Serum Interleukin-8 in Patients with Different Origin of Intra-Abdominal Infections in Perioperative Period
Abstract
1. Introduction
2. Methods
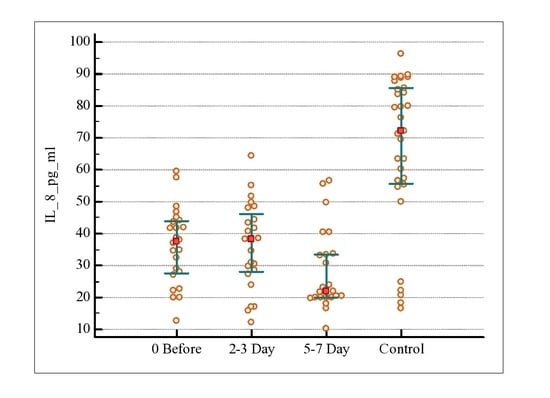
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Karantonis, F.F.; Nikiteas, N.; Perrea, D.; Vlachou, A.; Giamarellos-Bourboulis, E.J.; Tsigris, C.; Kostakis, A. Evaluation of the effects of laparotomy and laparoscopy on the immune system in intra-abdominal sepsis—A review. J. Investig. Surg. 2008, 21, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Riché, F.; Gayat, E.; Collet, C.; Matéo, J.; Laisné, M.; Launay, J.; Valleur, P.; Payen, D.; Cholley, B.P. Local and systemic innate immune response to secondary human peritonitis. Crit. Care 2013, 17, R201. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Pattern recognition theory and the launch of modern innate immunity. J. Immunol. 2013, 191, 4473. [Google Scholar] [CrossRef] [PubMed]
- Bickel, M. The role of interleukin-8 in inflammation and mechanisms of regulation. J. Periodontol. 1993, 64 (Suppl. S5), 456–460. [Google Scholar] [PubMed]
- Lin, E.; Calvano, S.E.; Stephen, F. Lowry, Flushing, and New Brunswick. Inflammatory cytokines and cell response in surgery. Surgery 2000, 127, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Loftus, T.J.; Mira, J.C.; Stortz, J.A.; Ozrazgat-Baslanti, T.; Ghita, G.L.; Wang, Z.; Brumback, B.A.; Ungaro, R.F.; Bihorac, A.; Leeuwenburgh, C.; et al. Persistent Inflammation and Anemia among Critically Ill Septic Patients. J. Trauma Acute Care Surg. 2018, 86, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, F.; Anderson, C. Sterile trauma to normal human dermis invariably induces IL1 beta, IL6 and IL8 in an innate response to “danger”. Acta Derm. Venereol. 2009, 89, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Norton, J.A.; Bollinger, R.R.; Chang, A.E.; Lowry, S.F.; Mulvihill, S.J. Surgery: Basic Science and Clinical Evidence; Springer: New York, NY, USA, 2008. [Google Scholar]
- Xiao, P.; Long, X.; Zhang, L.; Ye, Y.; Guo, J.; Liu, P.; Zhang, R.; Ning, J.; Yu, W.; Wei, F.; et al. Neurotensin/IL-8 pathway orchestrates local inflammatory response and tumor invasion by inducing M2 polarization of Tumor-Associated macrophages and epithelial-mesenchymal transition of hepatocellular carcinoma cells. Oncoimmunology 2018, 7, e1440166. [Google Scholar] [CrossRef]
- Pease, J.E.; Sabroe, I. The role of interleukin-8 and its receptors in inflammatory lung disease: Implications for therapy. Am. J. Respir. Med. 2002, 1, 19–25. [Google Scholar] [CrossRef]
- Zhao, F.X.; Liu, G.H.; Zhang, J. Value of IL-6 and IL-8 in the diagnosis of neonatal sepsis. Chin. J. Contemp. Pediatr. 2015, 17, 1311–1315. [Google Scholar]
