Alteration to Dopaminergic Synapses Following Exposure to Perfluorooctane Sulfonate (PFOS), in Vitro and in Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Culturing of SH-SY5Y Cells
2.3. Culturing of HEK-hVMAT2 Cells
2.4. Cell Viability
2.5. Vesicular 3H-DA Uptake in HEK-hVMAT2 Cells
2.6. Primary Culture of Mesencephalic Neurons
2.7. Animals and Treatment
2.8. Western Blot Analysis
2.9. Immunohistochemistry
2.10. Statistical Analysis
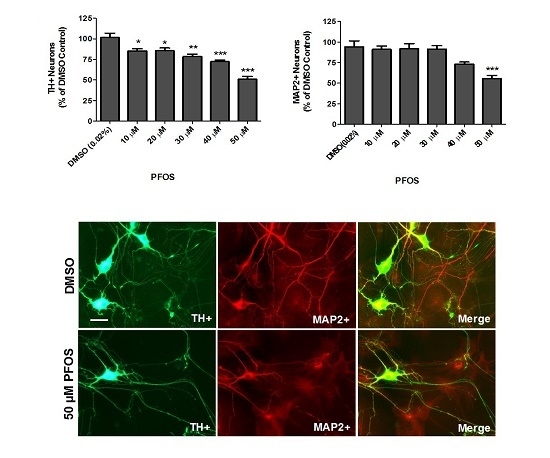
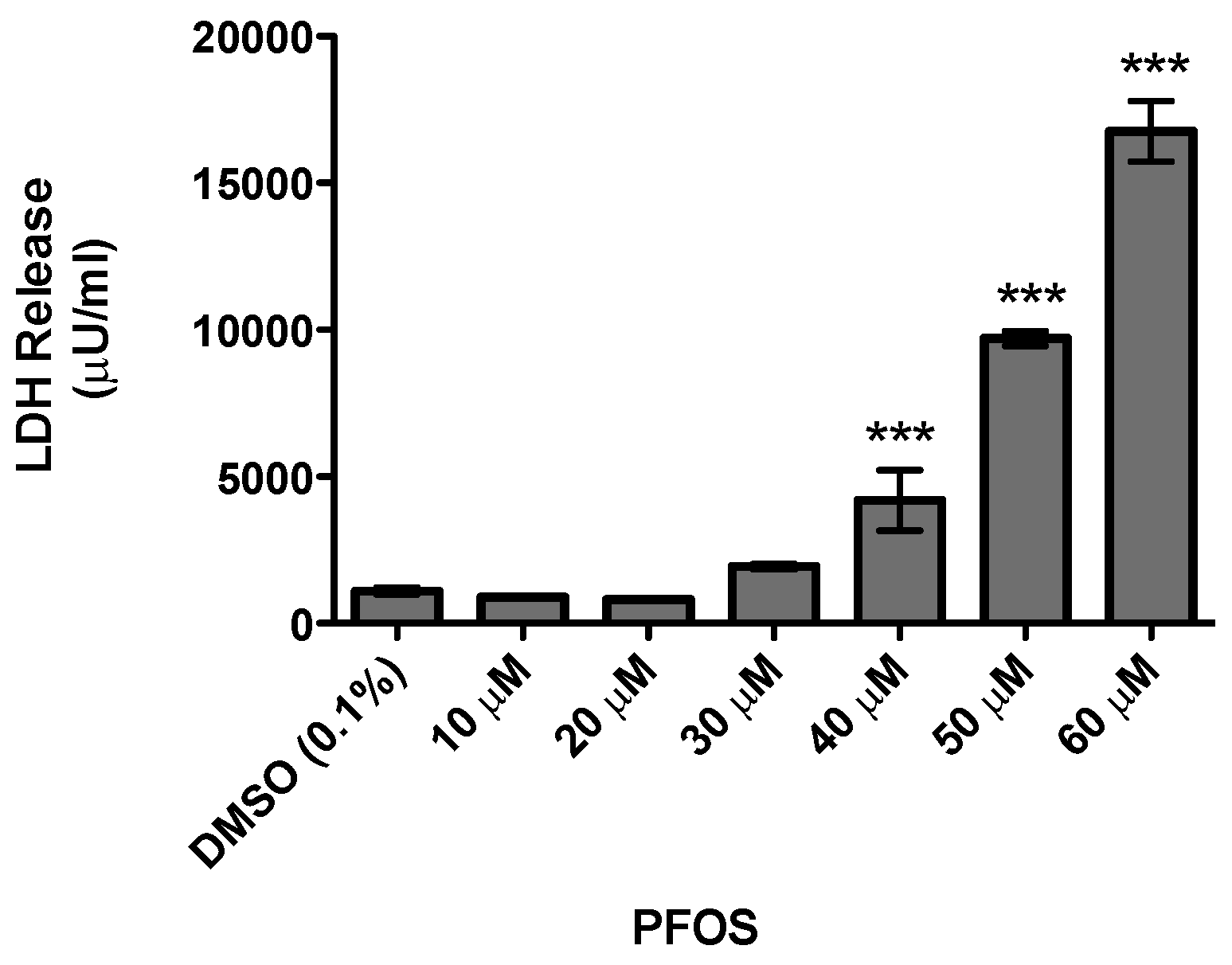
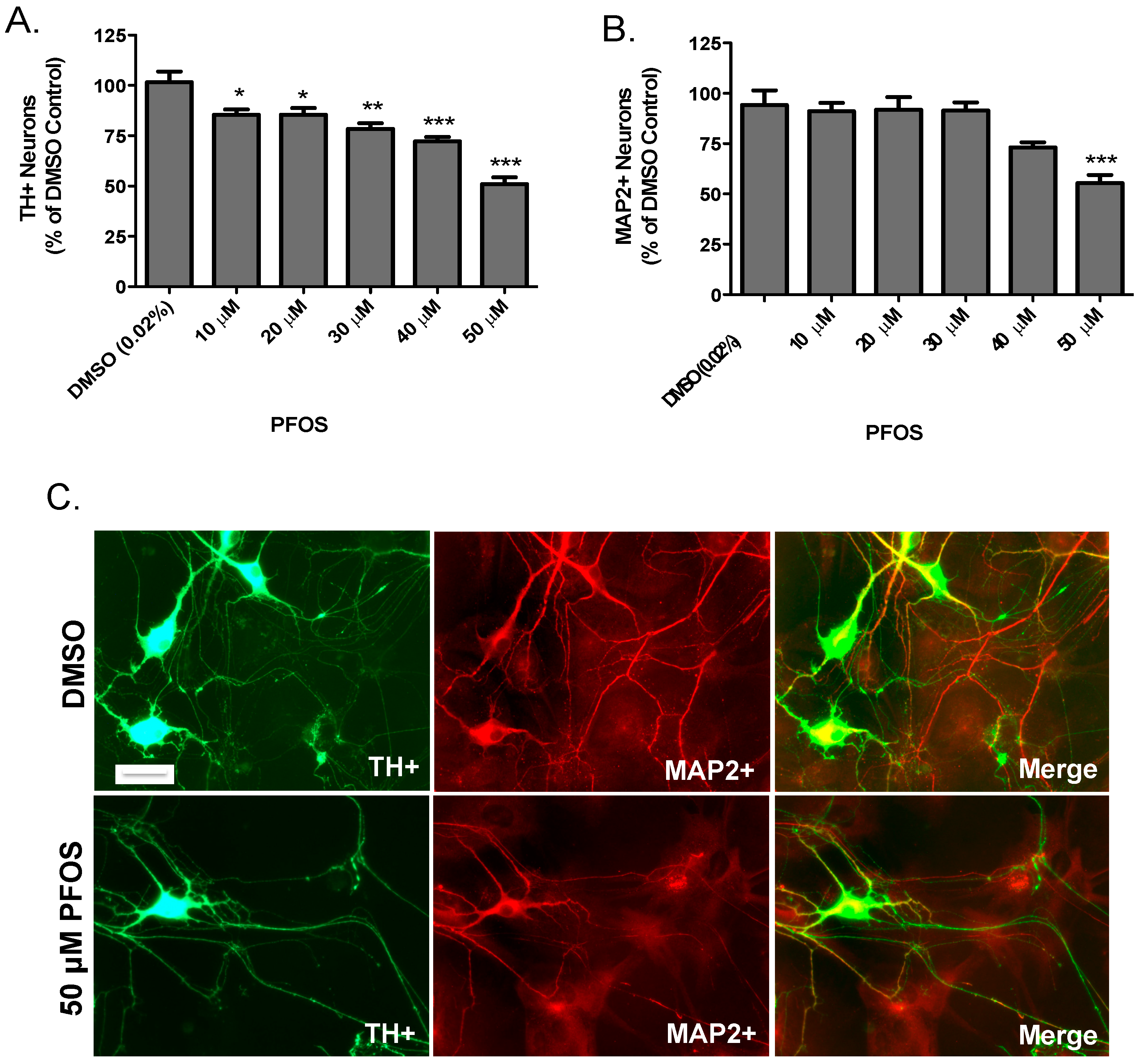
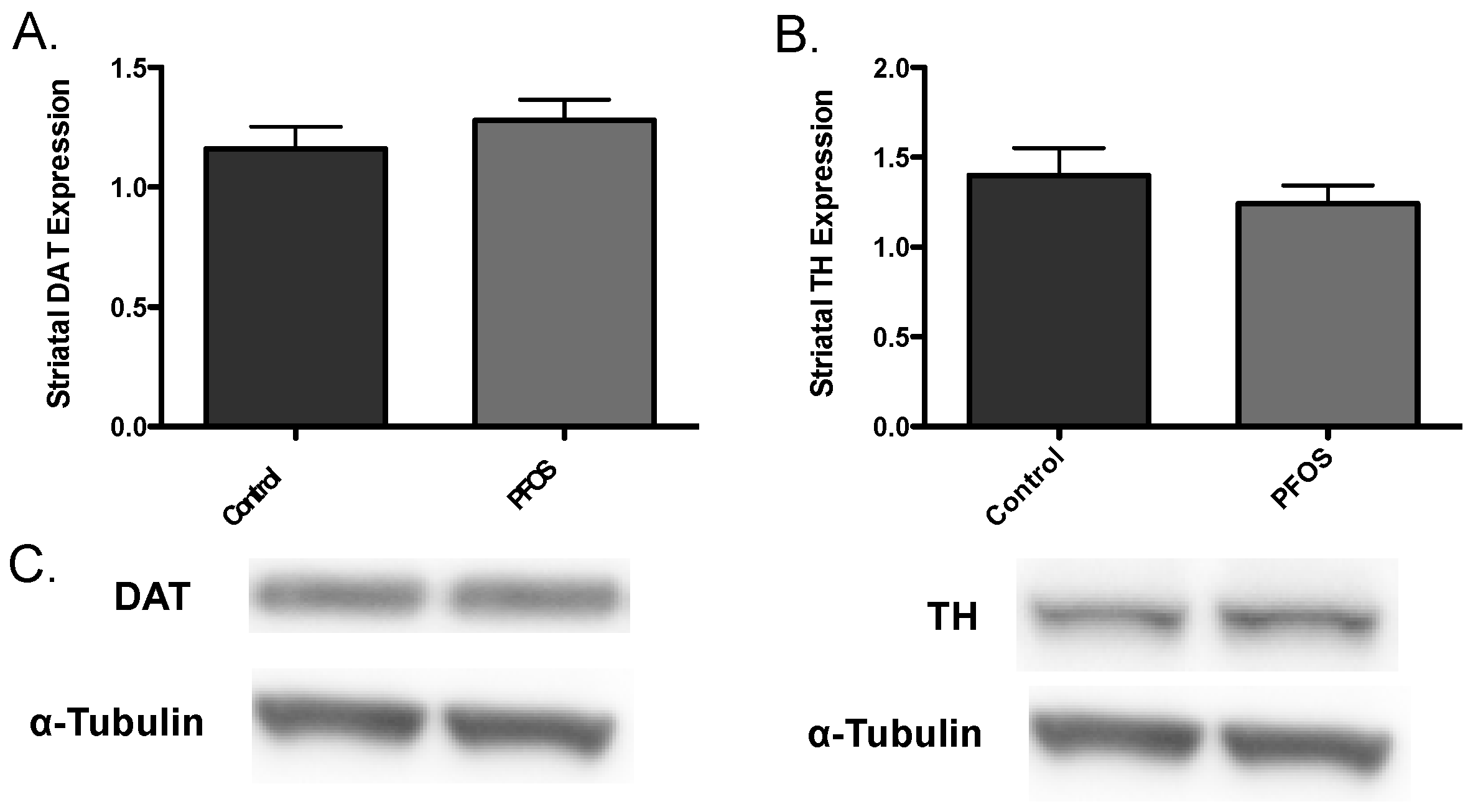
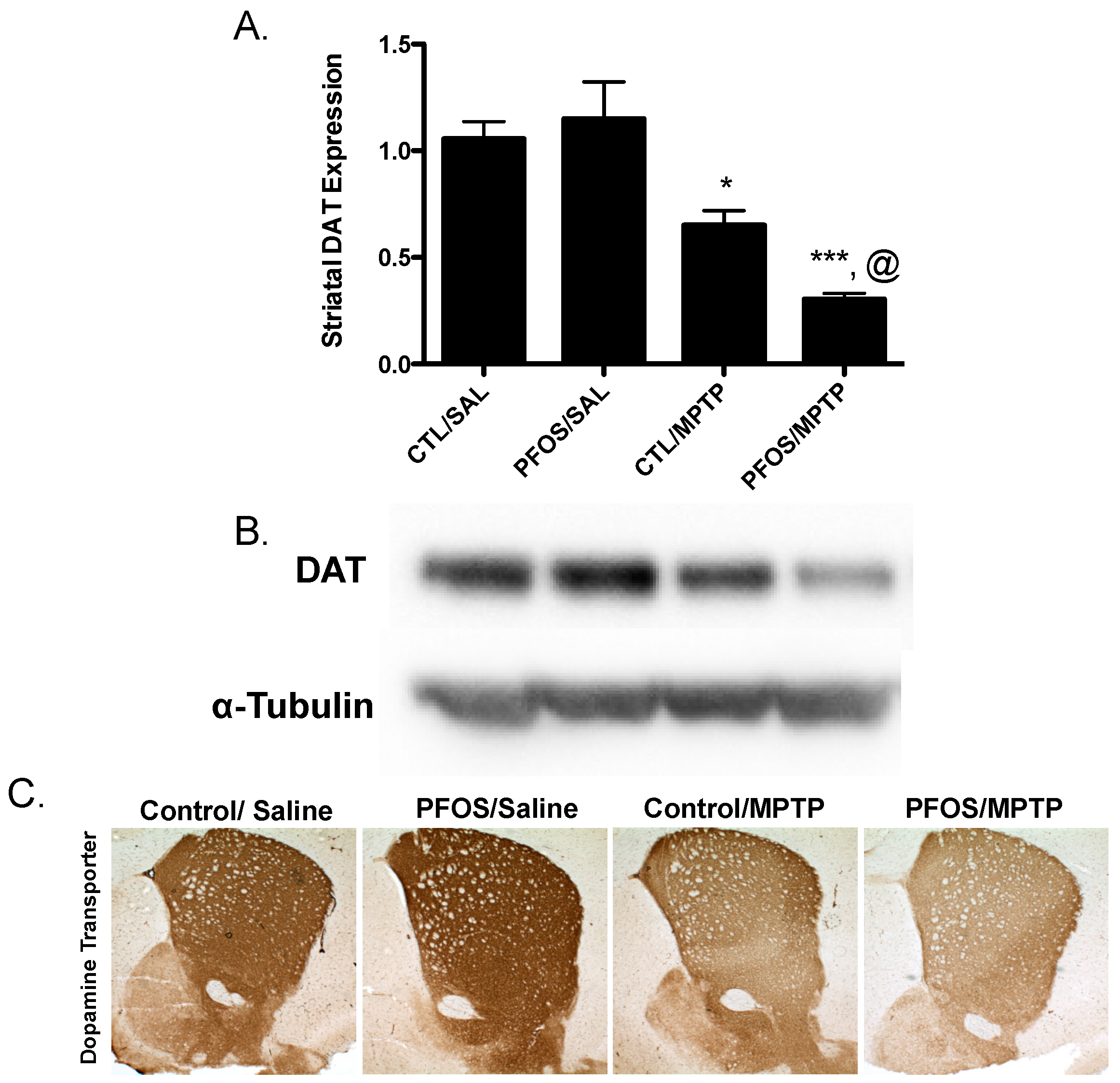
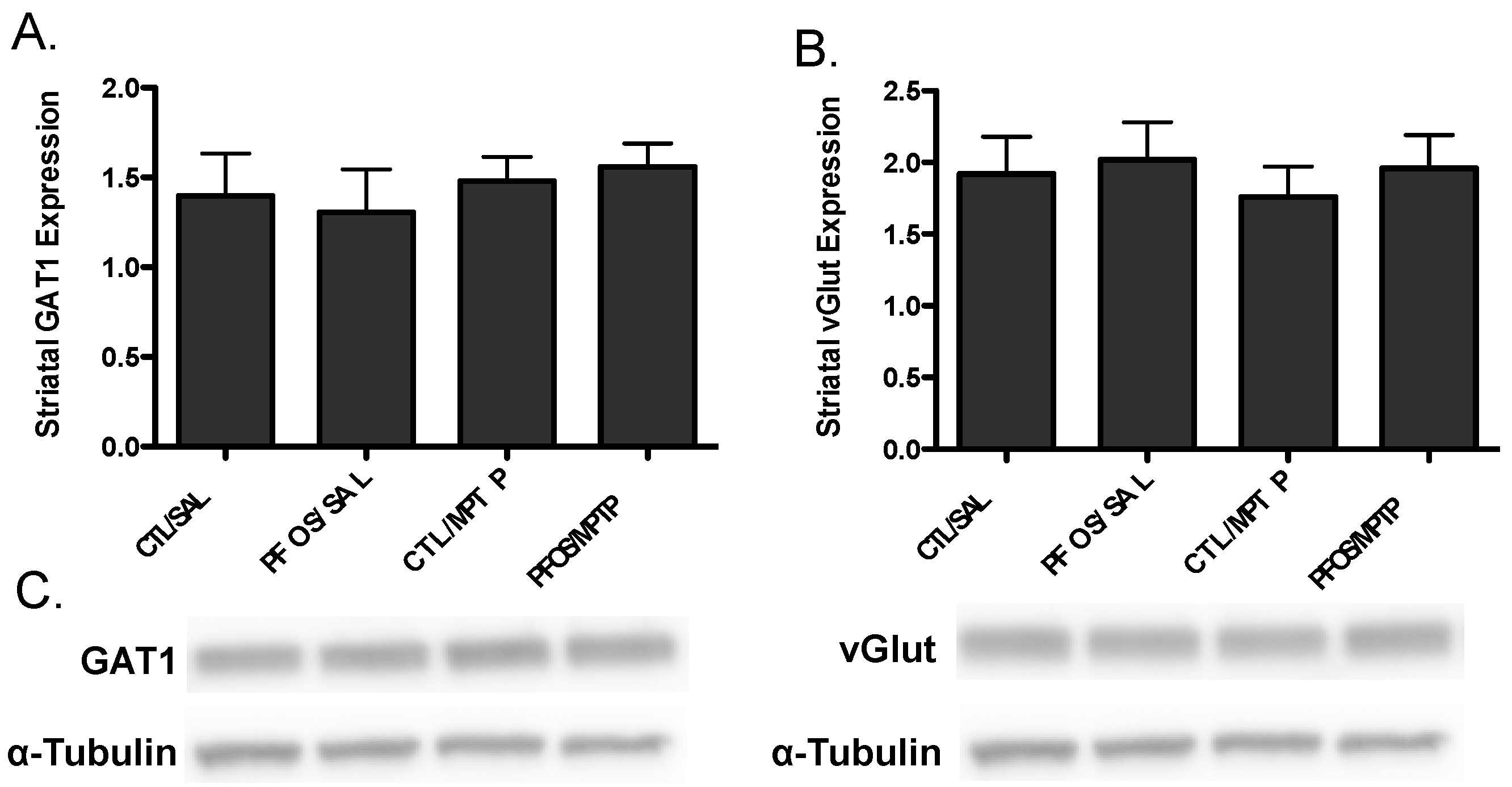
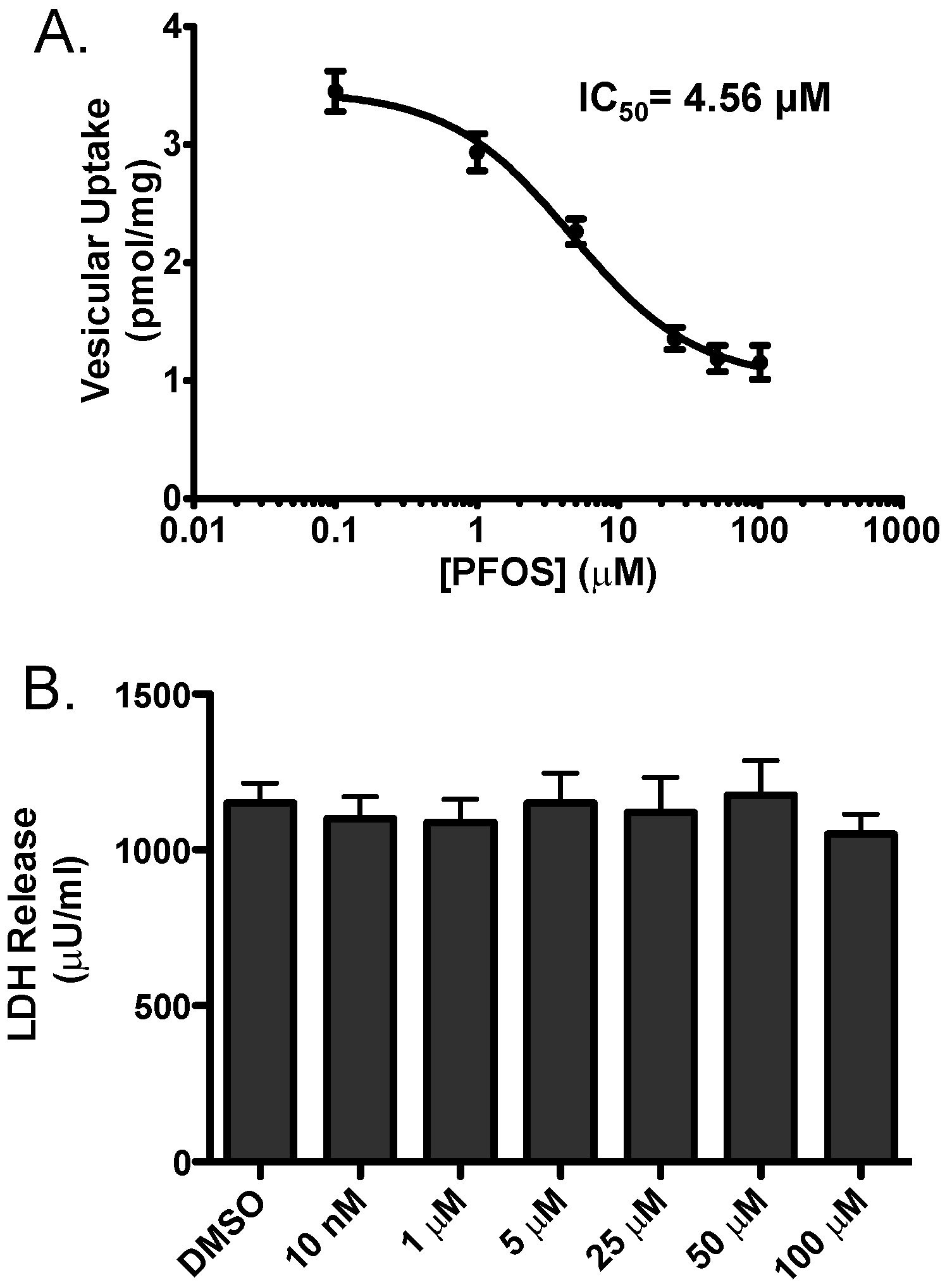
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fonnum, F.; Mariussen, E. Mechanisms involved in the neurotoxic effects of environmental toxicants such as polychlorinated biphenyls and brominated flame retardants. J. Neurochem. 2009, 111, 1327–1347. [Google Scholar] [CrossRef] [PubMed]
- Fonnum, F.; Mariussen, E.; Reistad, T. Molecular mechanisms involved in the toxic effects of polychlorinated biphenyls (PCBs) and brominated flame retardants (BFRs). J. Toxicol. Environ. Health A 2006, 69, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Bradner, J.M.; Suragh, T.A.; Caudle, W.M. Alterations to the circuitry of the frontal cortex following exposure to the polybrominated diphenyl ether mixture, DE-71. Toxicology 2013, 312, 48–55. [Google Scholar] [CrossRef]
- Bradner, J.M.; Suragh, T.A.; Wilson, W.W.; Lazo, C.R.; Stout, K.A.; Kim, H.M.; Wang, M.Z.; Walker, D.I.; Pennell, K.D.; Richardson, J.R.; et al. Exposure to the polybrominated diphenyl ether mixture DE-71 damages the nigrostriatal dopamine system: Role of dopamine handling in neurotoxicity. Exp. Neurol. 2013, 241, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Caudle, W.M.; Guillot, T.S.; Lazo, C.; Miller, G.W. Parkinson’s disease and the environment: Beyond pesticides. Neurotoxicology 2012, 33, 585. [Google Scholar] [CrossRef] [PubMed]
