The Counterbalance of Skin Metabolism on Orbits and Diseases
Abstract
:1. Introduction
1.1. The relationship between Water and Skin
1.2. Skin and the Nervous System
1.3. Regularity Reaction of the Autonomic Nervous System
1.4. Porphyra-334 and the Nervous System
1.5. Objectives
2. Experimental Section
2.1. Test Preparations
2.2. Participants and Experimental Design of the Treatment Study
2.3. Data Generation and Analysis
2.4. Autonomic Nervous System Performance Diagram Generation
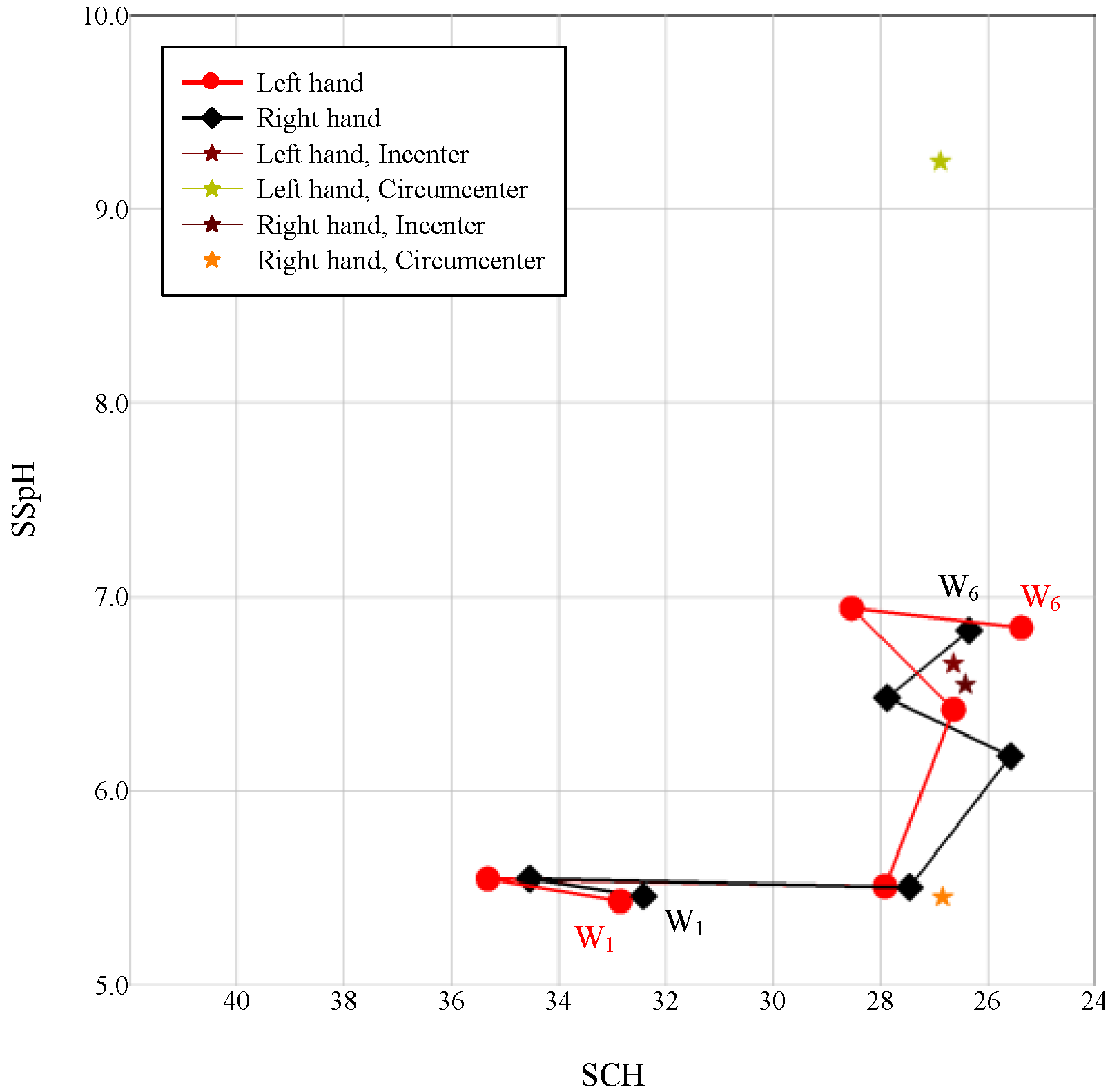
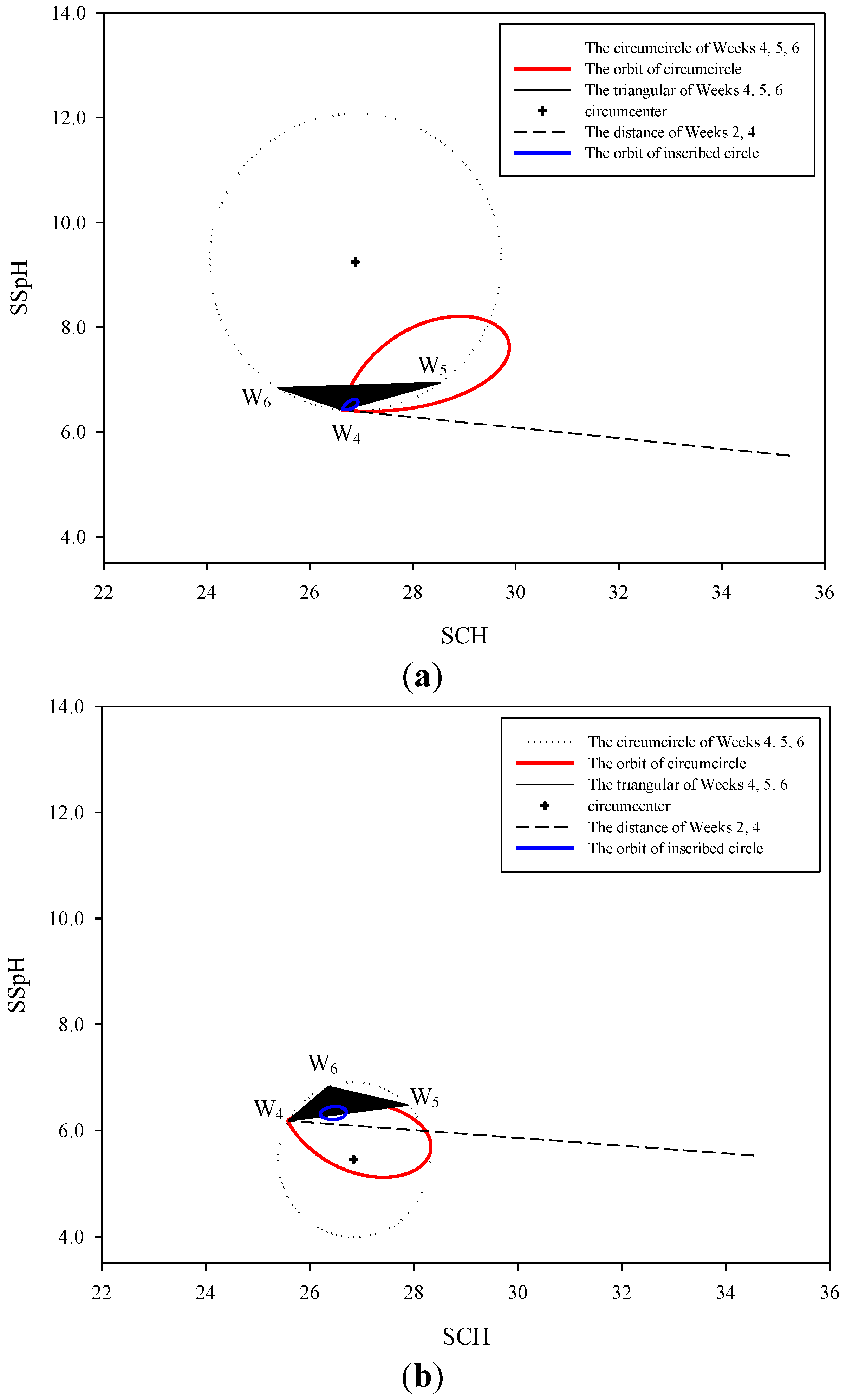
2.5. Signal Operation Diagram Generation
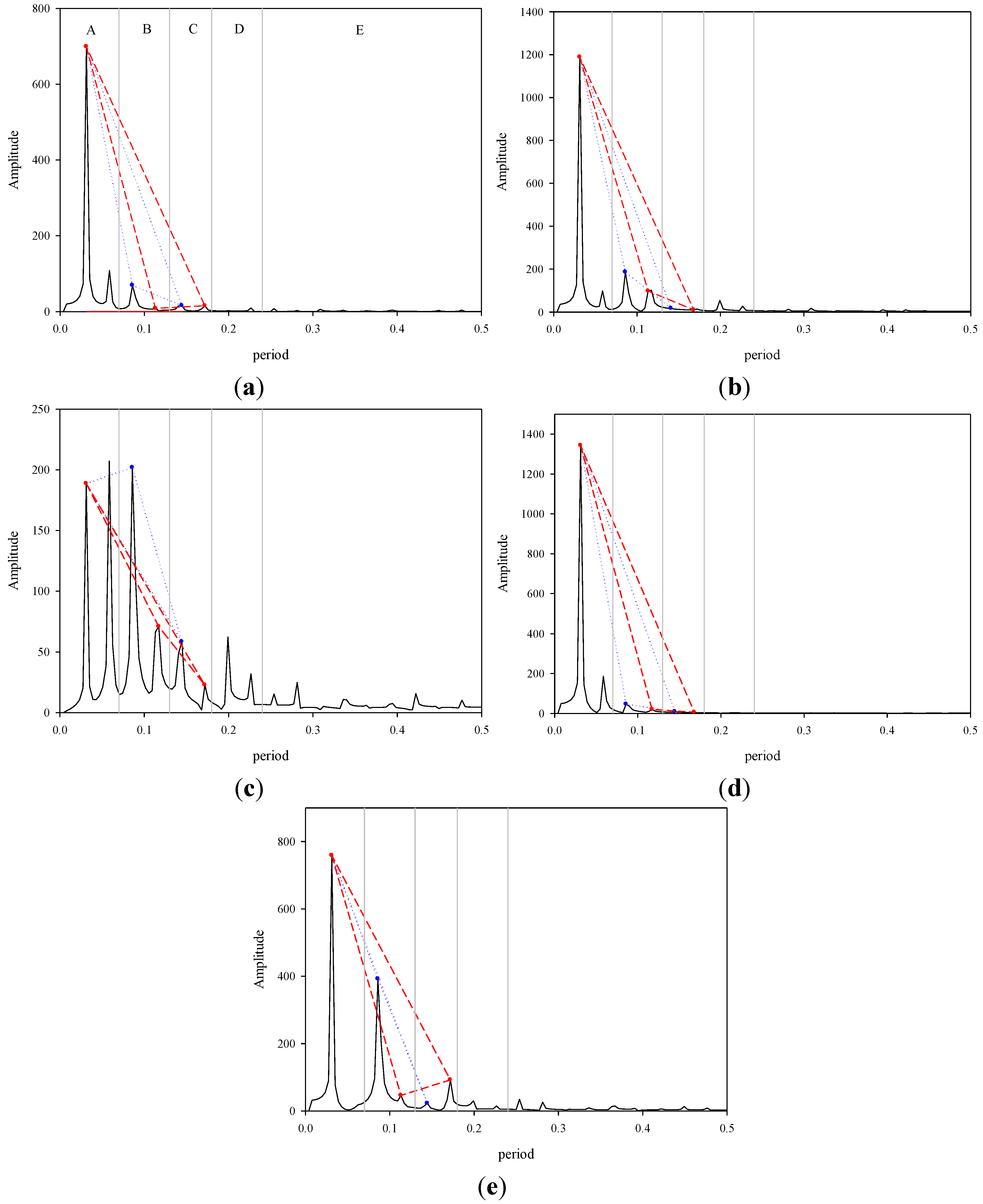
3. Results and Discussion
3.1. Changes in the Autonomic Nervous System and Orbits Observed in the Natural Environment
3.2. Signal Operation Diagram
3.3. Natural Orbits and a Diagram of Signal Operation in the Human Body
3.4. Discussion of the Signal Operation Diagrams of Four Participants
| Receptor | → | Sensory nerve | → | Spinal cord | → | Brain stem | → | Cerebrum | → | Brain stem | → | Spinal cord | → | Motor nerve | → | Effector | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 0.0273 | 0.0273 | 0.0273 | 0.0273 | 0.0313 | 0.0234 | 0.0313 | 0.0273 | |||||||||||||||||
| Case 1 | 0.0273 | 0.0273 | 0.0273 | 0.0313 | 0.0273 | 0.0273 | 0.0273 | 0.0273 | |||||||||||||||||
| Case 2 | 0.0234 | 0.0273 | 0.0313 | 0.0273 | 0.0273 | 0.0273 | 0.0234 | 0.0273 | |||||||||||||||||
| Case 3 | 0.0273 | 0.0313 | 0.0234 | 0.0273 | 0.0313 | 0.0273 | 0.0234 | 0.0313 | |||||||||||||||||
| Case 4 | 0.0234 | 0.0273 | 0.0273 | 0.0273 | 0.0273 | 0.0313 | 0.0273 | 0.0273 | |||||||||||||||||
| Case 5 | 0.0273 | 0.0313 | 0.0234 | 0.0313 | 0.0234 | 0.0313 | 0.0273 | 0.0273 | |||||||||||||||||
4. Conclusions
Acknowledgments
Author Contributions
Conflict of Interest
References
