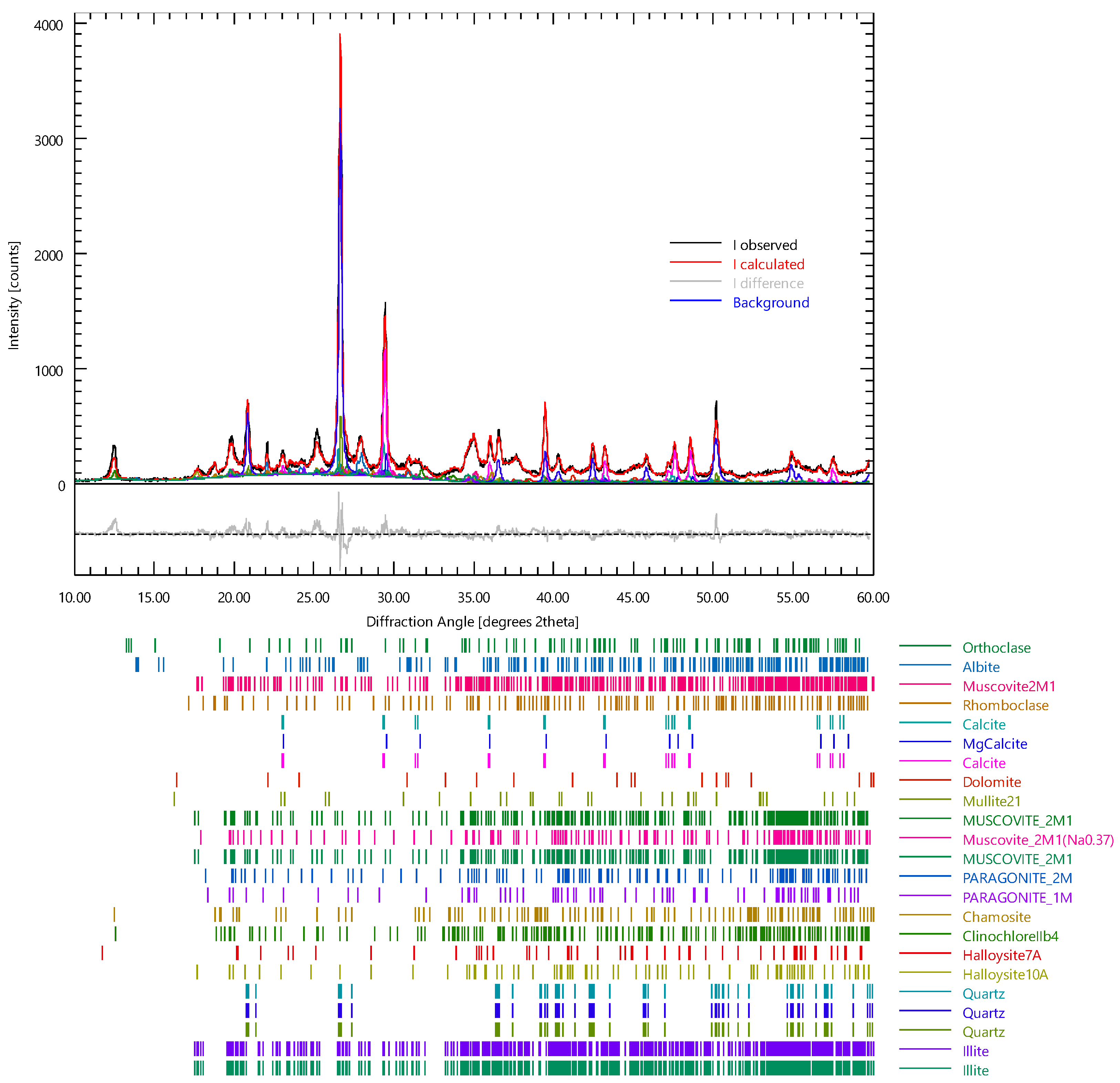
| Mineral | wt% | ESD |
|---|---|---|
| Orthoclase | 3.83 | 0.38 |
| Albite | 11.29 | 0.59 |
| Rhomboclase | 4.29 | 0.52 |
| Calcite | 23.97 | 1.29 |
| MgCalcite | 3.18 | 0.59 |
| Dolomite | 4.92 | 0.29 |
| Mullite21 | 3.55 | 0.41 |
| Musc2m1 | 3.21 | 0.61 |
| Muscovite2M1 | 1.64 | 0.39 |
| Muscovitena037 | 5.52 | 0.92 |
| Parago2m | 1.88 | 0.38 |
| Parago1m | 4.42 | 0.59 |
| Chamosite | 5.35 | 0.30 |
| ClinochloreIIb4 | 2.50 | 0.14 |
| Halloysite7A | 5.71 | 0.36 |
| Halloysite10A | 7.24 | 0.48 |
| Quartz | 4.50 | 1.12 |
| Illite | 2.99 | 0.65 |
| Rwp | 12.79 | |
| Rexp | 7.21 | |
| Chi2 | 3.1468 | |
| GOF | 1.7739 |
| Oxide | Spot A (wt%) | Spot B (wt%) |
|---|---|---|
| Na2O | 7.7 | 6.0 |
| MgO | 4.9 | 5.8 |
| Al2O3 | 16.6 | 14.7 |
| SiO2 | 43.1 | 40.4 |
| K2O | 2.8 | 2.7 |
| CaO | 10.2 | 11.2 |
| Fe O | 12.3 | 16.8 |
| TiO2 | 2.1 | 2.3 |
| Phase | wt% | ESD |
|---|---|---|
| Augite | 13.27 | 0.87 |
| Anorthite | 4.99 | 0.79 |
| Maghemite | 0.64 | 0.24 |
| Titanomaghemite | 0.33 | 0.12 |
| Muscovite2M1 | 7.16 | 0.91 |
| Merrillite | 4.91 | 0.90 |
| Stishovite | 13.57 | 0.59 |
| Magnetite | 0.03 | 0.07 |
| Maghemite | 0.64 | 0.24 |
| Sanidine | 0.16 | 0.33 |
| Aragonite | 3.33 | 0.50 |
| Calcite | 6.77 | 1.23 |
| Quartz | 1.00 | 0.41 |
| Hematite | 3.60 | 0.29 |
| Anortk33 (Albite high_k_33) | 39.43 | 1.57 |
| Rwp | 15.67 | |
| Rexp | 10.39 | |
| Chi2 | 2.27 | |
| GOF | 1.51 |
| Phase | wt% | ESD |
|---|---|---|
| Albite | 1.916885 | 0.33499 |
| Calcite | 1.284127 | 0.204716 |
| Clinochlore1A | 1.600506 | 0.3536 |
| Musc2m1 | 0.986358 | 0.279158 |
| Siderite | 2.140212 | 0.186105 |