- Caudle, W.M.; Richardson, J.R.; Delea, K.C.; Guillot, T.S.; Wang, M.; Pennell, K.D.; Miller, G.W. Polychlorinated biphenyl-induced reduction of dopamine transporter expression as a precursor to Parkinson’s disease-associated dopamine toxicity. Toxicol. Sci. 2006, 92, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Caudle, W.M.; Richardson, J.R.; Wang, M.; Miller, G.W. Perinatal heptachlor exposure increases expression of presynaptic dopaminergic markers in mouse striatum. Neurotoxicology 2005, 26, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Caudle, W.M.; Wang, M.; Dean, E.D.; Pennell, K.D.; Miller, G.W. Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson’s disease. FASEB J. 2006, 20, 1695–1697. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Caudle, W.M.; Wang, M.Z.; Dean, E.D.; Pennell, K.D.; Miller, G.W. Developmental heptachlor exposure increases susceptibility of dopamine neurons to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)in a gender-specific manner. Neurotoxicology 2008, 29, 855–863. [Google Scholar] [CrossRef]
- Wilson, W.W.; Onyenwe, W.; Bradner, J.M.; Nenning, S.E.; Caudle, W.M. Developmental exposure to the organochlorine insecticide endosulfan alters expression of proteins associated with neurotransmission in the frontal cortex. Synapse 2014, 68, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Wilson, W.W.; Shapiro, L.P.; Bradner, J.M.; Caudle, W.M. Developmental exposure to the organochlorine insecticide endosulfan damages the nigrostriatal dopamine system in male offspring. Neurotoxicology 2014, 44, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Genskow, K.R.; Bradner, J.M.; Hossain, M.M.; Richardson, J.R.; Caudle, W.M. Selective damage to dopaminergic transporters following exposure to the brominated flame retardant, HBCDD. Neurotoxicol. Teratol. 