- Widmaier, E.P.; Raff, H.; Strang, K.T. Chemical Composition of the Body. In Vander’s Human Physiology–The Mechanisms of Body Function, 13th ed.; McGraw-Hill: New York, NY, USA, 2014; p. 27. [Google Scholar]
- Rawlings, A.V.; Harding, C.R. Moisturization and skin barrier function. Dermatol. Ther. 2004, 17, 43–48. [Google Scholar] [PubMed]
- Elias, P.M. Stratum corneum architecture, metabolic activity and interactivity with subjacent cell layers. Exp. Dermatol. 1996, 5, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Lodén, M. Do moisturizers work? J. Cosmet. Sci. 2004, 2, 141–149. [Google Scholar] [CrossRef]
- Buraczewska, I.; Berne, B.; Lindberg, M.; Lodén, M.; Törmä, H. Moisturizers change the mRNA expression of enzymes synthesizing skin barrier lipids. Arch Dermatol. Res. 2009, 301, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Cicek, D.; Kandi, B.; Berilgen, M.S.; Bulut, S.; Tekatas, A.; Dertlioglu, S.B.; Ozel, S.; Saral, Y. Does autonomic dysfunction play a role in atopic dermatitis? Br. J. Dermatol. 2008, 159, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Charkoudian, N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin. Proc. 2003, 78, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Pérgola, P.E.; Kellogg, D.L.J.R.; Johnson, J.M.; Kosiba, W.A.; Solomon, D.E. Role of sympathetic nerves in the vascular effects of local temperature in human forearm skin. Am. J. Physiol. 1993, 265, 785–792. [Google Scholar]
- Dung, H.C. Acupuncture points of the brachial plexus. Am. J. Chin. Med. 1985, 13, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Stücker, M.; Struk, A.; Altmeyer, P.; Herde, M.; Baumgärtl, H.; Lübbers, D.W. The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. J. Physiol. 2002, 538, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Román, E.M.; Soriano, G.; Fuentes, M.; Gálvez, M.L.; Fernández, C. The influence of the full moon on the number of admissions related to gastrointestinal bleeding. Int. J. Nurs. Pract. 