Screening the Resilience of Short-Rotation Woody Crops to Climate Change
Abstract
:1. Introduction
2. Methods
2.1. Study Area and Field Sampling
2.2. Sample Processing and Dendrochronological Measurements
2.3. Growth-Climate Analyses
2.4. Growth Projections under Future Climate Change Scenarios
3. Results
| Full-Sib Family | Genetic Acc. No. | Diameter (cm) | Height (m) | Basal Area (m2) |
|---|---|---|---|---|
| CAL_WEX | 56-6-77 | 35.2 | 22.6 | 0.0983 |
| LAK_MAR | 27-38-27 | 31.1 | 24.6 | 0.0772 |
| BRA_CLA | 4-9-10 | 30.0 | 23.4 | 0.0729 |
| GLA_GLA | 14-21-13 | 29.6 | 22.2 | 0.0689 |
| IOS_GLA | 24-33-13 | 28.5 | 21.2 | 0.0671 |
| VAN_IRO | 78-44-34 | 28.1 | 18.5 | 0.0651 |
| SAG_CHI | 76-40-15 | 28.4 | 21.2 | 0.0631 |
| MAR_CLA1 | 32-45-10 | 27.5 | 19.7 | 0.0591 |
| CAL_IRO | 57-6-34 | 27.1 | 21.0 | 0.0585 |
| WEX_BEN | 81-46-8 | 27.2 | 20.7 | 0.0585 |
| MAR_OAK | 33-45-32 | 26.4 | 17.1 | 0.0567 |
| CHI_KAL | 8-14-21 | 25.8 | 20.0 | 0.0533 |
| ROS_OAK | 44-71-32 | 25.4 | 17.8 | 0.0517 |
