Neurocognitive Suicide and Homicide Markers in Patients with Schizophrenia Spectrum Disorders: A Systematic Review
Abstract
:1. Introduction
- (a)
- What is the relationship between cognitive functions and suicide and homicide behaviors in patients with schizophrenia spectrum disorders?
- (b)
- Does the performance of cognitive functions predispose to these behaviors?
- (c)
- Are there shared neurocognitive mechanisms?
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy and Selection Process
2.4. Data Collection Process
2.5. Risk of Bias Assessment of Individual Studies
2.6. Synthesis Methods
2.7. Certainty Assessment
3. Results
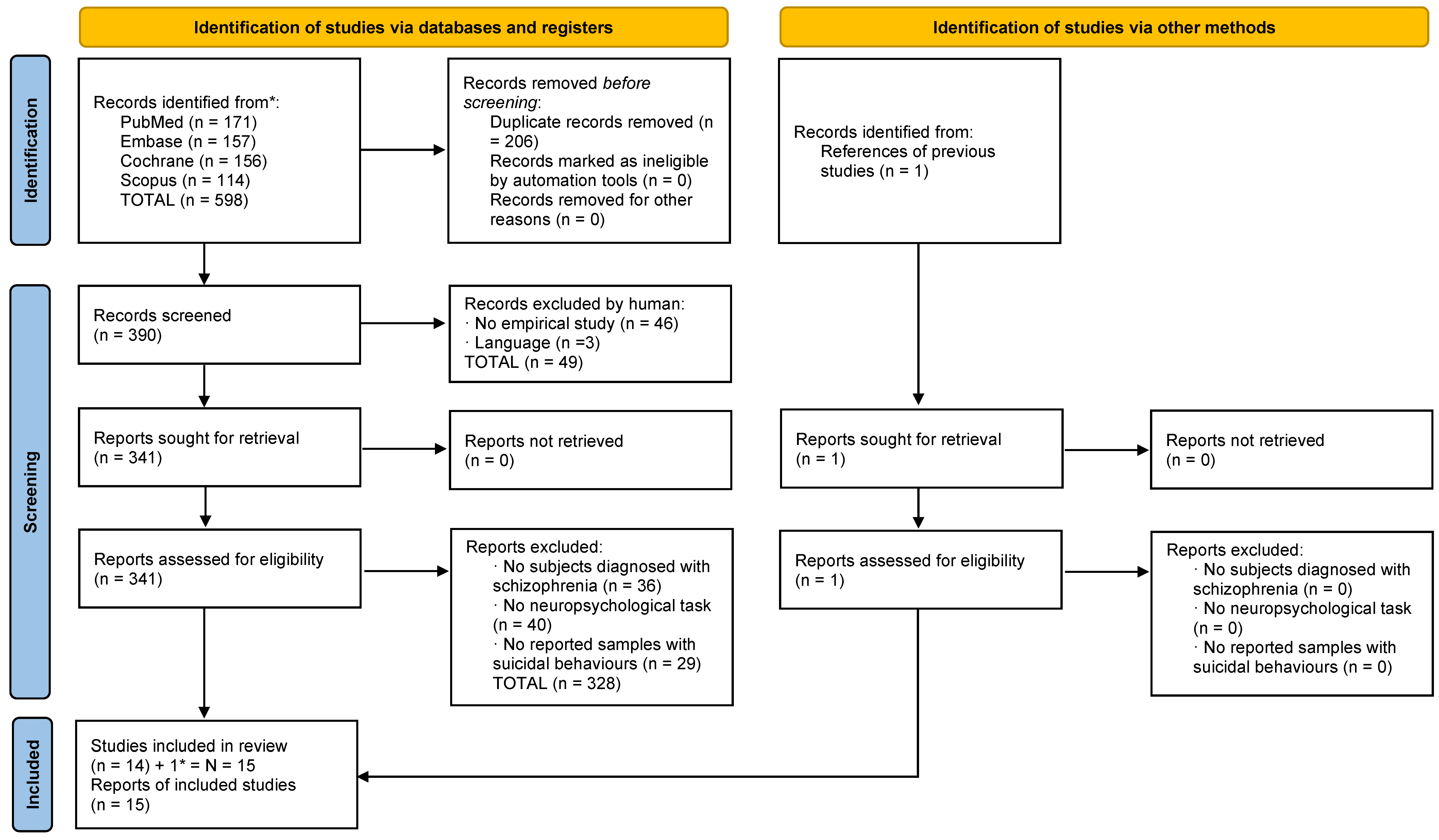
3.1. Selection of Studies: Suicide
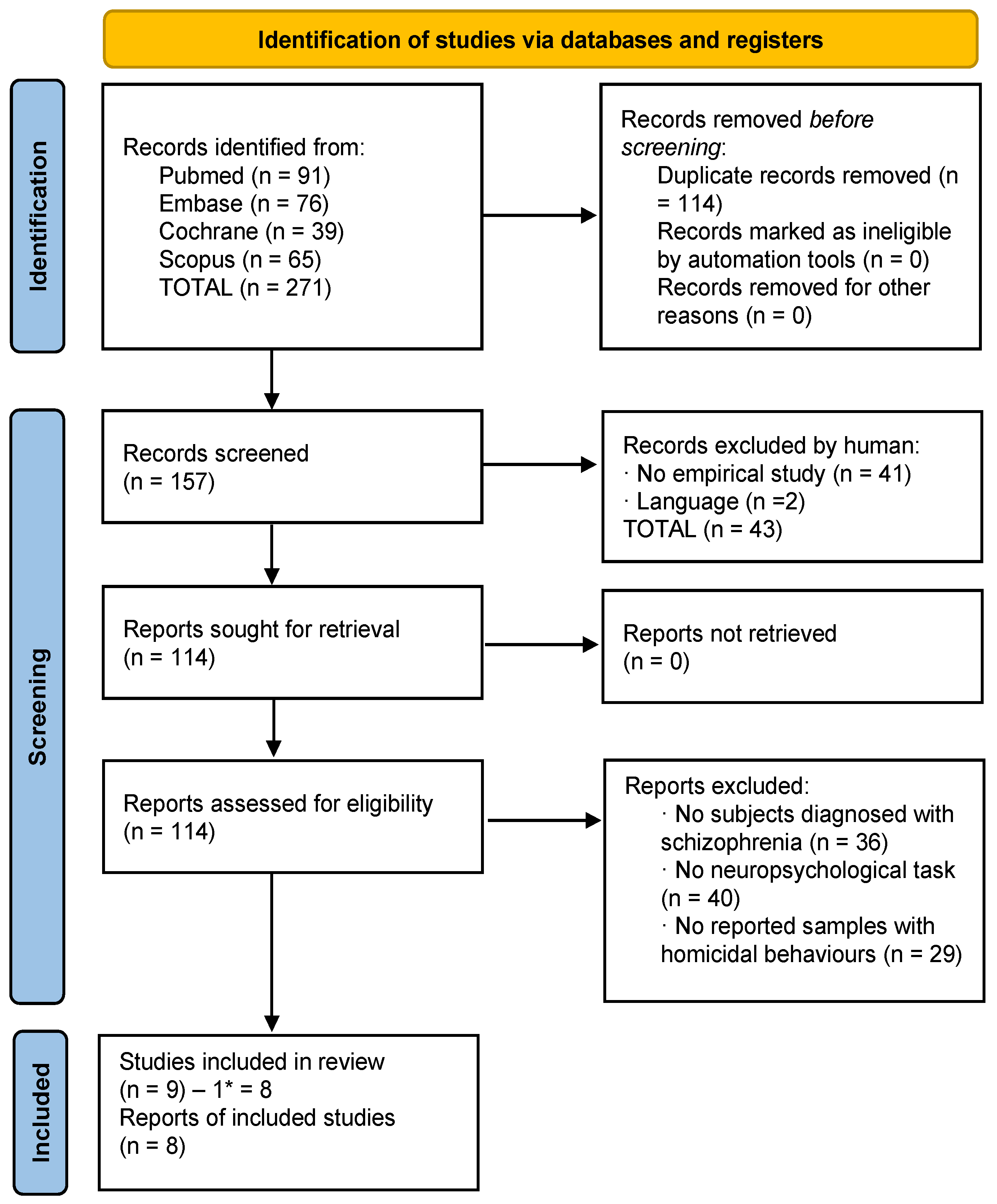
3.2. Selection of Studies: Homicide
3.3. Descriptive Data and Types of Studies
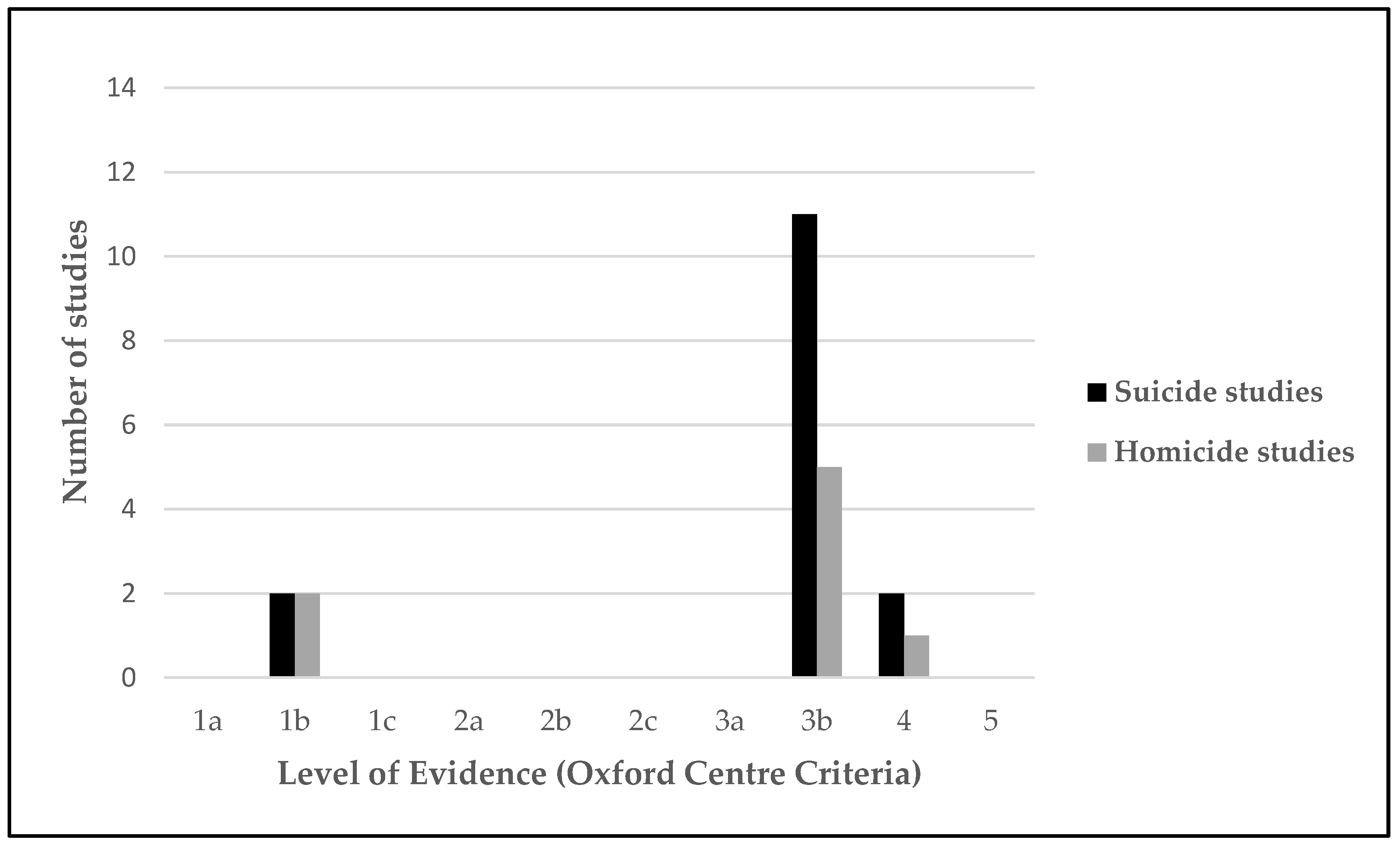
3.4. Risk of Bias in Studies
3.5. Certainty of Evidence
3.6. Neurocognitive Functioning in Relation to Homicidal or Suicidal Behavior in Schizophrenia
3.6.1. Neurocognitive Functioning and Suicidal Behaviors in Patients with Schizophrenia
3.6.2. Neurocognitive Functioning and Homicidal Behaviors in Patients with Schizophrenia
3.6.3. Shared Neuropsychological Impairments in Suicidal and Homicidal Patients
| Ref. | Country | OCEBM * | Year | Objective | Average Age | Sample No. (N) | Type of Study |
|---|---|---|---|---|---|---|---|
| [48] | China | Moderate | 2021 | To investigate the lifetime suicide attempt rate, clinical characteristics, and cognitive function of Chinese patients with chronic schizophrenia who had attempted suicide. | 46.4 | N = 908 (742M/166F) | Cross-sectional study |
| [53] | USA | Moderate | 2021 | To examine the relationships between positive and negative symptoms, symptoms of depression, clinical insight, cognitive functioning, and suicidal ideation among a first-episode sample of participants with psychosis. | 23.6 | N = 404 (293M/111F) | Cross-sectional study |
| [46] | Spain | Moderate | 2020 | To examine the relationship between neurocognitive functioning and the history of suicidality in violent offenders with schizophrenia. | 44.3 | N= 61 (M) | Cross-sectional study |
| [49] | China | Moderate | 2020 | To examine whether there is an effect of DβH 5′-insertion/deletion (Ins/Del) polymorphism on cognitive performance in suicide attempters with chronic schizophrenia. | 47.5 | N = 731 (615M/116F) | Cross-sectional study |
| [50] | China | Moderate | 2019 | To investigate the prevalence of suicidal ideation in patients with schizophrenia, and to identify which clinical symptoms and biochemical parameters were most strongly associated with suicidal ideation. | 36.2 | N = 174 (82M/92F) | Cross-sectional study |
| [51] | China | Low | 2018 | To examine the prevalence of suicide attempts and the association of this prevalence with demographic and clinical variables and cognitive function in Chinese first-episode, drug-naive (FEDN) schizophrenia patients. | 27.5 | N = 737 (352M/385F) | Cross-sectional, case control design |
| [45] | Spain | Very high | 2018 | To explore predictors of suicidal behavior, adjusting the analyses for a set of sociodemographic, clinical, and neurocognitive variables. Additionally, to examine potential long-term differences in clinical measures and neuro- cognitive functioning between patients who undertook suicidal acts and those who did not over the follow-up period. | 29.9 | N = 517 (297M/220F) | Longitudinal study |
| [54] | USA | Moderate | 2018 | To assess for cognitive ability, cognitive insight, and a history of suicidal ideation and behavior. | 50.6 | N = 162 (86M/76F) | Cross-sectional study |
| [43] | Spain | Moderate | 2017 | To explore the differences in executive functioning between suicide attempters and non-attempters in dual schizophrenia patients, and the possible premorbid and clinical-related factors. | 36.1 | N = 50 (M) | Cross-sectional study |
| [62] | India | Moderate | 2016 | To investigate the triangular relationship between suicide intent, insight, and cognitive competence in schizophrenia. | 33.5 | N = 175 (107M/68F) | Cross-sectional study |
| [44] | Spain | Very high | 2015 | To examine the premorbid, demographic, clinical, insight, and neurocognitive characteristics that are potentially related to suicide risk before the first presentation to psychiatric services and over the follow-up period. | 28.9 | N = 397 (226M/171F) | Longitudinal study |
| [52] | China | Moderate | 2014 | To test the hypothesis that higher cognitive function is associated with an increase in suicide attempts in a population of Han Chinese patients suffering from schizophrenia. | 51.6 | N = 316 (236M/80F) | Cross-sectional pilot study |
| [47] | China | Moderate | 2014 | To examine the prevalence of suicidal ideation and its relationship with clinical, neurocognitive, and psychological factors in first-episode psychosis patients. | 20.5 | N = 89 (43M/46F) | Cross-sectional study |
| [56] | UK | Moderate | 2013 | To investigate the demographic, clinical, and neuropsychological aspects of self-harm in schizophrenia, and to identify which of these aspects are independently predictive of and therefore the most relevant to clinical intervention. | 39.7 | N = 87 (78M/9F) | Cross-sectional study (prospective) |
| [13] | Ireland | Low | 2012 | To investigate whether the relationship between suicidality and neurocognition varied according to differences in suicidal ideation and behavior. | - | N = 310 | Cross-sectional study |
| Ref. | Country | OCEBM * | Year | Objective | Average Age | Sample No. (N) | Type of Study |
|---|---|---|---|---|---|---|---|
| [60] | Japan | Moderate | 2022 | To provide a resource for risk assessment and intervention studies by conducting multifaceted well-established assessments. | 35.3 | N = 1620 (834M/786F) | Cross-sectional study |