2004, 10, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, U.; Ghosh, T. A study on the physical fitness index, heart rate and blood pressure in different phases of lunar month on male human subjects. Int. J. Biometeorol. 2013, 57, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, U.; Ghosh, T. Autonomic neural activity in male human subjects during different phases of synodic period of moon. Biol. Rhythm Res. 2013, 44, 411–420. [Google Scholar] [CrossRef]
- Zimecki, M. The lunar cycle: effects on human and animal behavior and physiology. Postepy Hig. Med. Dosw. 2006, 60, 1–7. [Google Scholar]
- Chakraborty, U. Effects of different phases of lunar month on human. Biol. Rhythm Res. 2013, 45, 383–396. [Google Scholar] [CrossRef]
- Sinha, R.P.; Singh, S.P.; Häder, D.P. Database on mycosporines and mycosporine-like amino acids (MAAs) in fungi, cyanobacteria, macroalgae, phytoplankton and animals. Photochem. Photobiol. B 2007, 89, 29–35. [Google Scholar] [CrossRef]
- Hoyer, K.; Karsten, U.; Sawall, T.; Wiencke, C. Photoprotective substances in Antarctic macroalgae and their variation with respect to depth distribution, different tissues and developmental stages. Mar. Ecol. Prog. Ser. 2001, 211, 117–129. [Google Scholar] [CrossRef]
- Huovinen, P.; Gómez, I.; Figueroa, F.L.; Ulloa, N.; Morales, V.; Lovengreen, C. Ultraviolet-absorbing mycosporine-like amino acids in red macroalgae from Chile. Bot. Mar. 2004, 47, 21–29. [Google Scholar] [CrossRef]
- Oyamada, C.; Kaneniw, A.M.; Ebitani, K.; Murata, M.; Ishihara, K. Mycosporine-like amino acids extracted from scallop (Patinopecten yessoensis) ovaries: UV protection and growth stimulation activities on human cells. Mar. Biotechnol. 2008, 10, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Tao, C.; Sugawara, T.; Maeda, S.; Wang, X.; Hirata, T. Antioxidative activities of a mycosporine-like amino acid, porphyra-334. Fish. Sci. 2008, 74, 1161–1172. [Google Scholar] [CrossRef]