- Ku, Y.; Hong, S.M.; Fujikura, K.; Kim, S.J.; Akita, M.; Abe-Suzuki, S.; Shiomi, H.; Masuda, A.; Itoh, T.; Azuma, T.; et al. IL-8 Expression in Granulocytic Epithelial Lesions of Idiopathic Duct-centric Pancreatitis (Type 2 Autoimmune Pancreatitis). Am. J. Surg. Pathol. 2017, 41, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Namba, S.; Nakano, R.; Kitanaka, T.; Kitanaka, N.; Nakayama, T.; Sugiya, H. ERK2 and JNK1 contribute to TNF-α-induced IL-8 expression in synovial fibroblasts. PLoS ONE 2017, 12, e0182923. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, A.; Knapp, M.; Lang, I.; Köhler, G. Interleukin 8 (IL-8)—A universal biomarker? Int. Arch. Med. 2010, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Hikmet, K. Determination of diagnostic and prognostic values of urinary interleukin-8, tumor necrosis factor-, and leukocyte arylsulfatase-A activity in patients with bladder cancer. Clin. Biochem. 2004, 37, 673–678. [Google Scholar] [CrossRef]
- Sapin, F.; Biston, P.; Piagnerelli, M. Predictive value of C-reactive protein in critically ill patients after abdominal surgery. Clinics (Sao Paulo) 2017, 72, 23–29. [Google Scholar] [CrossRef]
- Xiao, Z.; Wilson, C.; Robertson, H.L.; Roberts, D.J.; Ball, C.G.; Jenne, C.N.; Andrew, W. Kirkpatrick Inflammatory mediators in intra-abdominal sepsis or injury—A scoping review. Crit. Care 2015, 19, 373. [Google Scholar] [CrossRef]
- Ferreira, J.F.G.; Rezende-Neto, J.B.; Pinto-Silva, R.A.; de Souza, J.G.; Farias, L.D.; de Carvalho, M.A.R.; Santiago, H.; Serufo, J.C.; Santos, S.G.D. Microbiota Evaluation and Extracellular Cytokine Profile in Patients Affected with Intraabdominal Infection. Br. J. Med. Med. Res. 2016, 15, 1–14. [Google Scholar] [CrossRef]
- Sartelli, M.; Catena, F.; Abu-Zidan, F.M. Management of intra-abdominal infections: Recommendations by the WSES 2016 consensus conference. World J. Emerg. Surg. 2017, 12, 22. [Google Scholar] [CrossRef]
- Tang, H.; Lu, W.; Yang, Z.; Jiang, K.; D, M.; Chen, Y.; Lu, S.; Dong, J. Risk factors and long-term outcome for postoperative intra-abdominal infection after hepatectomy for hepatocellular carcinoma. Medicine (Baltimore) 2017, 96, e6795. [Google Scholar] [CrossRef]
- Heizmann, W.R.; Dupont, H.; Montravers, P.; Guirao, X.; Eckmann, C.; Bassetti, M.; García, M.S.; Capparella, M.R.; Simoneau, D.; Bodmann, K.F. Resistance mechanisms and epidemiology of multiresistant pathogens in Europe and efficacy of tigecycline in observational studies. J. Antimicrob. Chemother. 2013, 68 (Suppl. S2), 45–55. [Google Scholar] [CrossRef][Green Version]
- Miller, B.; Popejoy, M.W.; Hershberger, E.; Steenbergen, J.N.; Alverdy, J. Characteristics and Outcomes of Complicated Intra-abdominal Infections Involving Pseudomonas aeruginosa from a Randomized, Double-Blind, Phase 3 Ceftolozane-Tazobactam Study. Antimicrob. Agents Chemother. 2016, 60, 4387–4390. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, M.; Cuesta, D.P.; Flórez, L.E.; Correa, A.; Llanos, C.E.; Isaza, B.; Vanegas, S.; Osorio, J.; Casanova, L.; Villegas, M.V. Clinical and microbiological characteristics of complicated intra-abdominal infection in Colombia: A multicenter study. Rev. Chil. Infectol. 