| [58] | Italy | Moderate | 2021 | To analyze the differential predictive potential of neurocognition and social cognition to identify patients with schizophrenia spectrum disorders with and without a history of severe violence. | Age 18–29 = 102; Age 30–41 = 153; Age 42–53 = 85; Age 54–65 = 58 | N = 398 (336M/62F) | Cross-sectional study |
| [55] | UK | Very high | 2020 | To investigate the association between neuropsychological test performance and a sensitive marker of violent behavior. | 26.8 | N = 891 (688M/203F) | Longitudinal study |
| [63] | Norway | Moderate | 2018 | To investigate global and specific cognition among homicide offenders with schizophrenia (HOS). | 36.3 | N = 205 (126M/79F) | Cross-sectional study |
| [57] | Italy | Moderate | 2017 | To investigate the relationship between clinical and neuropsychological factors and violence risk in patients with schizophrenia, taking into account current psychopathology and lifetime alcohol use. | 47.9 | N = 87 (78M/9F) | Cross-sectional study (prospective) |
| [64] | Turkey | Low | 2016 | To investigate factors associated with violent behavior in schizophrenia and to clarify the relationship between violent behavior, insight, and cognitive functions. | 42.2 | N = 68 (40M/28F) | Cross-sectional study |
| [61] | Japan | Moderate | 2015 | To examine the backgrounds and neurocognitive functions of violent and nonviolent patients with schizophrenia to identify factors associated with serious violence. | 42.2 | N = 54 (M) | Cross-sectional study |
| [59] | Ireland | Very high | 2015 | To examine whether neurocognition and social cognition predicts inpatient violence amongst patients with schizophrenia and schizoaffective disorder over a 12-month period. | 40 | N = 89 (84M/5F) | Longitudinal study |
| Ref. | Year | Sample Groups | Diagnoses | Cognitive Domains/Task | Main Results |
|---|---|---|---|---|---|
| [48] | 2021 | 908 individuals. Suicide: n = 97; non-suicide: n = 811 | Schizophrenia (DSM-IV). | RBANS (repeatable battery for the assessment of neuropsychological status): immediate memory, visuospatial skills, language, attention, and delayed memory. | There were no significant statistical differences between the suicidal and non-suicidal groups. |
| [53] | 2021 | 404 individuals. Suicide attempters: n = 106; non-suicide attempters: n = 298 | Schizophrenia/schizoaffective disorder/schizophreniform disorder/brief psychotic disorder/delusional disorder or psychotic disorder. (DSM-IV). | BACS (brief assessment of cognition in schizophrenia): verbal memory, working memory, motor speed, verbal fluency, attention and speed of information processing, and executive function. | Clinical insight (p = 0.031; OR = 0.73) and working memory (p = 0.041; OR = 0.04) were associated with increased odds of suicide ideation after baseline. |
| [46] | 2020 | Suicide attempters: n = 26; suicide non-attempters: n = 35 | Schizophrenia, schizoaffective disorder, or delusional disorder (DSM-IV-TR). | Premorbid IQ; national adult reading test (NART); current IQ (namely, verbal (vocabulary KBIT); nonverbal (matrix KBIT); attentional control (d2); episodic, verbal, and working memory (subsets of the Wechsler Memory Scale, third edition (WMS-III); subscale of letters and numbers (WAIS-III)); executive functioning (the Wisconsin Card-Sorting task (WCST); the computerized Tower of London test; the Zoo Map subset (BADS); the trail making test; the Stroop color–word task); planning abilities (the computerized Tower of London test; the Zoo Map subset (BADS); the Stroop color–word task); verbal fluency (controlled oral word fluency task (FAS)). | There were no significant statistical differences between the two groups in neurocognitive functioning. However, after controlling for the effects of demographic and clinical variables, suicide attempters performed better than suicide non-attempters in two planning-related tasks (in both tasks: p < 0.001): the Zoo Map Part 1 (p = 0.000; Cohen’s d = 1.25), Part 2 (p = 0.006; Cohen’s d = 0.73), the Tower of London extra moves (p = 0.000; Cohen’s d = 2.73), and the Tower of London time: seconds (p = 0.002). |
| [49] | 2020 | 731 patients. Attempters: n = 114; non-attempters: n = 617 | Schizophrenia (DSM-IV). | RBANS (repeatable battery for the assessment of neuropsychological status): immediate memory, list learning, story memory, attention, digit span, coding, language, picture fluency, visuospatial, figure copy, line orientation, delayed memory, list recall, story recall, figure recall, and list recognition | There were no significant statistical differences between attempters and non-attempters. |
| [50] | 2019 | Suicidal ideation: n = 26; no suicidal ideation: n = 148 | Schizophrenia disorder (DSM-IV). | Total score of RBANS (repeatable battery for the assessment of neuropsychological status): immediate memory (list learning and story memory tasks); visuospatial/constructional (figure copy and line orientation tasks); language (picture naming and semantic fluency tasks); attention (digit span and coding tasks); delayed memory (list recall; story recall; figure recall; list recognition tasks). | No significant statistical differences were found between those with suicidal ideation and those with no suicidal ideation in terms of performance on the RBANS test (total score and cognitive domains). |
| [51] | 2018 | Schizophrenia: n = 123; suicide attempters: n = 28; suicide non-attempters: n = 95; healthy controls: n = 151 | Schizophrenia (DSM-IV—SCID). | Total score of RBANS (repeatable battery for the assessment of neuropsychological status): immediate memory (list learning and story memory tasks); attention (digits span and coding tasks); language (picture naming and semantic fluency tasks); visuospatial/constructional (figure copy and line orientation tasks); delayed memory (list recall, story recall, figure recall, and list recognition tasks). | Both groups with schizophrenia showed significantly lower cognitive scores on RBANS total, immediate memory, attention, delayed memory (all p < 0.001) and language (p = 0.002), than healthy controls. However, when suicide attempters were compared with non-attempters within the schizophrenia group, attempters performed better only on the attention domain (p = 0.025; ηP2 = 0.49). |