| MON_VAN | 70-31-73 | 22.4 | 16.8 | 0.0401 |
| GLA_CHI | 12-21-8 | 22.4 | 19.9 | 0.0393 |
| OGE_GLA | 73-33-22 | 20.8 | 15.5 | 0.0375 |
| MAR_ING | 28-43-16 | 19.3 | 16.7 | 0.0299 |
| MAR_CLA2 | 29-43-10 | 19.3 | 17.6 | 0.0294 |
| Mean All | -- | 26.3 | 19.8 | 0.0570 |
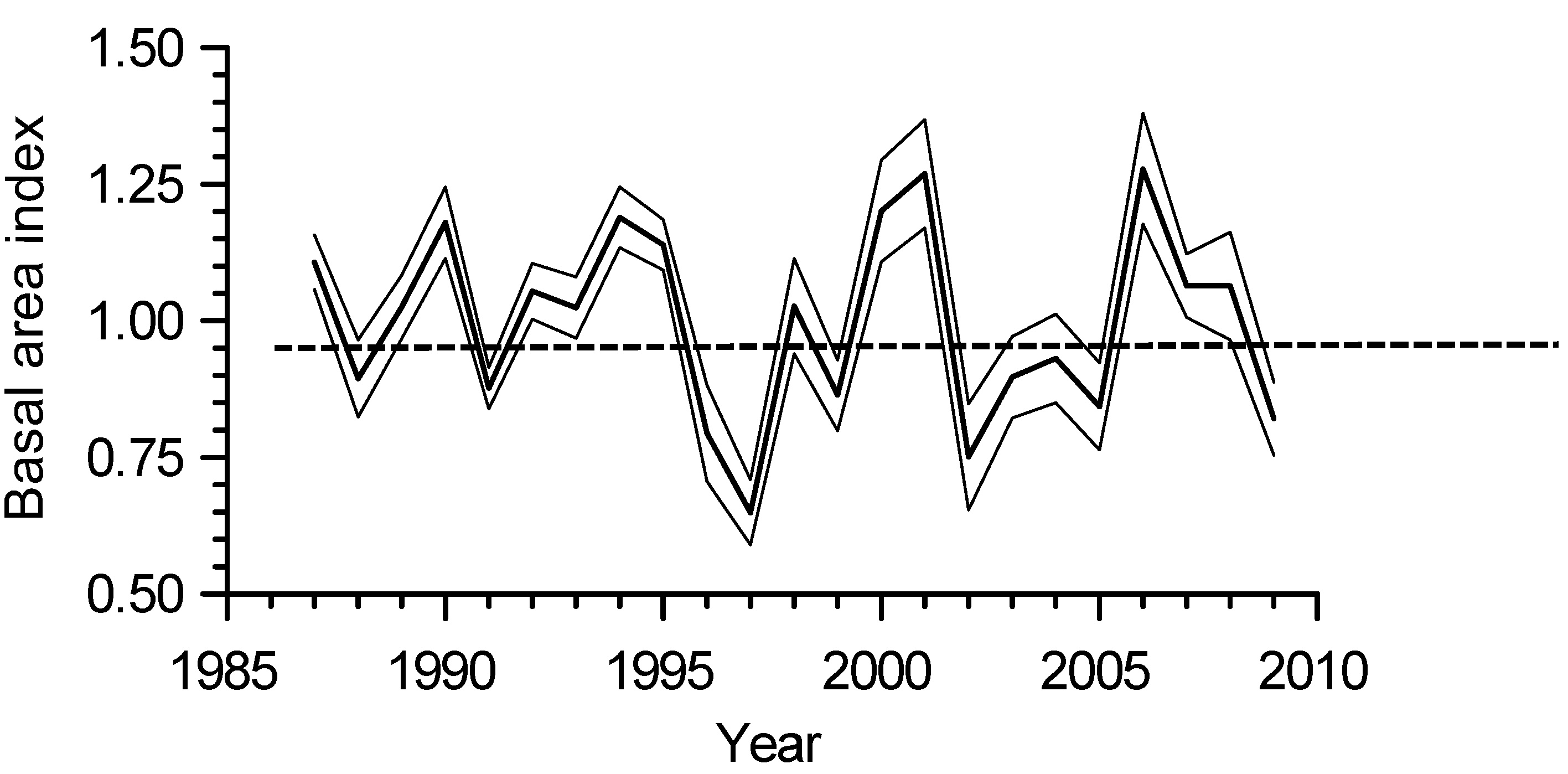
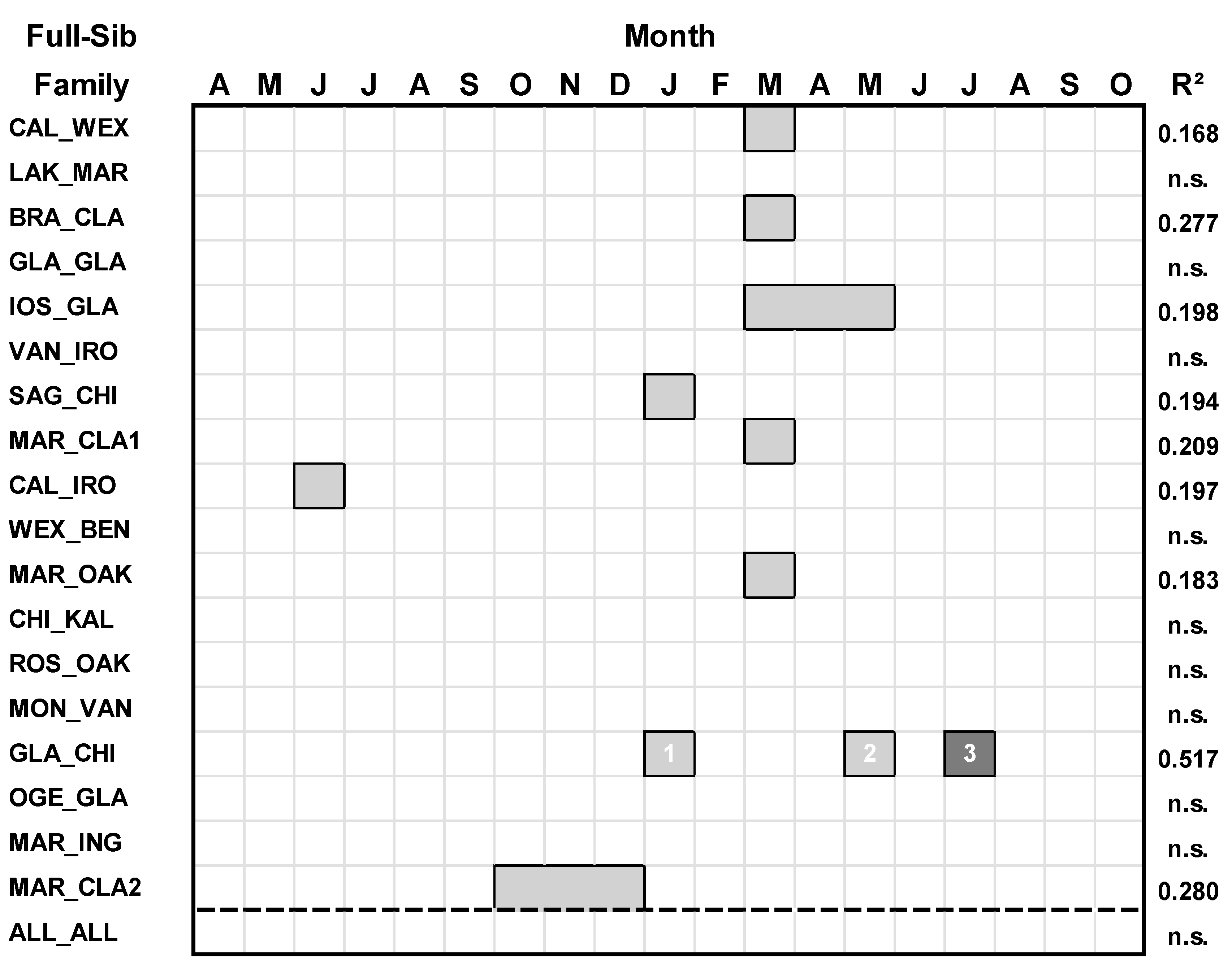
| Family | C | V1 | V2 | V3 | V4 |
|---|---|---|---|---|---|
| CAL_WEX | 0.987 | 0.455 (0.0494) | -- | -- | -- |
| LAK_MAR | n.s. | -- | -- | -- | -- |
| BRA_CLA | 0.992 | 0.558 (0.0661) | -- | -- | -- |
| GLA_GLA | n.s. | -- | -- | -- | -- |
| IOS_GLA | 0.984 | 0.486 (0.0825) | -- | -- | -- |
| VAN_IRO | n.s. | -- | -- | -- | -- |
| SAG_CHI | 0.951 | 0.482 (0.0381) | -- | -- | -- |
| MAR_CLA1 | 1.005 | 0.497 (0.0527) | -- | -- | -- |
| CAL_IRO | 0.968 | 0.485 (0.0892) | -- | -- | -- |
| WEX_BEN | n.s. | -- | -- | -- | -- |
| MAR_OAK | 0.975 | 0.471 (0.0671) | -- | -- | -- |
| CHI_KAL | n.s. | -- | -- | -- | -- |