- Torres, A.; Enk, C.D.; Hochberg, M.; Srebnik, M. Porphyra-334, a potential natural source for UVA protective sunscreens. Photochem. Photobiol. Sci. 2006, 5, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C 2007, 146, 60–78. [Google Scholar] [CrossRef]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The photoprotector mechanism of mycosporine-like amino acids. Excited-state properties and photostability of porphyra-334 in aqueous solution. J. Photochem. Photobiol. B 2000, 56, 2139–2144. [Google Scholar]
- Conde, F.R.; Churio, M.S.; Previtali, C.M. The deactivation pathways of the excited-states of the mycosporine-like amino acids shinorine and porphyra-334 in aqueous solution. Photochem. Photobiol. Sci. 2004, 3, 960–967. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.F.; Chou, H.N.; Sung, P.J. Porphyra-334 isolated from the marine algae Bangia atropurpurea: Conformational performance for energy conversion. Mar. Drugs 2014, 12, 4732–4740. [Google Scholar] [CrossRef] [PubMed]
- Kligfield, P.; Gettes, L.S.; Bailey, J.J.; Childers, R.; Deal, B.J.; Hancock, E.W.; Herpen, G.V.; Kors, J.A.; Macfarlane, P.; Mirvis, D.M.; et al. Recommendations for the standardization and interpretation of the electrocardiogram: Part I: the electrocardiogram and its technology a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society endorsed by the International Society for Computerized Electrocardiology. J. Am. Coll. Cardiol. 2007, 49, 1109–1127. [Google Scholar] [CrossRef] [PubMed]
- Volkmann, M.; Gorbushina, A.A. A broadly applicable method for extraction and characterization of mycosporines and mycosporine-like amino acids of terrestrial, marine and freshwater origin. FEMS Microbiol. Lett. 2006, 255, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Schmid, D.; Schürch, C.; Zülli, F. UVA-screening compounds from red algae protect against photoageing. Personal Care 2004, 1, 29–31. [Google Scholar]