| [45] | 2018 | Non-suicidal behavior: n = 466; suicidal behavior: n = 51 | Schizophrenia, brief psychotic disorder, not otherwise specified (NOS) psychosis, schizophreniform disorder, schizoaffective disorder, delusional disorder (structured clinical interview for DSM-IV—SCID). | Global cognitive functioning (GCF); verbal memory (Rey auditory verbal learning test (RAVLT)); visual memory (Rey complex figure (RCF); delayed reproduction); executive functioning (trail making test (TMT)); working memory (WAIS-III backward digits subset); processing speed (WAIS-III digit symbol subtest); motor dexterity (grooved pegboard handedness (GP)); attention (continuous performance test (CPT)); premorbid IQ (WAIS-III vocabulary subtest). | Patients with suicidal behaviors presented worse scores in visual memory (p < 0.01; F(1.186) = 8.16) and global cognitive functioning (p < 0.01; F(1.134) = 7.10). In addition, global cognitive functioning (GCF) was the most important predictor of lifetime suicidality. |
| [54] | 2018 | No actual attempt: n = 95; attempt: n = 66 | Schizophrenia/schizoaffective disorder (DSM-IV) | Total score of consensus cognitive battery (MCCB); verbal learning (Hopkins Verbal Learning Test); speed of processing (Trail Making Test A; symbol coding (Brief Assessment of Cognition in Schizophrenia); animal naming (category fluency)); working memory (letter–number span (WMS)); reasoning and problem solving (mazes (neuropsychological assessment battery)). | Patients with active suicidal ideation presented a greater MBCC total score (p = 0.025; Cohen’s d = −0.363), verbal learning (p = 0.003; Cohen’s d = −0.482), speed of processing (p = 0.038; Cohen’s d = 0.334), and working memory scores (p = 0.013; Cohen’s d = −0.397) than patients with non-active suicidal ideation. Additionally, patients with suicidal attempts performed better in verbal learning (p = 0.002; Cohen’s d = −0.49) than those without suicide attempts. |
| [43] | 2017 | Non-attempters: n = 26; Suicide attempters: n = 24 | Dual schizophrenia/schizoaffective disorder (DSM-IV-TR). | Total score of premorbid IQ; vocabulary (WAIS-III); block design (WAIS-III); total score of executive functioning; working memory (backward digits (WAIS-III)); cognitive flexibility (trail making test (TMT) B); planning abilities (Tower of Hanoi); abstract reasoning/problem solving (WCST) and decision-making (Iowa gambling task). | Suicide attempters presented lower composite summary scores in executive function (p < 0.05; ηP2 = 0.10), problem solving skills (p < 0.01; ηP2 = 0.14), and decision-making (p < 0.01; ηP2 = 0.19) compared to non-attempters. However, after controlling for the effects of alcohol dependence, only decision-making showed significant differences. |
| [62] | 2016 | Never attempted: n = 136 ever attempted: n = 39 | Schizophrenia/schizoaffective disorder (DSM-IV) | Executive function (Trail Making Test (TMT) A and B) | The attempters scored significantly better in executive function on both TMT A (p = 0.026) and TMT B (p = 0.012) than those who had never attempted. |
| [44] | 2015 | Suicide attempters: n = 60; suicide non-attempters: n = 337 | Schizophrenia, schizophreniform disorder, schizoaffective disorder, brief psychotic disorder, psychosis NOS, and delusional disorder. (DSM-IV). | Premorbid IQ (WAIS-III vocabulary); information processing speed (WAIS-III digit symbol); motor dexterity (grooved pegboard dominant hand); working memory (WAIS-III digits backward digits scale); verbal memory (RAVLT list delayed recall); visual memory (Rey figure delayed recall); attention (CPT); executive function (TMT B–A). | Processing speed was significantly (p = 0.046) more impaired in attempters. No significant differences were found in the other domains. |
| [52] | 2014 | Attempted suicide: n = 25 Non-attempters: n = 291 | Schizophrenia disorder (structured clinical interview for DSM-IV—SCID). | Total score of RBANS (repeatable battery for the assessment of neuropsychological status): immediate memory (list learning and story memory tasks); visuospatial/constructional (figure copy and line orientation tasks); language (picture naming and semantic fluency tasks); attention (digit span and coding tasks); delayed memory (list recall; story recall; figure recall; list recognition tasks). | There were no significant statistical differences between the suicide attempters and non-attempters. |
| [47] | 2014 | Suicidal ideation: n = 37 No suicidal ideation: n = 52 | Schizophrenia, schizophreniform disorder, delusional disorder, brief psychotic disorder, or psychosis not otherwise specified (DSM-IV). | Cognitive inflexibility (modified Wisconsin Card-Sorting test (MWCS)) and dyscontrol of executive inhibition (Hayline sentence completion test (HSCT) Part B). | There were no significant statistical differences between the suicidal and non-suicidal groups. |
| [56] | 2013 | 87 patients. Self-harm: n = 59; no self-harm: n = 28 | Schizophrenia (DSM-IV). | Nart (premorbid iq), trail making test (frontal executive function), computerized auditory continuous performance test (sustained attention and vigilance), computerized visual go/no-go reaction time task (cognitive–motor impulsivity) (240 go stimuli and 60 no-go stimuli). | Those with past self-harm, compared to those without, were significantly more likely to report impulsivity (p < 0.01; F = 7.97) and had higher premorbid IQs (p < 0.01; F = 7.34). |