| ROS_OAK | n.s. | -- | -- | -- | -- |
| MON_VAN | n.s. | -- | -- | -- | -- |
| GLA_CHI | 0.944 | 0.593 (0.0419) | −0.46 (0.0741) | 0.347 (0.0346) | 0.294 (0.0475) |
| OGE_GLA | n.s. | -- | -- | -- | -- |
| MAR_ING | n.s. | -- | -- | -- | -- |
| MAR_CLA2 | 0.983 | 0.56 (0.0695) | -- | -- | -- |
| ALL_ALL | n.s. | -- | -- | -- | -- |
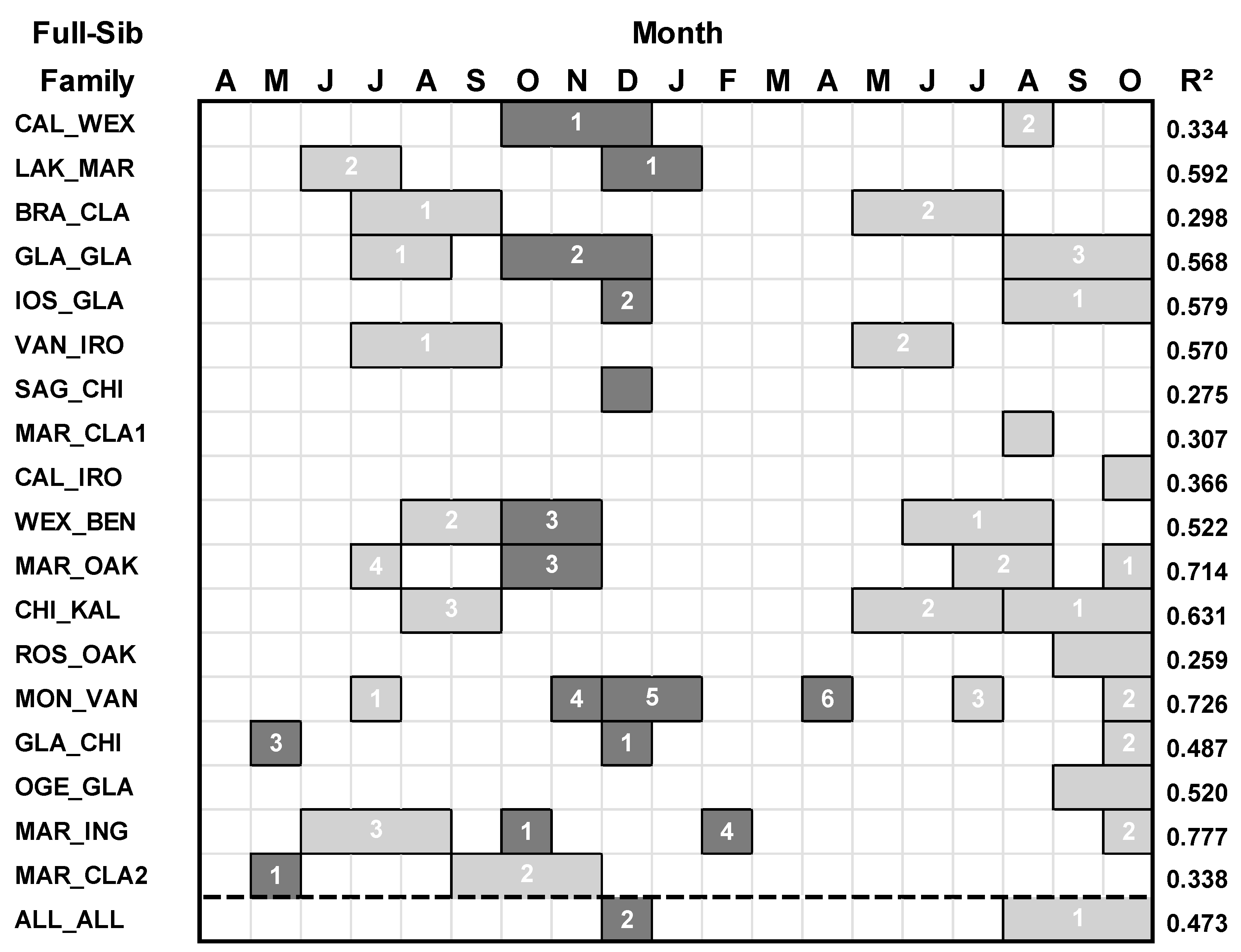
| Family | C | V1 | V2 | V3 | V4 | V5 | V6 |
|---|---|---|---|---|---|---|---|
| CAL_WEX | 0.985 | −0.45 (0.017) | 0.422 (0.0185) | -- | -- | -- | -- |
| LAK_MAR | 0.950 | −0.651 (0.0455) | 0.617 (0.0182) | -- | -- | -- | -- |
| BRA_CLA | 0.998 | 0.549 (0.0146) | 0.432 (0.0105) | -- | -- | -- | -- |
| GLA_GLA | 0.977 | 0.485 (0.0167) | −0.342 (0.013) | 0.325 (0.0078) | -- | -- | -- |
| IOS_GLA | 0.929 | 0.61 (0.0164) | −0.42 (0.0671) | -- | -- | -- | -- |
| VAN_IRO | 0.995 | 0.735 (0.0167) | 0.507 (0.0115) | -- | -- | -- | -- |
| SAG_CHI | 0.886 | −0.556 (0.0865) | -- | -- | -- | -- | -- |