2015, 52, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Conder, J.M.; Hoke, R.A.; De Wolf, W.; Russell, M.H.; Buck, R.C. Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ. Sci. Technol. 2008, 42, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Smithwick, M.; Muir, D.C.; Mabury, S.A.; Solomon, K.R.; Martin, J.W.; Sonne, C.; Born, E.W.; Letcher, R.J.; Dietz, R. Perflouroalkyl contaminants in liver tissue from East Greenland polar bears (Ursus maritimus). Environ. Toxicol. Chem. 2005, 24, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Verreault, J.; Houde, M.; Gabrielsen, G.W.; Berger, U.; Haukas, M.; Letcher, R.J.; Muir, D.C. Perfluorinated alkyl substances in plasma, liver, brain, and eggs of glaucous gulls (Larus hyperboreus) from the Norwegian arctic. Environ. Sci. Technol. 2005, 39, 7439–7445. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; He, X.; Liu, W.; Zhang, H.; Saito, N.; Tsuda, S. Distribution of perfluoroalkyl compounds in rats: Indication for using hair as bioindicator of exposure. J. Expo. Sci. Environ. Epidemiol. 2015, 25, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Greaves, A.K.; Letcher, R.J.; Sonne, C.; Dietz, R. Brain region distribution and patterns of bioaccumulative perfluoroalkyl carboxylates and sulfonates in east greenland polar bears (Ursus maritimus). Environ. Toxicol. Chem. 2013, 32, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Maestri, L.; Negri, S.; Ferrari, M.; Ghittori, S.; Fabris, F.; Danesino, P.; Imbriani, M. Determination of perfluorooctanoic acid and perfluorooctanesulfonate in human tissues by liquid chromatography/single quadrupole mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2728–2734. [Google Scholar] [CrossRef] [PubMed]