| [13] | 2012 | Non-attempters with no ideation: n = 172; history of ideation without having made a suicide attempt: n = 63; a single attempt: n = 48; multiple attempts: n = 27 | Schizophrenia/schizoaffective disorder (Structured Clinical Interview for DSM-IV- SCID). | Cognition (full scale IQ (WAIS-III); verbal IQ (WAIS-III); performance IQ (WAIS-III)); memory (logical memory 1 (WMS-III); logical memory 2 (WMS-III); PAL stages (CANTAB); PAL total errors (CANTAB)); working memory (letter–number (WMS-III); SWM errors (CANTAB)); attention (CANTAB IDED; CANTAB IDED-ED). | The “single attempters” group outperformed those in the “No Ideation, No Attempts” group in terms of current full-scale IQ (p = 0.02; H = 8.33) and verbal IQ (p < 0.05; H = 7.75). The “ideation only” group out-performed the “no ideation, no attempts” group in episodic memory (p < 0.03; H1.381). After regrouping, the “ideation only + single attempters” outperformed the “no ideation, no attempters” group in full-scale IQ (p = 0.01; H = 6.18), working memory (p = 0.04; H1.02), and episodic memory (p = 0.04; H = 0.92). |
| Ref. | Year | Sample Groups | Diagnoses | Cognitive Domains/Task | Main Results |
|---|---|---|---|---|---|
| [60] | 2022 | 1620 individuals. Healthy subjects (HS): n = 1265; 355 patients with schizophrenia: history of violence (V-SZ): n = 112; without a history of violence (NV-SZ): n = 243 | Schizophrenia. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I). | Wechsler Adult Intelligence Scale (WAIS)-III, Wechsler Memory Scale–Revised (WMS-R), Wisconsin Card-Sorting Test (WCST), Verbal Fluency Test (VFT), Rey Auditory Verbal Learning Test (AVLT), and Continuous Performance Test–Identical Pairs version (CPT-IP). | There were significant differences between “Violent” and “Non-violent” in visual memory function (p = 1.9 × 10−5, Cohen’s d = 0.34), being lower in the V-SZ group. |
| [58] | 2021 | 398 patients. Forensic patients: n = 221; non-forensic patients: N = 177 | Schizophrenia/schizoaffective disorder. (DSM-V). | The Brief Assessment of Cognition in Schizophrenia (BACS). Verbal (list learning) and Working memory (Digit Sequencing Task), Motor speed (Token Motor Task), Verbal fluency (semantic and letter fluency), Attention and speed information processing (Symbol Coding Task) and Executive functions (Tower of London). | There were significant differences between “Forensic Group” and “Control group” in processing speed (BACS Symbol Coding Task: p < 0.01; ηP2 = 0.49) with larger impairments in the Forensic group. |
| [55] | 2020 | 891 patients. Violent: n = 183; non-violent: n = 708 | Schizophrenia/schizoaffective disorder/psychotic disorder/others. (DSM-IV-TR). | Continuous Performance Test-HQ (CPT-HQ) (inhibition); Response Shifting Task (RST) (cognitive flexibility); Wechsler Adult Intelligence Scale, third edition (WAIS-III); Block Design subtest (fluid intelligence); (IV) Neuropsychological Assessment Battery (NAB): Mazes Test (planning); (v) Degraded Facial Affect Recognition Task (DFAR) (affective ToM); and (vi) Hinting Task (cognitive ToM). | Violent patients performed significantly worse than non-violent patients in fluid intelligence (p = 0.02; ηP2 = 0.006), planning (p = 0.02; ηP2 = 0.01), and theory of mind (cognitive part) (p < 0.01; ηP2 = 0.01). |
| [63] | 2018 | Homicide offenders (HOS): n = 26; no history of violence (non-HOS): n = 28 | Schizophrenia/schizoaffective disorder (ICD-10). | Global cognition (Vocabulary (WASI); Matrix Reasoning (WASI); MCCB comp); MCCB total score; Speed of processing (Trail Making Test (TMT)); Attention/vigilance (Continuous Performance Test–Identical Pairs); Working memory (Spatial Span and Letter–Number Span (WMS-III)); Verbal learning (Hopkins Verbal Learning Test–Revised (HVLT-R)); Visual learning (Brief Visuospatial Memory Test–Revised (BVMT-R)); Reasoning/problem solving Neuropsychological Assessment Battery (NAB): Mazes; Social cognition (Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT): Managing Emotions); Color naming, Word Reading, Inhibition, inhibition/switching (Color–Word Interference Test (CWIT)). | The effects sizes for IQ were medium (Cohen’s d = 0.52), but the difference was no longer statistically significant (p = 0.13). For verbal learning, the group difference between HOS and non-HOS remained statistically significant (p = 0.03) and the effect size was large (Cohen’s d = 0.82). |
| [57] | 2017 | 87 patients. Violent (vSZ): n = 50; non-violent (nvSZ): n = 37 | Schizophrenia (DSM-IV). | Brief Assessment of Cognition in Schizophrenia (BACS): verbal memory, working memory, motor speed, verbal fluency, attention, and speed of information processing and planning. Wisconsin Card-Sorting test (WCST) (executive function: flexibility and inhibition). Iowa Gambling Test (IGT) (decision making). | The vSZ subjects had significantly higher motor speed scores (p = 0.007; ηP2 = 0.03), lower executive function: flexibility (p = 0.023; ηP2 = 0.03), and inhibition (p = 0.025; ηP2 = 0.03) compared to the nvSZ group. However, entering the BPRS negative score and a lifetime problematic use of alcohol into the ANCOVA model, no significant differences between groups were found (p = 0.130, p = 0.122, and p = 0.114, respectively). |
| [64] | 2016 | 68 individuals. Violent: n = 30; non-violent: n = 38 | Schizophrenia. (Structured Clinical interview for DSM-IV axis I disorders (SCID-I)). | The California Verbal Learning Test (CVLT), Trail-Making Test (TMT), Wisconsin Card-Sorting Test (WCST) and Stroop test. | The non-violent group performed significantly better than violent group in verbal memory (CVLT long-delayed response: p < 0.05). |