| MAR_CLA1 | 1.028 | 0.583 (0.0249) | -- | -- | -- | -- | -- |
| CAL_IRO | 0.991 | 0.63 (0.0387) | -- | -- | -- | -- | -- |
| WEX_BEN | 1.007 | 0.515 (0.0117) | 0.481 (0.0117) | −0.307 (0.0117) | -- | -- | -- |
| MAR_OAK | 0.972 | 0.592 (0.0436) | 0.501 (0.0223) | −0.354 (0.0195) | 0.262 (0.0151) | -- | -- |
| CHI_KAL | 0.982 | 0.451 (0.0139) | 0.594 (0.0173) | 0.402 (0.0141) | -- | -- | -- |
| ROS_OAK | 1.016 | 0.542 (0.0123) | -- | -- | -- | -- | -- |
| MON_VAN | 0.926 | 0.569 (0.0384) | 0.582 (0.0309) | −0.306 (0.0262) | 0.323 (0.017) | −0.307 (0.0229) | −0.211 (0.0119) |
| GLA_CHI | 0.919 | −0.526 (0.073) | 0.384 (0.0212) | −0.364 (0.0121) | -- | -- | -- |
| OGE_GLA | 1.030 | 0.737 (0.0316) | -- | -- | -- | -- | -- |
| MAR_ING | 1.004 | 0.429 (0.0244) | −0.498 (0.0286) | 0.386 (0.0094) | −0.303 (0.0338) | -- | -- |
| MAR_CLA2 | 1.027 | −0.41 (0.0095) | 0.403 (0.0074) | -- | -- | -- | -- |
| ALL_ALL | 0.953 | 0.554 (0.0106) | −0.396 (0.0452) | -- | -- | -- | -- |