2016, 33, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Solomkin, J.S.; Meakins, J.L., Jr.; Allo, M.D.; Dellinger, E.P.; Simmons, R.L. Antibiotic trials in intra-abdominal infections. A critical evaluation of study design and outcome reporting. Ann. Surg. 1984, 200, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Easton, R.; Balogh, Z.J. Peri-operative changes in serum immune markers after trauma: a systematic review. Injury 2014, 45, 934–941. [Google Scholar] [CrossRef] [PubMed]
- Kraft, R.; Herndon, D.N.; Finnerty, C.C.; Cox, R.A.; Song, J.; Jeschke, M.G. Predictive Value of IL-8 for Sepsis and Severe Infections After Burn Injury: A Clinical Study. Shock 2015, 43, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Sartelli, M.; Abu-Zidan, F.M.; Catena, F.; Griffiths, E.A.; di Saverio, S.; Coimbra, R.; Ordoñez, C.A.; Leppaniemi, A.; Gustavo, P.; Fraga, F.C.; et al. Global validation of the WSES Sepsis Severity Score for patients with complicated intra-abdominal infections: A prospective multicentre study (WISS Study). World J. Emerg. Surg. 2015, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Sinapidis, D.; Kosmas, V.; Vittoros, V.; Koutelidakis, I.M.; Pantazi, A.; Stefos, A.; Katsaros, K.E.; Akinosoglou, K.; Bristianou, M.; Toutouzas, K.; et al. Progression into sepsis: An individualized process varying by the interaction of comorbidities with the underlying infection. BMC Infect. Dis. 2018, 18, 242. [Google Scholar] [CrossRef] [PubMed]
- Mazuski, J.E.; Tessier, J.M.; May, A.K.; Sawyer, R.G.; Nadler, E.P.; Rosengart, M.R.; Chang, P.K.; O’Neill, P.J.; Mollen, K.P.; Huston, J.M.; et al. The Surgical Infection Society Revised Guidelines on the Management of Intra-Abdominal Infection. Surg. Infect. 2017, 18, 1–76. [Google Scholar] [CrossRef]
- Casqueiro, J.; Casqueiro, J.; Alves, C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J. Endocrinol. Metab. 2012, 16 (Suppl. S1), S27–S36. [Google Scholar] [CrossRef]
- Geerlings, S.E.; Hoepelman, A.I.M. Immune dysfunction in patients with diabetes mellitus (DM). FEMS Immunol. Med. Microbiol. 1999, 26, 259–265. [Google Scholar] [CrossRef]
- Cimini, F.A.; Barchetta, I.; Porzia, A.; Mainiero, F.; Costantino, C.; Bertoccini, L.; Ceccarelli, V.; Morini, S.; Baroni, M.G.; Lenzi, A.; et al. Circulating IL-8 levels are increased in patients with type 2 diabetes and associated with worse inflammatory and cardiometabolic profile. Acta Diabetol. 2017, 54, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.M.; Gorter, K.J.; Hak, E.; Goudzwaard, W.L.; Schellevis, F.G.; Hoepelman, A.I.; Rutten, G.E.H.M. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin. Infect. Dis. 2005, 41, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A.G.; Page, K.; Campbell, M.; Martin, E.; Rashleigh-Rolls, R.; Halton, K.; Paterson, D.L.; Hall, L.; Jimmieson, N.; White, K.; et al. The increased risks of death and extra lengths of hospital and ICU stay from hospital-acquired bloodstream infections: A case-control study. BMJ Open 2013, 3, e003587. [Google Scholar] [CrossRef] [PubMed]