| [61] | 2015 | Violent: n =30; non-violent: n = 24 | Schizophrenia (DSM-IV-TR). | The Brief Assessment of Cognition in Schizophrenia (BACS): verbal memory (digit sequencing test); working memory (digit sequencing test); motor speed (token motor test); verbal fluency (symbol coding test); attention; executive functioning (BACS total score)). | The violent group performed significantly better than the control group on working memory (p = 0.047; Cohen’s d = 0.61) and executive function (p < 0.001; Cohen’s d = 1.00). |
| [59] | 2015 | 89 patients. Violent: n = 10; non-violent: n = 79 | Schizophrenia/schizoaffective disorder. Structured clinical interview for DSM-IV-TR. | The MATRICS Consensus Cognitive Battery (MCCB), which covers: processing speed, attention/vigilance, working memory, verbal learning, visual learning, and reasoning and problem solving; Test of Premorbid Functioning TOPF-UK. | The violent and non-violent groups differed only in the verbal learning domain (p = 0.007; ηP2 = 0.08; Cohen’s d = 0.92). |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Reference | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| [60] | Yes | Yes | Yes | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | No |
| [58] | Yes | Yes | Yes | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | Yes |
| [48] | Yes | Yes | No | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | Yes |
| [53] | Yes | Yes | Yes | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | No |
| [46] | Yes | Yes | No | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | No |
| [47] | Yes | Yes | Yes | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | Yes |
| [50] | Yes | Yes | No | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | Yes |
| [51] | Yes | Yes | No | Yes | Yes | - | Don’t know/comment | Yes | Yes | Yes |
| [63] | Yes | Yes | No | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | Yes |
| [54] | Yes | Yes | No | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | Yes |
| [43] | Yes | Yes | Yes | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | Yes |
| [57] | Yes | Yes | Yes | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | Yes |
| [62] | Yes | Yes | Yes | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | No |
| [64] | Yes | Yes | Yes | Yes | Don’t know/comment | Don’t know/comment | Don’t know/comment | Yes | Yes | No |
| [61] | Yes | Yes | No | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | Yes |
| [52] | Yes | Yes | Yes | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | Yes |
| [47] | Yes | Yes | No | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | Yes |
| [56] | Yes | Yes | Yes | Yes | Yes | Yes | Don’t know/comment | Yes | Yes | No |
| [13] | Yes | Yes | No | Yes | Don’t know/comment | Don’t know/comment | Don’t know/comment | Yes | Yes | No |
| Reference | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| [60] | Yes | Yes | No | Don’t know/ comment | Don’t know/ comment | Yes | Yes | Yes | No | Yes |
| [58] | Yes | Yes | Don’t know/ comment | Don’t know/ comment | Yes | No | Yes | Yes | No | Don’t know/ comment |
| [48] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes |
| [53] | Yes | Yes | Don’t know/ comment | Don’t know/ comment | Don’t know/ comment | Yes | Yes | Yes | No | Yes |
| [46] | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | No | Yes |
| [47] | Yes | Yes | Don’t know/ comment | Don’t know/ comment | Don’t know/ comment | Yes | Yes | No | No | Don’t know/ comment |
| [50] | Yes | Yes | Don’t know/ comment | Don’t know /comment | Don’t know/ comment | Yes | Yes | Yes | Yes | Yes |
| [51] | Yes | Yes | Don’t know/ comment | Don’t know/ comment | No | No | Yes | Yes | No | Yes |
| [63] | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes |
| [54] | Yes | Yes | No | No | Yes | Yes | Yes | Yes | No | Yes |
| [43] | Yes | Yes | Don’t know/ comment | Don’t know/ comment | Don’t know/ comment | Yes | Yes | Yes | No | Yes |
| [57] | Yes | Yes | Don’t know/ comment | Don’t know/ comment | Yes | Yes | Yes | Yes | Don’t know/ comment | Yes |
| [62] | Yes | Yes | Don’t know/ comment | Don’t know/ comment | Yes | No | Yes | Yes | No | Yes |
| [64] | No | Yes | Don’t know/ comment | Don’t know/ comment | Yes | Don’t know/ comment | Yes | Yes | No | Yes |
| [61] | Yes | Yes | Don’t know/ comment | Don’t know/ comment | Don’t know/ comment | Yes | Yes | No | Yes | Yes |
| [52] | Yes | Yes | Don’t know/ comment | Don’t know/ comment | Don’t know/ comment | Yes | Yes | Yes | No | Yes |
| [47] | Yes | Yes | Don’t know/ comment | Don’t know/ comment | Don’t know/ comment | Yes | Yes | Yes | No | Yes |
| [56] | Yes | Yes | Don’t know/ comment | Don’t know/ comment | Don’t know/ comment | Yes | Yes | Yes | No | Yes |
| [13] | Yes | Yes | Don’t know/ comment | Don’t know/ comment | Don’t know/ comment | Yes | Yes | Yes | Yes | Yes |
Appendix B
| Reference | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [45] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes |
| [44] | Yes | Yes | Yes | Unclear | No | Yes | Yes | Yes | Yes | No | Yes |
| [59] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Reference | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| [55] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
References
- Fazel, S.; Runeson, B. Suicide. N. Engl. J. Med. 2020, 382, 266–274. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240005105 (accessed on 24 September 2022).
- Matsubayashi, T.; Ueda, M. The effect of national suicide prevention programs on suicide rates in 21 OECD nations. Soc. Sci. Med. 2011, 73, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M. Global, regional, and national burden of suicide mortality 1990 to 2016: Systematic analysis for the Global Burden of Disease Study 2016. BMJ 2019, 364, l94. [Google Scholar] [CrossRef] [PubMed]
- UNODC. Estudio Mundial Sobre el Homicidio. 2019. Available online: https://www.unodc.org/documents/ropan/2021/HOMICIOS_EN_ESPANOL.pdf (accessed on 13 March 2023).