| Seasonal and Future Projection Period | Temperature (°C) | Climate Moisture Index (cm) |
|---|---|---|
| (a) Summer | ||
| 2011–2040 | 1.1 | 0.4 |
| 2041–2070 | 2.8 | −9.9 |
| 2071–2100 | 3.8 | −10.1 |
| (b) Winter | ||
| 2011–2040 | 1.8 | 2.9 |
| 2041–2070 | 3.6 | 0.2 |
| 2071–2100 | 4.4 | 2.1 |
| (c) Annual | ||
| 2011–2040 | 1.6 | 2.7 |
| 2041–2070 | 3.2 | −9.3 |
| 2071–2100 | 4.1 | −9.8 |
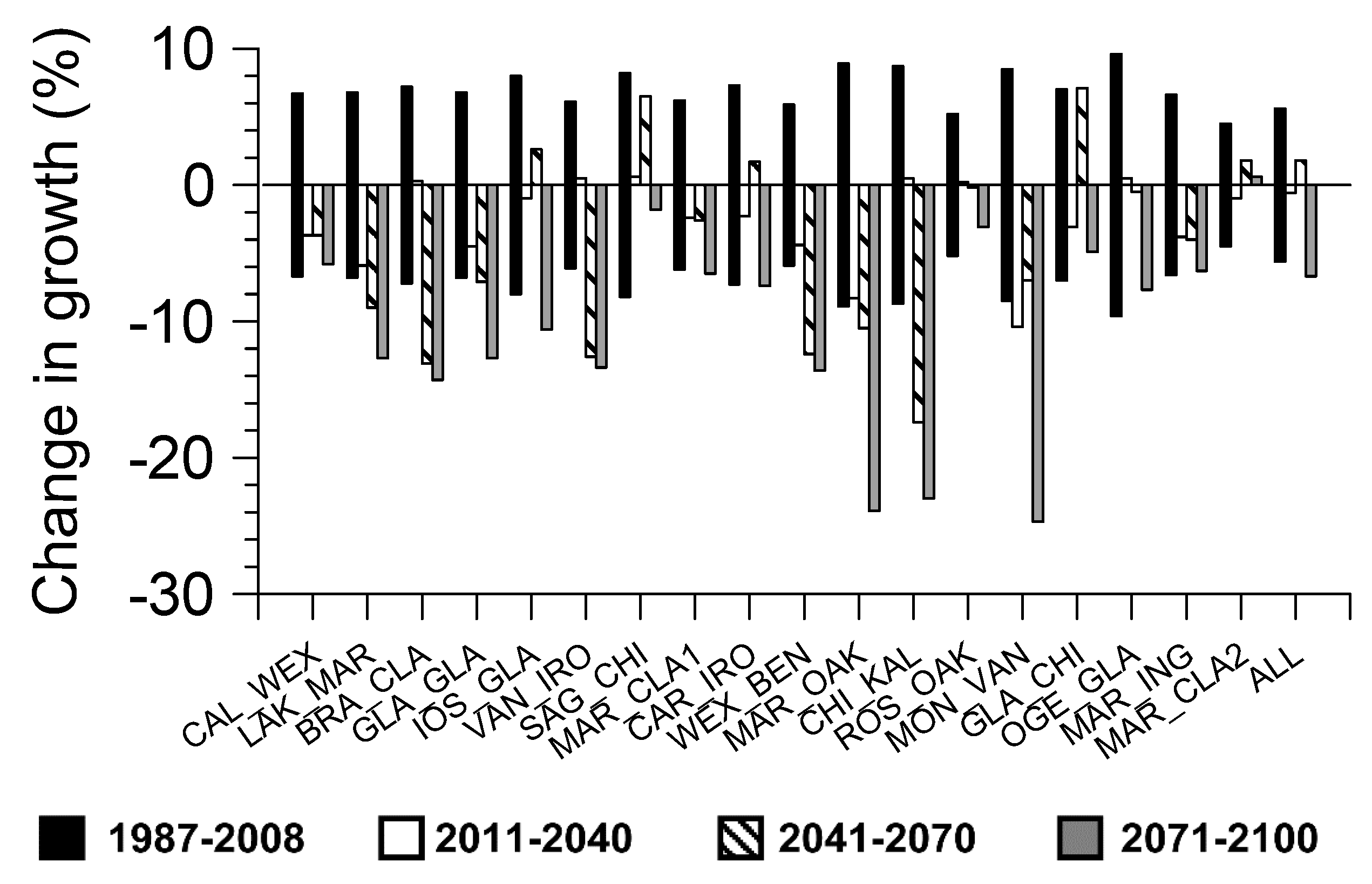
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Eriksson, H.M.; Hall, J.P.; Helynen, S. Rationale for forest energy production. In Bioenergy from Sustainable Forestry; Richardson, J., Bjorheden, R., Hakkila, P., Lowe, A.T., Smith, C.T., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 1–17. [Google Scholar]
- Ohlrogge, J.; Allen, D.; Berguson, B.; DellaPenna, D.; Shachar-Hill, Y.; Stymne, S. Driving on biomass. Science 2009, 324, 1019–1020. [Google Scholar] [CrossRef] [PubMed]
- Lasch, P.; Kollas, C.; Rock, J.; Suckow, F. Potentials and impacts of short-rotation coppice plantation with aspen in Eastern Germany under conditions of climate change. Reg. Environ. Chang. 2010, 10, 83–94. [Google Scholar] [CrossRef]
- Quinn, L.D.; Straker, K.C.; Guo, J.; Kim, S.; Thapa, S.; Kling, G.; Lee, D.K.; Voigt, T.B. Stress-tolerant feedstocks for sustainable bioenergy production on marginal land. Bioenerg. Res. 2015, 8, 1081–1100. [Google Scholar] [CrossRef]
- Calfapietra, C.; Gielen, B.; Karnosky, D.; Ceulemans, R.; Mugnozza, G.S. Response and potential of agroforestry crops under global change. Environ. Pollut. 2010, 158, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Dickmann, D.I. Silviculture and biology of short-rotation woody crops in temperature regions: Then and now. Biomass Bioenergy 2006, 30, 696–705. [Google Scholar] [CrossRef]
- Demeritt, M.E., Jr. Poplar Hybrids (Populus L.). Available online: http://www.na.fs.fed.us/spfo/pubs/silvics_manual/volume_2/populus/populus.htm (accessed on 1 August 2010).