- Perez, F.; Nadal, M.; Navarro-Ortega, A.; Fàbrega, F.; Domingo, J.L.; Barceló, D.; Farré, M. Accumulation of perfluoroalkyl substances in human tissues. Environ. Int. 2013, 59, 354–362. [Google Scholar] [CrossRef] [PubMed]
- De Cock, M.; de Boer, M.R.; Lamoree, M.; Legler, J.; van de Bor, M. Prenatal exposure to endocrine disrupting chemicals in relation to thyroid hormone levels in infants—A Dutch prospective cohort study. Environ. Health 2014, 13, 106. [Google Scholar] [CrossRef] [PubMed]
- Lehmler, H.J. Synthesis of environmentally relevant fluorinated surfactants—A review. Chemosphere 2005, 58, 1471–1496. [Google Scholar] [CrossRef] [PubMed]
- Renner, R. Growing concern over perfluorinated chemicals. Environ. Sci. Technol. 2001, 35, 154A–160A. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; López-Doval, S.; Pereiro, N.; Lafuente, A. Perfluorooctane sulfonate (PFOS) exposure could modify the dopaminergic system in several limbic brain regions. Toxicol. Lett. 2016, 240, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Viberg, H.; Lee, I.; Eriksson, P. Adult dose-dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology 2013, 304, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Zhang, Q.; Zhao, H.; Quan, X. Effects of developmental perfluorooctane sulfonate exposure on spatial learning and memory ability of rats and mechanism associated with synaptic plasticity. Food Chem. Toxicol. 2015, 76, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, S.; Viberg, H. Postnatal exposure to PFOS, but not PBDE 99, disturb dopaminergic gene transcription in the mouse CNS. Environ. Toxicol. Pharmacol. 2016, 41, 121–126. [Google Scholar] [CrossRef]