- Favril, L.; Yu, R.; Uyar, A.; Sharpe, M.; Fazel, S. Risk factors for suicide in adults: Systematic review and meta-analysis of psychological autopsy studies. É;vid. Based Ment. Health 2022, 25, 148–155. [Google Scholar] [CrossRef]
- Fazel, S.; Smith, E.N.; Chang, Z.; Geddes, J.R. Risk factors for interpersonal violence: An umbrella review of meta-analyses. Br. J. Psychiatry 2018, 213, 609–614. [Google Scholar] [CrossRef]
- Fazel, S.; Gulati, G.; Linsell, L.; Geddes, J.R.; Grann, M. Schizophrenia and violence: Systematic review and meta-analysis. PLoS Med. 2009, 6, e1000120. [Google Scholar] [CrossRef]
- Large, M.; Smith, G.; Nielssen, O. The relationship between the rate of homicide by those with schizophrenia and the overall homicide rate: A systematic review and meta-analysis. Schizophr. Res. 2009, 112, 123–129. [Google Scholar] [CrossRef]
- Iozzino, L.; Ferrari, C.; Large, M.; Nielssen, O.; de Girolamo, G. Prevalence and Risk Factors of Violence by Psychiatric Acute Inpatients: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0128536. [Google Scholar] [CrossRef]
- American Psychiatric Association—APA. Manual Diagnóstico y Estadístico de los Trastornos Mentales DSM-5, 5th ed.; Editorial Médica Panamericana: Madrid, Spain, 2014. [Google Scholar]
- Palmer, E.J.; Connelly, R. Depression, hopelessness and suicide ideation among vulnerable prisoners. Crim. Behav. Ment. Health 2005, 15, 164–170. [Google Scholar] [CrossRef]
- Delaney, C.; McGrane, J.; Cummings, E.; Morris, D.; Tropea, D.; Gill, M.; Corvin, A.; Donohoe, G. Preserved cognitive function is associated with suicidal ideation and single suicide attempts in schizophrenia. Schizophr. Res. 2012, 140, 232–236. [Google Scholar] [CrossRef]
- Stratton, J.; Brook, M.; Hanlon, R.E. Murder and psychosis: Neuropsychological profiles of homicide offenders with schiz-ophrenia. Crim. Behav. Ment. Health 2017, 27, 146–161. [Google Scholar] [CrossRef] [PubMed]
- Hor, K.; Taylor, M. Suicide and schizophrenia: A systematic review of rates and risk factors. J. Psychopharmacol. 2010, 24, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Caqueo-Urízar, A.; Fond, G.; Urzúa, A.; Boyer, L.; Williams, D.R. Violent behavior and aggression in schizophrenia: Prevalence and risk factors. A multicentric study from three Latin-America countries. Schizophr. Res. 2016, 178, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Whiting, D.; Lichtenstein, P.; Fazel, S. Violence and mental disorders: A structured review of associations by individual di-agnoses, risk factors, and risk assessment. Lancet Psychiatry 2021, 8, 150–161. [Google Scholar] [CrossRef]
- Belli, H.; Ozcetin, A.; Ertem, U.; Tuyluoglu, E.; Namli, M.; Bayik, Y.; Simsek, D. Perpetrators of homicide with schizophrenia: Sociodemographic characteristics and clinical factors in the eastern region of Turkey. Compr. Psychiatry 2010, 51, 135–141. [Google Scholar] [CrossRef]
- Buchanan, A.; Sint, K.; Swanson, J.; Rosenheck, R. Correlates of Future Violence in People Being Treated for Schizophre-nia. Am. J. Psychiatry 2019, 176, 694–701. [Google Scholar] [CrossRef]
- Rund, B.R. A review of factors associated with severe violence in schizophrenia. Nord. J. Psychiatry 2018, 72, 561–571. [Google Scholar] [CrossRef]
- Schug, R.A.; Raine, A. Comparative meta-analyses of neuropsychological functioning in antisocial schizophrenic persons. Clin. Psychol. Rev. 2009, 29, 230–242. [Google Scholar] [CrossRef]
- Stratton, J.; Cobia, D.J.; Reilly, J.; Brook, M.; Hanlon, R.E. Differences in Neuropsychological Functioning between Homicidal and Nonviolent Schizophrenia Samples. J. Forensic Sci. 2018, 63, 1435–1443. [Google Scholar] [CrossRef]
- Richard-Devantoy, S.; Orsat, M.; Dumais, A.; Turecki, G.; Jollant, F. Neurocognitive vulnerability: Suicidal and homicidal behaviors in patients with schizophrenia. Can. J. Psychiatry 2014, 59, 18–25. [Google Scholar] [CrossRef]
- Kim, C.-H.; Jayathilake, K.; Meltzer, H.Y. Hopelessness, neurocognitive function, and insight in schizophrenia: Relationship to suicidal behavior. Schizophr. Res. 2003, 60, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Potkin, S.G.; Anand, R.; Alphs, L.; Fleming, K. Neurocognitive performance does not correlate with suicidality in schizophrenic and schizoaffective patients at risk for suicide. Schizophr. Res. 2003, 59, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Barrett, E.A.; Sundet, K.; Simonsen, C.; Agartz, I.; Lorentzen, S.; Mehlum, L.; Mork, E.; Andreassen, O.A.; Melle, I. Neurocognitive functioning and suicidality in schizophrenia spectrum disorders. Compr. Psychiatry 2011, 52, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Schoretsanitis, G.; Stegmayer, K.; Razavi, N.; Federspiel, A.; Müller, T.J.; Horn, H.; Wiest, R.; Strik, W.; Walther, S. Inferior frontal gyrus gray matter volume is associated with aggressive behavior in schizophrenia spectrum disorders. Psychiatry Res. Neuroimaging 2019, 290, 14–21. [Google Scholar] [CrossRef]
- Shinko, Y.; Otsuka, I.; Okazaki, S.; Horai, T.; Boku, S.; Takahashi, M.; Ueno, Y.; Sora, I.; Hishimoto, A. Chemokine alterations in the postmortem brains of suicide completers. J. Psychiatr. Res. 2020, 120, 29–33. [Google Scholar] [CrossRef]
- Jung, S.J.; Vlasov, K.; D’ambra, A.F.; Parigi, A.; Baya, M.; Frez, E.P.; Villalobos, J.; Fernandez-Frentzel, M.; Anguiano, M.; Ideguchi, Y.; et al. Novel Cerebello-Amygdala Connections Provide Missing Link between Cerebellum and Limbic System. Front. Syst. Neurosci. 2022, 16, 879634. [Google Scholar] [CrossRef]
- Cristofori, I.; Cohen-Zimerman, S.; Grafman, J. Executive functions. Handb. Clin. Neurol. 2019, 163, 197–219. [Google Scholar] [PubMed]
- Rudebeck, P.H.; Rich, E.L. Orbitofrontal cortex. Curr. Biol. 2018, 28, 1083–1088. [Google Scholar] [CrossRef]
- Redlich, R.; Opel, N.; Bürger, C.; Dohm, K.; Grotegerd, D.; Förster, K.; Zaremba, D.; Meinert, S.; Repple, J.; Enneking, V.; et al. The Limbic System in Youth Depression: Brain Structural and Functional Alterations in Adolescent In-patients with Severe Depression. Neuropsychopharmacology 2017, 43, 546–554. [Google Scholar] [CrossRef]
- Saiz Ruiz, J.; Vega Sánchez, D.C.; Sánchez Páez, P. Bases neurobiológicas de la Esquizofrenia. Clín. Salud 2010, 21, 235–254. [Google Scholar] [CrossRef]
- Hanlon, R.E.; Coda, J.J.; Cobia, D.; Rubin, L.H. Psychotic domestic murder: Neuropsychological differences between homicidal and nonhomicidal schizophrenic men. J. Fam. Violence 2012, 27, 105–113. [Google Scholar] [CrossRef]
- Fjellvang, M.; Grøning, L.; Haukvik, U.K. Imaging Violence in Schizophrenia: A Systematic Review and Critical Discussion of the MRI Literature. Front. Psychiatry 2018, 9, 333. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.J. Neurobiology of suicidal behaviour. Nat. Rev. Neurosci. 2003, 4, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Harmer, B.; Lee, S.; Duong, T.; Saadabadi, A. Suicidal Ideation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Smit, P.R.; de Jong, R.R.; Bijleveld, C.C.J.H. Homicide Data in Europe: Definitions, Sources, and Statistics. In Handbook of Eu-ropean Homicide Research: Patterns, Explanations, and Country Studies; Springer: New York, NY, USA, 2011; pp. 5–23. [Google Scholar] [CrossRef]
- Jacquin, K.M. Violence. Updated 19 February 2022. Encyclopedia Britannica. Available online: https://www.britannica.com/topic/violence (accessed on 15 August 2022).