- Riemenschneider, D.E.; Berguson, W.E.; Dickmann, D.I.; Hall, R.B.; Isebrands, J.G.; Mohn, C.A.; Stanosz, G.C.; Tuskan, G.A. Poplar breeding and testing strategies in the north-central U.S.: Demonstration of potential yield and consideration of future research needs. For. Chron. 2001, 77, 245–253. [Google Scholar] [CrossRef]
- Pliura, A.; Zhang, S.Y.; MacKay, J.; Bousquet, J. Genotypic variation in wood density and growth traits of poplar hybrids at four clonal trials. For. Ecol. Manag. 2007, 238, 92–106. [Google Scholar] [CrossRef]
- White, T.L.; Addams, W.T.; Neale, D.B. Forest Genetics; CABI Publishing: Oxfordshire, UK, 2007. [Google Scholar]
- Robison, D.; Raffa, K.F. Productivity, drought tolerance and pest status of hybrid Populus: Tree improvement and silvicultural implications. Biomass Bioenergy 1998, 14, 1–20. [Google Scholar] [CrossRef]
- Zalesny, R.S., Jr.; Hall, R.B.; Zalesny, J.A.; McMahon, B.G.; Berguson, W.E.; Stonosz, G.R. Biomass and genotype × environment interactions of Poplulus energy crops in the midwestern United States. Bioenergy Res. 2009, 2, 106–1222. [Google Scholar] [CrossRef]
- Zalesny, R.S., Jr.; Hall, R.B.; Bauer, E.O.; Riemenschneider, D.E. Soil temperature and precipitation affect the rooting ability of dormant hardwood cuttings of Populus. Silvae Genet. 2005, 54, 47–58. [Google Scholar]
- Monclus, R.; Dreyer, E.; Villar, M.; Delmotte, F.M.; Delay, D.; Petit, J.-M.; Barbaroux, C.; le Thiec, D.; Brechet, C.; Brignolas, F. Impact of drought on productivity and water use efficiency in 29 genotypes of Populus deltoides × Populus nigra. New Phytol. 2006, 169, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, A.; Deslauriers, A.; Fragnelli, G.; Scaletti, L.; Castro, G.; Rossi, S.; Crivellaro, A. Evaluation of drought response of two poplar clones through high resolution analysis of stem growth. J. Exp. Bot. 2007, 58, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Monclus, R.; Villar, M.; Barbaroux, C.; Bastien, C.; Fichot, R.; Delmotte, F.M.; Delay, D.; Petit, J.-M.; Brechet, C.; Dreyer, E. Productivity, water-use efficiency and tolerance to moderate water deficit correlate in 33 poplar genotypes from a Populus deltoides × Populus trichocarpa F1 progeny. Tree Physiol. 2009, 29, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Pulkkinen, P.; Vaario, L.-M.; Koivuranta, L.; Stenvall, N. Elevated temperature effects on germination and early growth of European aspen (Populus tremula), hybrid aspen (P. tremula × P. tremuloides) and their F2-hybrids. Eur. J. For. Res. 2013, 132, 791–800. [Google Scholar] [CrossRef]
- Headlee, W.L.; Zalesny, R.S., Jr.; Donner, D.M.; Hall, R.B. Using a process-based model (3-PG) to predict and map hybrid poplar biomass productivity in Minnesota and Wisconsin, USA. Bioenergy Res. 2013, 6, 196–210. [Google Scholar] [CrossRef]
- Vaganov, E.A.; Hughes, M.K.; Shashkin, A.V. Growth Dynamics of Conifer Tree Rings: Images of Past and Future Environments; Ecological Studies 183; Springer-Verlag: Berlin, Germany, 2006. [Google Scholar]
- Fritts, H.C. Tree Rings and Climate; Academic Press: London, UK, 1976. [Google Scholar]
- Chhin, S.; Hogg, E.H.; Lieffers, V.J.; Huang, S. Potential effects of climate change on the growth of lodgepole pine across diameter size classes and ecological regions. For. Ecol. Manag. 2008, 256, 1692–1703. [Google Scholar] [CrossRef]
- Hogg, E.H.; Brandt, J.P.; Michaelian, M. Impacts of a regional drought on the productivity, dieback, and biomass of western Canadian aspen forests. Can. J. For. Res. 2008, 38, 1373–1384. [Google Scholar] [CrossRef]
- Leonelli, G.; Denneler, B.; Bergeron, Y. Climate sensitivity of trembling aspen radial growth along a productivity gradient in northeastern British Columbia, Canada. Can. J. For. Res. 2008, 38, 1211–1222. [Google Scholar] [CrossRef]
- Huang, J.; Tardif, J.C.; Bergeron, Y.; Denneler, B.; Berninger, F.; Girardin, M.P. Radial growth response of four dominant boreal tree species to climate along a latitudinal gradient in the eastern Canadian boreal forest. Glob. Chang. Biol. 2009, 16, 711–731. [Google Scholar] [CrossRef]
- Chhin, S. Influence of climate on the growth of hybrid poplar in Michigan. Forests 2010, 1, 209–229. [Google Scholar] [CrossRef]
- Pawson, S.M.; Brin, A.; Brockerhoff, E.G.; Lamb, D.; Payn, T.W.; Paquette, A.; Parrotta, J.A. Plantation forests, climate change and biodiversity. Biodivers. Conserv. 2013, 22, 1203–1227. [Google Scholar] [CrossRef]
- Zalesny, R.S., Jr.; Headlee, W.L. Developing woody crops for the enhancement of ecosystem services under changing climates in the north central United States. J. For. Environ. Sci. 2015, 31, 78–90. [Google Scholar] [CrossRef]
- Laidly, P.R. Bigtooth Aspen (Populus grandidentata Michx.). Available online: http://www.na.fs.fed.us/pubs/silvics_manual/volume_2/populus/grandidentata.htm (accessed on 3 December 2015).