| FerrosiliteMg06Fe13Ca | 1.637727 | 0.409432 |
| Hematite | 1.833138 | 0.178661 |
| Magnetite | 9.677479 | 0.725811 |
| Augite | 21.73711 | 1.712169 |
| Maghemite | 1.098022 | 0.428042 |
| Titanomaghemite | 1.507454 | 0.279158 |
| Anorthoclase | 5.601772 | 0.7072 |
| Oligoclase | 8.746953 | 0.949137 |
| Silica | 26.79917 | 4.466529 |
| Cristobalite | 6.885899 | 0.595537 |
| Dolomite | 3.666276 | 0.372211 |
| Enstatite | 2.493812 | 0.558316 |
| Rwp | 14.93 | |
| Rexp | 9.96 | |
| Chi2 | 2.247 | |
| GOF | 1.499 |
| Element | (wt%) |
|---|---|
| O | 68.5 |
| Mg | 1.1 |
| Al | 7.8 |
| Si | 14.5 |
| Sr | 4.0 |
| Ca | 0.7 |
| Na | 2.5 |
| Fe | 1.0 |
| Oxide | Spot A (wt%) | Spot B (wt%) | Spot C (wt%) |
|---|---|---|---|
| Na2O | 1.08 | - | 6.8 |
| MgO | 27.85 | - | - |
| Al2O3 | 3.60 | - | 19.8 |
| SiO2 | 0.09 | 1.9 | 64.1 |
| K2O | 51.78 | 98.1 | 9.3 |
| CaO | 1.47 | - | - |
| Fe O | 1.64 | - |
| Phase | wt% | ESD |
|---|---|---|
| Goethite | 1.08 | 0.23 |
| Augite | 27.85 | 1.89 |
| Hematite | 3.60 | 0.53 |
| Calcite | 0.09 | 0.22 |
| Anorthoclase | 51.78 | 3.11 |
| Stishovite | 1.47 | 0.39 |
| Ferrihydrite | 1.64 | 0.39 |
| Maghemite | 3.00 | 0.50 |
| Quartz | 8.00 | 1.07 |
| Stishovite | 1.47 | 0.39 |
| Rwp | 20.62 | |
| Rexp | 14.58 | |
| Chi2 | 2.0001 | |
| GOF | 1.4143 |
| Oxide | Spot A (wt%) | Spot B (wt%) | Spot C (wt%) |
|---|---|---|---|
| Na2O | 3.0 | 1.3 | 5.6 |
| MgO | 6.1 | 13.8 | - |
| Al2O3 | 16.5 | 4.8 | 29.8 |
| SiO2 | 48.8 | 47.3 | 49.9 |
| P2O5 | 0.5 | - | 9.3 |
| Cl | 0.2 | - | - |
| K2O | 4.0 | - | 0.5 |
| CaO | 9.1 | 23.6 | 13.2 |
| TiO2 | 2.2 | 1.7 | - |
| FeO | 9.6 | 7.5 | 1.0 |