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef] [PubMed]
- Oxford Centre for Evidence-Based Medicine. Levels of Evidence. May 2001. Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence (accessed on 5 May 2023).
- Adan, A.; Capella, M.D.; Prat, G.; Forero, D.A.; López-Vera, S.; Navarro, J.F. Executive Functioning in Men with Schizo-phrenia and Substance Use Disorders. Influence of Lifetime Suicide Attempts. PLoS ONE 2017, 12, e0169943. [Google Scholar] [CrossRef] [PubMed]
- Ayesa-Arriola, R.; Alcaraz, E.G.; Hernández, B.V.; Pérez-Iglesias, R.; Moríñigo, J.D.L.; Duta, R.; David, A.S.; Tabares-Seisdedos, R.; Crespo-Facorro, B. Suicidal behaviour in first-episode non-affective psychosis: Specific risk periods and stage-related factors. Eur. Neuropsychopharmacol. 2015, 25, 2278–2288. [Google Scholar] [CrossRef]
- Canal-Rivero, M.; López-Moríñigo, J.D.; Setién-Suero, E.; Ruiz-Veguilla, M.; Ayuso-Mateos, J.L.; Ayesa-Arriola, R.; Crespo-Facorro, B. Predicting suicidal behaviour after first episode of non-affective psychosis: The role of neurocognitive functioning. Eur. Psychiatry 2018, 53, 52–57. [Google Scholar] [CrossRef]
- Sánchez-Sansegundo, M.; Portilla-Tamarit, I.; Rubio-Aparicio, M.; Albaladejo-Blazquez, N.; Ruiz-Robledillo, N.; Ferrer-Cascales, R.; Zaragoza-Martí, A. Neurocognitive Functioning and Suicidal Behavior in Violent Offenders with Schizophrenia Spectrum Disorders. Diagnostics 2020, 10, 10–91. [Google Scholar] [CrossRef]
- Chang, W.C.; Chen, E.S.M.; Hui, C.L.M.; Chan, S.K.W.; Lee, E.H.M.; Chen, E.Y.H. The relationships of suicidal ideation with symptoms, neurocognitive function, and psychological factors in patients with first-episode psychosis. Schizophr. Res. 2014, 157, 12–18. [Google Scholar] [CrossRef]
- Dai, Q.; Wang, D.; Wang, J.; Xu, H.; Andriescue, E.C.; Wu, H.E.; Xiu, M.; Chen, D.; Zhang, X. Suicide attempts in Chinese Han patients with schizophrenia: Cognitive, demographic, and clinical variables. Rev. Bras. Psiquiatr. 2021, 43, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Hu, W.M.; Zhu, Z.H.; Gao, S.T.; Han, M.; Fan, Y.; Tian, Q.; Yin, X.Y.; Yuan, Y.; Jiang, C.X.; et al. Association between dopamine beta-hydroxylase polymorphism and attention function in suicide attempters with chronic schizophrenia. Hum. Psychopharmacol. Clin. Exp. 2020, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Chen, Y.; Wang, Y.; Zhang, C. Identification of risk factors for suicidal ideation in patients with schizophrenia. Psychiatry Res. 2019, 271, 195–199. [Google Scholar]
- Zhang, X.Y.; Du, X.; Yin, G.; Zhang, Y.; Chen, D.; Xiu, M.; Wang, C.; Zhang, R.; Cassidy, R.M.; Ning, Y.; et al. Prevalence and Clinical Correlates of and Cognitive Function at the Time of Suicide Attempts in First-Episode and Drug-Naive Patients with Schizophrenia. J. Clin. Psychiatry 2018, 79, 17m11797. [Google Scholar] [CrossRef]
- Zoghbi, A.W.; Al Jurdi, R.K.; Deshmukh, P.R.; Chen, D.C.; Xiu, M.H.; Tan, Y.L.; Yang, F.D.; Zhang, X.Y. Cognitive function and suicide risk in Han Chinese inpatients with schizophrenia. Psychiatry Res. 2014, 220, 188–192. [Google Scholar] [CrossRef]
- Bornheimer, L.A.; Wojtalik, J.A.; Li, J.; Cobia, D.; Smith, M.J. Suicidal ideation in first-episode psychosis: Considerations for depression, positive symptoms, clinical insight, and cognition. Schizophr. Res. 2021, 228, 298–304. [Google Scholar] [CrossRef]
- Villa, J.; Choi, J.; Kangas, J.L.; Kaufmann, C.N.; Harvey, P.D.; Depp, C.A. Associations of suicidality with cognitive ability and cognitive insight in outpatients with Schizophrenia. Schizophr. Res. 2018, 192, 340–344. [Google Scholar] [CrossRef]
- Lamsma, J.; Cahn, W.; Fazel, S.; Genetic Risk and Outcome of Psychosis (GROUP) investigators. Cognition and violent behavior in psychotic disorders: A nationwide case-control study. Schizophr. Res. Cogn. 2019, 19, 100–166. [Google Scholar] [CrossRef] [PubMed]
- Pluck, G.; Lekka, N.; Sarkar, S.; Lee, K.; Bath, P.; Sharif, O.; Woodruff, P. Clinical and neuropsychological aspects of non-fatal self-harm in schizophrenia. Eur. Psychiatry 2013, 28, 344–348. [Google Scholar] [CrossRef]
- Bulgari, V.; Iozzino, L.; Ferrari, C.; Picchioni, M.; Candini, V.; De Francesco, A.; Maggi, P.; Segalini, B.; de Girolamo, G. Clinical and neuropsychological features of violence in schizophrenia: A prospective cohort study. Schizophr. Res. 2017, 181, 124–130. [Google Scholar] [CrossRef]
- Iozzino, L.; Harvey, P.D.; Canessa, N.; Gosek, P.; Heitzman, J.; Macis, A.; Picchioni, M.; Salize, H.J.; Wancata, J.; Koch, M.; et al. Neurocognition and social cognition in patients with schizophrenia spectrum disorders with and without a history of violence: Results of a multinational European study. Transl. Psychiatry 2021, 11, 620. [Google Scholar] [CrossRef]
- O’Reilly, K.; Donohoe, G.; Coyle, C.; O’Sullivan, D.; Rowe, A.; Losty, M.; McDonagh, T.; McGuinness, L.; Ennis, Y.; Watts, E.; et al. Prospective cohort study of the relationship between neuro-cognition, social cognition and violence in forensic patients with schizophrenia and schizoaffective disor-der. BMC Psychiatry 2015, 15, 155. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, H.; Matsumoto, J.; Miura, K.; Takeda, K.; Yamada, Y.; Fujimoto, M.; Yasuda, Y.; Yamamori, H.; Ikeda, M.; Hirabayashi, N.; et al. Neurocognitive features, personality traits, and social function in patients with schizophrenia with a history of violence. J. Psychiatr. Res. 2022, 147, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, H.; Kuroki, N.; Ikezawa, S.; Matsushita, M.; Ishikawa, M.; Nakagome, K.; Hirabayashi, N.; Ikeda, M. Neurocog-nitive features in male patients with schizophrenia exhibiting serious violence: A case control study. Ann. Gen. Psychiatry 2015, 14, 46. [Google Scholar] [CrossRef]
- Verma, D.; Srivastava, M.; Singh, S.K.; Bhatia, T.; Deshpande, S.N. Lifetime suicide intent, executive function and insight in schizophrenia and schizoaffective disorders. Schizophr. Res. 2016, 178, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Engelstad, K.N.; Vaskinn, A.; Torgalsbøen, A.-K.; Mohn, C.; Lau, B.; Rund, B.R. Impaired neuropsychological profile in homicide offenders with schizophrenia. Compr. Psychiatry 2018, 85, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Köşger, F.; Eşsizoğlu, A.; Sönmez, İ.; Güleç, G.; Genek, M.; Akarsu, Ö. The Relationship between Violence and Clinical Features, Insight and Cognitive Functions in Patients with Schizophrenia. Turk. Psikiyatr. Derg. 2016, 27, 1–8. [Google Scholar]
- Keilp, J.G.; Gorlyn, M.; Russell, M.; Oquendo, M.A.; Burke, A.K.; Harkavy-Friedman, J.; Mann, J.J. Neuropsychological function and suicidal behavior: Attention control, memory and executive dysfunction in suicide attempt. Psychol. Med. 2013, 43, 539–551. [Google Scholar] [CrossRef]