- Delamaire, M.; Maugendre, D.; Moreno, M.; Le Goff, M.-C.; Allannic, H.; Genetet, B. Impaired leucocyte functions in diabetic patients. Diabet. Med. 1997, 14, 29–34. [Google Scholar] [CrossRef]



| Data | POA n = 24 | PIAA n = 12 | PC n = 20 | Total n = 56 |
|---|---|---|---|---|
| Age, years (Median, (min; max)) | 58 (30; 78) | 63 (52; 79) | 48 (19; 83) | 56 (19; 83) |
| Male | 10 | 4 | 12 | 26 |
| Female | 14 | 8 | 8 | 30 |
| Diabetes mellitus type 2 | 2 | 3 | 2 | 7 |
| Systemic arterial hypertension | 2 | 1 | 2 | 7 |
| Previous abdominal surgical interventions | 24 | 1 | 1 | 26 |
| Duration in hospital, days (Median, (min; max)) | 14 (7; 98) | 18 (12; 34) | 11 (6; 33) | 13 (6; 98) |
| Routine surgical intervention (laparotomy) | 6 | 4 | 20 | 30 |
| Minimally invasive surgical intervention (abscess punction) | 18 | 8 | 0 | 26 |
| Abscess or peritoneum culture | ||||
| Gram positive | 3 | 9 | 13 | 25 |
| Enterococcus fecalis | 1 | 3 | 6 | 10 |
| Staphilococcus aureus | - | 4 | 6 | 10 |
| Staphilococcus epidermidis | 1 | 1 | 3 | 5 |
| Gram negative | 19 | 1 | 2 | 22 |
| Escherihia coli | 4 | 3 | 11 | 18 |
| Enterobacter aerogenes | 2 | - | 1 | 3 |
| Enterobacter spp. | 3 | - | - | 3 |
| Pseudomonas aeruginosa | 1 | - | 2 | 3 |
| Edwardsiella tarda | 1 | - | - | 1 |
| Klebsiella spp. | 1 | - | - | 1 |
| Poly | 2 | 2 | 3 | 7 |
| IAI | AUC | Concentration, pg/mL | p | Sensitivity (95% Confidential Interval) | Specificity 95% Confidential Interval) | Youden Index J |
|---|---|---|---|---|---|---|
| Post-operative abscesses | 0.860 | ≤49.71 | <0.0001 | 90.28 (81.0–96.0) | 83.87 (66.3–94.5) | 0.7415 |
| Primary intra-abdominal abscesses | 0.888 | ≤48.88 | <0.0001 | 97.22 (85.5–99.9) | 83.87 (66.3–94.5) | 0.8109 |
| Diffuse peritoneal collection | 0.864 | ≤54.35 | <0.0001 | 95.00 (86.1–99.0) | 80.65 (62.5–92.5) | 0.7565 |
| Total | 0.850 | ≤51.52 | <0.0001 | 94.64 (85.1–98.9) | 80.65 (62.5–92.5) | 0.7529 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riga, A.; Boyko, V.; Grirorov, Y. Serum Interleukin-8 in Patients with Different Origin of Intra-Abdominal Infections in Perioperative Period. Med. Sci. 2019, 7, 94. https://doi.org/10.3390/medsci7090094
Riga A, Boyko V, Grirorov Y. Serum Interleukin-8 in Patients with Different Origin of Intra-Abdominal Infections in Perioperative Period. Medical Sciences. 2019; 7(9):94. https://doi.org/10.3390/medsci7090094
Chicago/Turabian StyleRiga, Artem, Valeriy Boyko, and Yuriy Grirorov. 2019. "Serum Interleukin-8 in Patients with Different Origin of Intra-Abdominal Infections in Perioperative Period" Medical Sciences 7, no. 9: 94. https://doi.org/10.3390/medsci7090094
APA StyleRiga, A., Boyko, V., & Grirorov, Y. (2019). Serum Interleukin-8 in Patients with Different Origin of Intra-Abdominal Infections in Perioperative Period. Medical Sciences, 7(9), 94. https://doi.org/10.3390/medsci7090094