- Lee, I.; Viberg, H. A. single neonatal exposure to perfluorohexane sulfonate (PFHxS) affects the levels of important neuroproteins in the developing mouse brain. Neurotoxicology 2013, 37, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Hatcher, J.M.; Pennell, K.D.; Miller, G.W. Parkinson’s disease and pesticides: A toxicological perspective. Trends Pharmacol. Sci. 2008, 29, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Blandini, F.; Nappi, G.; Tassorelli, C.; Martignoni, E. Functional changes of the basal ganglia circuitry in Parkinson’s disease. Prog. Neurobiol. 2000, 62, 63–88. [Google Scholar] [CrossRef]
- Elwan, M.A.; Richardson, J.R.; Guillot, T.S.; Caudle, W.M.; Miller, G.W. Pyrethroid pesticide-induced alterations in dopamine transporter function. Toxicol. Appl. Pharmacol. 2006, 211, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Taylor, M.M.; Shalat, S.L.; Guillot, T.S., 3rd; Caudle, W.M.; Hossain, M.M.; Mathews, T.A.; Jones, S.R.; Cory-Slechta, D.A.; Miller, G.W. Developmental pesticide exposure reproduces features of attention deficit hyperactivity disorder. FASEB J. 2015, 29, 1960–1972. [Google Scholar] [CrossRef] [PubMed]
- Caudle, W.M.; Richardson, J.R.; Wang, M.Z.; Taylor, T.N.; Guillot, T.S.; McCormack, A.L.; Colebrooke, R.E.; Di Monte, D.A.; Emson, P.C.; Miller, G.W. Reduced vesicular storage of dopamine causes progressive nigrostriatal neurodegeneration. J. Neurosci. 2007, 27, 8138–8148. [Google Scholar] [CrossRef] [PubMed]
- Krishna, A.; Biryukov, M.; Trefois, C.; Antony, P.M.; Hussong, R.; Lin, J.; Heinäniemi, M.; Glusman, G.; Köglsberger, S.; Boyd, O.; et al. Systems genomics evaluation of the SH-SY5Y neuroblastoma cell line as a model for Parkinson’s disease. BMC Genom. 2014, 15, 1154. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinsons Dis. 2013, 3, 461–491. [Google Scholar] [PubMed]
- Jenner, P.; Olanow, C.W. The pathogenesis of cell death in Parkinson’s disease. Neurology 2006, 66, 24–36. [Google Scholar] [CrossRef]