| Phase | wt% | ESD |
|---|---|---|
| Augite | 13.27 | 0.87 |
| Anorthite | 4.99 | 0.79 |
| Maghemite | 0.64 | 0.24 |
| Titanomaghemite | 0.33 | 0.12 |
| Muscovite2M1 | 7.16 | 0.91 |
| Merrillite | 4.91 | 0.90 |
| Stishovite | 13.57 | 0.59 |
| Magnetite | 0.03 | 0.07 |
| Maghemite | 0.64 | 0.24 |
| Sanidine | 0.16 | 0.33 |
| Aragonite | 3.33 | 0.50 |
| Calcite | 6.77 | 1.23 |
| Quartz | 1.00 | 0.41 |
| Hematite | 3.60 | 0.29 |
| anortk33 (Albite high_k_33) | 39.43 | 1.57 |
| Rwp | 15.67 | |
| Rexp | 10.39 | |
| Chi2 | 2.27 | |
| GOF | 1.51 |
| Sample | S (kg/m2min0.5) | a0 (kg/m2) | Average 24-h Water Absorption (%) |
|---|---|---|---|
| B | 1.87 | −0.612 | 16.05 |
| BVA10 | 1.28 | −0.95 | 13.52 |
| BVA30 | 1.82 | −0.67535 | 14.86 |
| Sample | Density (g/cm3) | Dynamic Modulus of Elasticity (GPa) |
|---|---|---|
| B | 1.89 | 25.9 |
| BVA10 | 1.85 | 19.6 |
| BVA30 | 1.80 | 15.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candamano, S.; De Luca, P.; Garofalo, P.; Crea, F. Ceramic Materials Containing Volcanic Ash and Characterized by Photoluminescent Activity. Environments 2023, 10, 172. https://doi.org/10.3390/environments10100172
Candamano S, De Luca P, Garofalo P, Crea F. Ceramic Materials Containing Volcanic Ash and Characterized by Photoluminescent Activity. Environments. 2023; 10(10):172. https://doi.org/10.3390/environments10100172
Chicago/Turabian StyleCandamano, Sebastiano, Pierantonio De Luca, Pietro Garofalo, and Fortunato Crea. 2023. "Ceramic Materials Containing Volcanic Ash and Characterized by Photoluminescent Activity" Environments 10, no. 10: 172. https://doi.org/10.3390/environments10100172