- Perala, D.A. Quaking Aspen (Populus tremuloides Michx.). Available online: http://www.na.fs.fed.us/pubs/silvics_manual/volume_2/populus/tremuloides.htm (accessed on 3 December 2015).
- Reighard, G.L. Physiological Genetics Studies of Populus Grandidentata, Populus Tremuloides, and Their Hybrid, Populus X smithii. Ph.D. Thesis, Michigan State University, East Lansing, MI, USA, 1984. [Google Scholar]
- Reighard, G.L.; Hanover, J.W. Progeny testing of native aspens and their hybrids for biomass production in Michigan. In Tree Improvement and Genetics—Northeastern Forest Tree Improvement Conference—1985; West Virginia University: Morgantown, WV, USA, 1985; pp. 5–22. [Google Scholar]
- National Climatic Data Center. Monthly Suface Data; National Climatic Data Center: Asheville, NC, USA, 2010. [Google Scholar]
- Stokes, M.A.; Smiley, T.L. An Introduction to Tree-Ring Dating; The University of Arizona Press: Tuscon, AZ, USA, 1996. [Google Scholar]
- Yamaguchi, D.K. A simple method for cross-dating increment cores from living trees. Can. J. For. Res. 1991, 21, 414–416. [Google Scholar] [CrossRef]
- Metsaranta, J.M.; Lieffers, V.J. Using dendrochronology to obtain annual data for modelling stand development: A supplement to permanent sample plots. Forestry 2009, 82, 163–173. [Google Scholar] [CrossRef]
- Hogg, E.H. Temporal scaling of moisture and the forest-grassland boundary in western Canada. Agric. For. Meteorol. 1997, 84, 115–122. [Google Scholar] [CrossRef]
- Jones, P.D.; Hulme, M. Calculating regional climatic time series for temperature and precipitation: methods and illustrations. Int. J. Clim. 1996, 16, 361–377. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S, 4th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2014. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer-Verlag: New York, NY, USA, 2002; p. 488. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Upper Saddle River, NI, USA, 1999; p. 663. [Google Scholar]
- Flato, G.M.; Boer, G.J. Warming asymmetry in climate change simulations. Geophys. Res. Lett. 2001, 28, 195–198. [Google Scholar] [CrossRef]
- Intergovernmental Panel On Climate Change. Climate Change 2007: The Physical Science Basis; IPCC WGI Fourth Assessment Report; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Magruder, M.; Chhin, S.; Palik, B.; Bradford, J.B. Thinning increases climatic resilience of red pine. Can. J. For. Res. 2013, 43, 878–889. [Google Scholar] [CrossRef]
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chhin, S. Screening the Resilience of Short-Rotation Woody Crops to Climate Change. Geosciences 2016, 6, 7. https://doi.org/10.3390/geosciences6010007
Chhin S. Screening the Resilience of Short-Rotation Woody Crops to Climate Change. Geosciences. 2016; 6(1):7. https://doi.org/10.3390/geosciences6010007
Chicago/Turabian StyleChhin, Sophan. 2016. "Screening the Resilience of Short-Rotation Woody Crops to Climate Change" Geosciences 6, no. 1: 7. https://doi.org/10.3390/geosciences6010007