- Izzo, J.L.J.R.; Larrabee, P.S.; Sander, E.; Lillis, L.M. Hemodynamics of seasonal adaptation. Am. J. Hypertens. 1990, 3, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Yosipovitch, G.; Xiong, G.L.; Haus, E.; Sackett-lundeen, L.; Ashkenazi, I.; Maibach, H.I. Time-dependent variations of the skin barrier function in humans: transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J. Invest. Dermatol. 1998, 110, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.M. Why we sleep. Sci. Am. 2003, 289, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Brück, K.; Zeisberger, E. Adaptive changes in thermoregulation and their neuropharmacological basis. Pharmacol. Ther. 1987, 35, 163–215. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A.; Kater, S.B. Neurotransmitter regulation of neuronal outgrowth, plasticity and survival. Trends Neurosci. 1989, 12, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Kiernan, M.C.; Vucic, S.; Cheah, B.C.; Turner, M.R.; Eisen, A.; Hardiman, O.; Burrell, J.R.; Zoing, M.C. Amyotrophic lateral sclerosis. Lancet 2011, 377, 942–955. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, L.-F.; Hsu, C.-K.; Chou, H.-N.; Chou, H.-S.; Sung, P.-J.; Chen, C.-C.; Chen, F.-G. The Counterbalance of Skin Metabolism on Orbits and Diseases. Med. Sci. 2015, 3, 25-37. https://doi.org/10.3390/medsci3020025
Chuang L-F, Hsu C-K, Chou H-N, Chou H-S, Sung P-J, Chen C-C, Chen F-G. The Counterbalance of Skin Metabolism on Orbits and Diseases. Medical Sciences. 2015; 3(2):25-37. https://doi.org/10.3390/medsci3020025
Chicago/Turabian StyleChuang, Li-Fan, Chin-Kong Hsu, Hong-Nong Chou, Hung-Shih Chou, Ping-Jyun Sung, Chia-Ching Chen, and Fu-Gin Chen. 2015. "The Counterbalance of Skin Metabolism on Orbits and Diseases" Medical Sciences 3, no. 2: 25-37. https://doi.org/10.3390/medsci3020025