- Bohaterewicz, B.; Sobczak, A.M.; Podolak, I.; Wójcik, B.; Mȩtel, D.; Chrobak, A.A.; Fa Frowicz, M.; Siwek, M.; Dudek, D.; Marek, T. Machine Learning-Based Identification of Suicidal Risk in Patients With Schizophrenia Using Multi-Level Rest-ing-State fMRI Features. Front. Neurosci. 2021, 14, 605697. [Google Scholar] [CrossRef]
- Potvin, S.; Tikasz, A.; Richard-Devantoy, S.; Lungu, O.; Dumais, A. History of Suicide Attempt Is Associated with Reduced Medial Prefrontal Cortex Activity during Emotional Decision-Making among Men with Schizophrenia: An Exploratory fMRI Study. Schizophr. Res. Treat. 2018, 2018, 9898654. [Google Scholar] [CrossRef]
- Reutfors, J.; Brandt, L.; Jönsson, E.G.; Ekbom, A.; Sparén, P.; Ösby, U. Risk factors for suicide in schizophrenia: Findings from a Swedish population-based case-control study. Schizophr. Res. 2009, 108, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.C. Insight and Suicidality in Schizophrenia: A Replication Study. J. Nerv. Ment. Dis. 2000, 188, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, G.; Corvin, A.; Robertson, I.H. Are the Cognitive Deficits Associated with Impaired Insight in Schizophrenia Specific to Executive Task Performance? J. Nerv. Ment. Dis. 2005, 193, 803–808. [Google Scholar] [CrossRef]
- Nangle, J.-M.; Clarke, S.; Morris, D.W.; Schwaiger, S.; McGhee, K.A.; Kenny, N.; Murphy, K.; Gill, M.; Corvin, A.; Donohoe, G. Neurocognition and suicidal behaviour in an Irish population with major psychotic disorders. Schizophr. Res. 2006, 85, 196–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Raine, A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiatry Res. Neuroimaging 2009, 174, 81–88. [Google Scholar] [CrossRef]
- Tikàsz, A.; Potvin, S.; Richard-Devantoy, S.; Lipp, O.; Hodgins, S.; Lalonde, P.; Lungu, O.; Dumais, A. Reduced dorsolateral prefrontal cortex activation during affective Go/NoGo in violent schizophrenia patients: An fMRI study. Schizophr. Res. 2018, 197, 249–252. [Google Scholar] [CrossRef]
- Athanassiou, M.; Dumais, A.; Tikasz, A.; Lipp, O.; Dubreucq, J.-L.; Potvin, S. Increased cingulo-orbital connectivity is associated with violent behaviours in schizophrenia. J. Psychiatr. Res. 2022, 147, 183–189. [Google Scholar] [CrossRef]
- Barbey, A.K.; Koenigs, M.; Grafman, J. Dorsolateral prefrontal contributions to human working memory. Cortex 2013, 49, 1195–1205. [Google Scholar] [CrossRef]
- Naudts, K.; Hodgins, S. Neurobiological Correlates of Violent Behavior Among Persons with Schizophrenia. Schizophr. Bull. 2006, 32, 562–572. [Google Scholar] [CrossRef]
- Kaller, C.P.; Rahm, B.; Spreer, J.; Weiller, C.; Unterrainer, J.M. Dissociable contributions of left and right dorsolateral prefrontal cortex in planning. Cereb Cortex. 2011, 21, 307–317. [Google Scholar] [CrossRef]
- Kumari, V.; Das, M.; Taylor, P.J.; Barkataki, I.; Andrew, C.; Sumich, A.; Williams, S.C.; Ffytche, D.H. Neural and behavioural responses to threat in men with a history of serious violence and schizophrenia or antisocial personality disorder. Schizophr. Res. 2009, 110, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Tesli, N.; Westlye, L.T.; Storvestre, G.B.; Gurholt, T.P.; Agartz, I.; Melle, I.; Andreassen, O.A.; Haukvik, U.K. White matter microstructure in schizophrenia patients with a history of violence. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 271, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Antonova, E.; Kumari, V.; Morris, R.; Halari, R.; Anilkumar, A.; Mehrotra, R.; Sharma, T. The relationship of structural alterations to cognitive deficits in schizophrenia: A voxel-based morphometry study. Biol. Psychiatry 2005, 58, 457–467. [Google Scholar] [CrossRef]
- Dolan, M.C.; Fullam, R.S. Psychopathy and functional magnetic resonance imaging blood oxygenation level-dependent re-sponses to emotional faces in violent patients with schizophrenia. Biol. Psychiatry 2009, 66, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Joyal, C.C.; Putkonen, A.; Mancini-Marïe, A.; Hodgins, S.; Kononen, M.; Boulay, L.; Pihlajamaki, M.; Soininen, H.; Stip, E.; Tiihonen, J.; et al. Violent persons with schizophrenia and comorbid disorders: A functional magnetic resonance imaging study. Schizophr. Res. 2007, 91, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Sanfilipo, M.; LaFargue, T.; Arena, L.; Rusinek, H.; Kushner, K.; Lautin, A.; Loneragan, C.; Vaid, G.; Rotrosen, J.; Wolkin, A. Fine Volumetric Analysis of the Cerebral Ventricular System in Schizophrenia: Further Evidence for Multifocal Mild to Moderate Enlargement. Schizophr. Bull. 2000, 26, 201–216. [Google Scholar] [CrossRef]
| Search Strategy | |
|---|---|
| 1 | “schizophrenia” OR “psychotic disorder” |
| 2 | “suicide” OR “attempted suicide” |
| 3 | “cognition” OR “neuropsychology” OR “neuropsychological test” OR “executive function” OR “decision making” OR “problem solving” OR “prefrontal cortex” OR “neuropsychological functions” OR “executive functioning” OR “executive performance” |
| 4 | #1 AND #2 AND #3 |
| Search Strategy | |
|---|---|
| 1 | “schizophrenia” OR “psychotic disorder” |
| 2 | “homicide” OR “violence” |
| 3 | “cognition” OR “neuropsychology” OR “neuropsychological test” OR “executive function” OR “decision making” OR “problem solving” OR “prefrontal cortex” OR “neuropsychological functions” OR “executive functioning” OR “executive performance” |
| 4 | #1 AND #2 AND #3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomé-Fernández, M.; Berbegal-Bernabeu, M.; Sánchez-Sansegundo, M.; Zaragoza-Martí, A.; Rubio-Aparicio, M.; Portilla-Tamarit, I.; Rumbo-Rodríguez, L.; Hurtado-Sánchez, J.A. Neurocognitive Suicide and Homicide Markers in Patients with Schizophrenia Spectrum Disorders: A Systematic Review. Behav. Sci. 2023, 13, 446. https://doi.org/10.3390/bs13060446
Tomé-Fernández M, Berbegal-Bernabeu M, Sánchez-Sansegundo M, Zaragoza-Martí A, Rubio-Aparicio M, Portilla-Tamarit I, Rumbo-Rodríguez L, Hurtado-Sánchez JA. Neurocognitive Suicide and Homicide Markers in Patients with Schizophrenia Spectrum Disorders: A Systematic Review. Behavioral Sciences. 2023; 13(6):446. https://doi.org/10.3390/bs13060446
Chicago/Turabian StyleTomé-Fernández, Mario, Marina Berbegal-Bernabeu, Miriam Sánchez-Sansegundo, Ana Zaragoza-Martí, María Rubio-Aparicio, Irene Portilla-Tamarit, Lorena Rumbo-Rodríguez, and Jose Antonio Hurtado-Sánchez. 2023. "Neurocognitive Suicide and Homicide Markers in Patients with Schizophrenia Spectrum Disorders: A Systematic Review" Behavioral Sciences 13, no. 6: 446. https://doi.org/10.3390/bs13060446