- Chen, N.; Li, J.; Li, D.; Yang, Y.; He, D. Chronic exposure to perfluorooctane sulfonate induces behavior defects and neurotoxicity through oxidative damages, in vivo and in vitro. PLoS ONE 2014, 9, e113453. [Google Scholar] [CrossRef] [PubMed]
- Reistad, T.; Fonnum, F.; Mariussen, E. Perfluoroalkylated compounds induce cell death and formation of reactive oxygen species in cultured cerebellar granule cells. Toxicol. Lett. 2013, 218, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Lee, Y.J.; Yang, J.H. Perfluorooctane sulfonate induces apoptosis of cerebellar granule cells via a ROS-dependent protein kinase C signaling pathway. Neurotoxicology 2012, 33, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Wang, G.; Zhao, J.; Wang, E.; Yin, B.; Fang, D.; Zhao, J.; Zhang, H.; Chen, Y.Q.; Chen, W. Toxicity assessment of perfluorooctane sulfonate using acute and subchronic male C57BL/6J mouse models. Environ. Pollut. 2016, 210, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Wong, L.Y.; Kuklenyiz, Z.; Reidy, J.A.; Needham, L.L. Polyfluoroalkyl chemicals in the U.S. population: Data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons with NHANES 1999–2000. Environ. Health Perspect. 2007, 115, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Kraft, A.D.; Aschner, M.; Cory-Slechta, D.A.; Bilbo, S.D.; Caudle, W.M.; Makris, S.L. Unmasking silent neurotoxicity following developmental exposure to environmental toxicants. Neurotoxicol. Teratol. 2016, 55, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Caudle, W.M.; Guillot, T.S.; Watson, J.L.; Nakamaru-Ogiso, E.; Seo, B.B.; Sherer, T.B.; Greenamyre, J.T.; Yagi, T.; Matsuno-Yagi, A.; et al. Obligatory role for complex I inhibition in the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Toxicol. Sci. 2007, 95, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Caudle, W.M.; Colebrooke, R.E.; Emson, P.C.; Miller, G.W. Altered vesicular dopamine storage in Parkinson’s disease: A premature demise. Trends Neurosci. 2008, 31, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Chalon, S.; Emond, P.; Bodard, S.; Vilar, M.P.; Thiercelin, C.; Besnard, J.C.; Guilloteau, D. Time course of changes in striatal dopamine transporters and D2 receptors with specific iodinated markers in a rat model of Parkinson’s disease. Synapse 1999, 31, 134–139. [Google Scholar] [CrossRef]
- Chou, K.L.; Hurtig, H.I.; Stern, M.B.; Colcher, A.; Ravina, B.; Newberg, A.; Mozley, P.D.; Siderowf, A. Diagnostic accuracy of [99mTc]TRODAT-1 SPECT imaging in early Parkinson’s disease. Parkinsonism Relat. Disord. 2004, 10, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, H.H.; Friedman, J.H.; Fischman, A.J.; Noto, R.B.; Lannon, M.C. Is altropane SPECT more sensitive to fluoroDOPA PET for detecting early Parkinson’s disease? Med. Sci. Monit. 2001, 7, 1339–1343. [Google Scholar] [PubMed]
- Prunier, C.; Bézard, E.; Montharu, J.; Mantzarides, M.; Besnard, J.C.; Baulieu, J.L.; Gross, C.; Guilloteau, D.; Chalon, S. Presymptomatic diagnosis of experimental Parkinsonism with 123I-PE2I SPECT. Neuroimage 2003, 19, 810–816. [Google Scholar] [CrossRef]
- Bemis, J.C.; Seegal, R.F. PCB-induced inhibition of the vesicular monoamine transporter predicts reductions in synaptosomal dopamine content. Toxicol. Sci. 2004, 80, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Mariussen, E.; Fonnum, F. The effect of brominated flame retardants on neurotransmitter uptake into rat brain synaptosomes and vesicles. Neurochem. Int. 2003, 43, 533–542. [Google Scholar] [CrossRef]
- Mariussen, E.; Morch Andersen, J.; Fonnum, F. The effect of polychlorinated biphenyls on the uptake of dopamine and other neurotransmitters into rat brain synaptic vesicles. Toxicol. Appl. Pharmacol. 1999, 161, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Gainetdinov, R.R.; Fumagalli, F.; Wang, Y.M.; Jones, S.R.; Levey, A.I.; Miller, G.W.; Caron, M.G. Increased MPTP neurotoxicity in vesicular monoamine transporter 2 heterozygote knockout mice. J. Neurochem. 1998, 70, 1973–1978. [Google Scholar] [CrossRef] [PubMed]
- Lohr, K.M.; Bernstein, A.I.; Stout, K.A.; Dunn, A.R.; Lazo, C.R.; Alter, S.P.; Wang, M.; Li, Y.; Fan, X.; Hess, E.J.; et al. Increased vesicular monoamine transporter enhances dopamine release and opposes Parkinson disease-related neurodegeneration in vivo. Proc. Natl. Acad. Sci. USA 2014, 111, 9977–9982. [Google Scholar] [CrossRef] [PubMed]
- Colebrooke, R.E.; Humby, T.; Lynch, P.J.; McGowan, D.P.; Xia, J.; Emson, P.C. Age-related decline in striatal dopamine content and motor performance occurs in the absence of nigral cell loss in a genetic mouse model of Parkinson’s disease. Eur. J. Neurosci. 2006, 24, 2622–2630. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, R.; Bradner, J.M.; Stout, K.A.; Caudle, W.M. Alteration to Dopaminergic Synapses Following Exposure to Perfluorooctane Sulfonate (PFOS), in Vitro and in Vivo. Med. Sci. 2016, 4, 13. https://doi.org/10.3390/medsci4030013
Patel R, Bradner JM, Stout KA, Caudle WM. Alteration to Dopaminergic Synapses Following Exposure to Perfluorooctane Sulfonate (PFOS), in Vitro and in Vivo. Medical Sciences. 2016; 4(3):13. https://doi.org/10.3390/medsci4030013
Chicago/Turabian StylePatel, Rahul, Joshua M. Bradner, Kristen A. Stout, and William Michael Caudle. 2016. "Alteration to Dopaminergic Synapses Following Exposure to Perfluorooctane Sulfonate (PFOS), in Vitro and in Vivo" Medical Sciences 4, no. 3: 13. https://doi.org/10.3390/medsci4030013