Establishing a Model for Evaluating Chicken Coccidiosis Resistance Based on Principal Component Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Coccidiosis Challenge
2.3. Detection of Resistance-Associated Parameters
2.4. Statistical Analysis
2.4.1. Principal Component Analysis
2.4.2. Correlation Analysis
3. Results
3.1. The Selection of Valid Resistant Parameters
3.2. Contribution Rate and the Cumulative Contribution Rate of the Eigenvalues
3.3. Establishing the Coccidiosis Resistance Evaluation Model
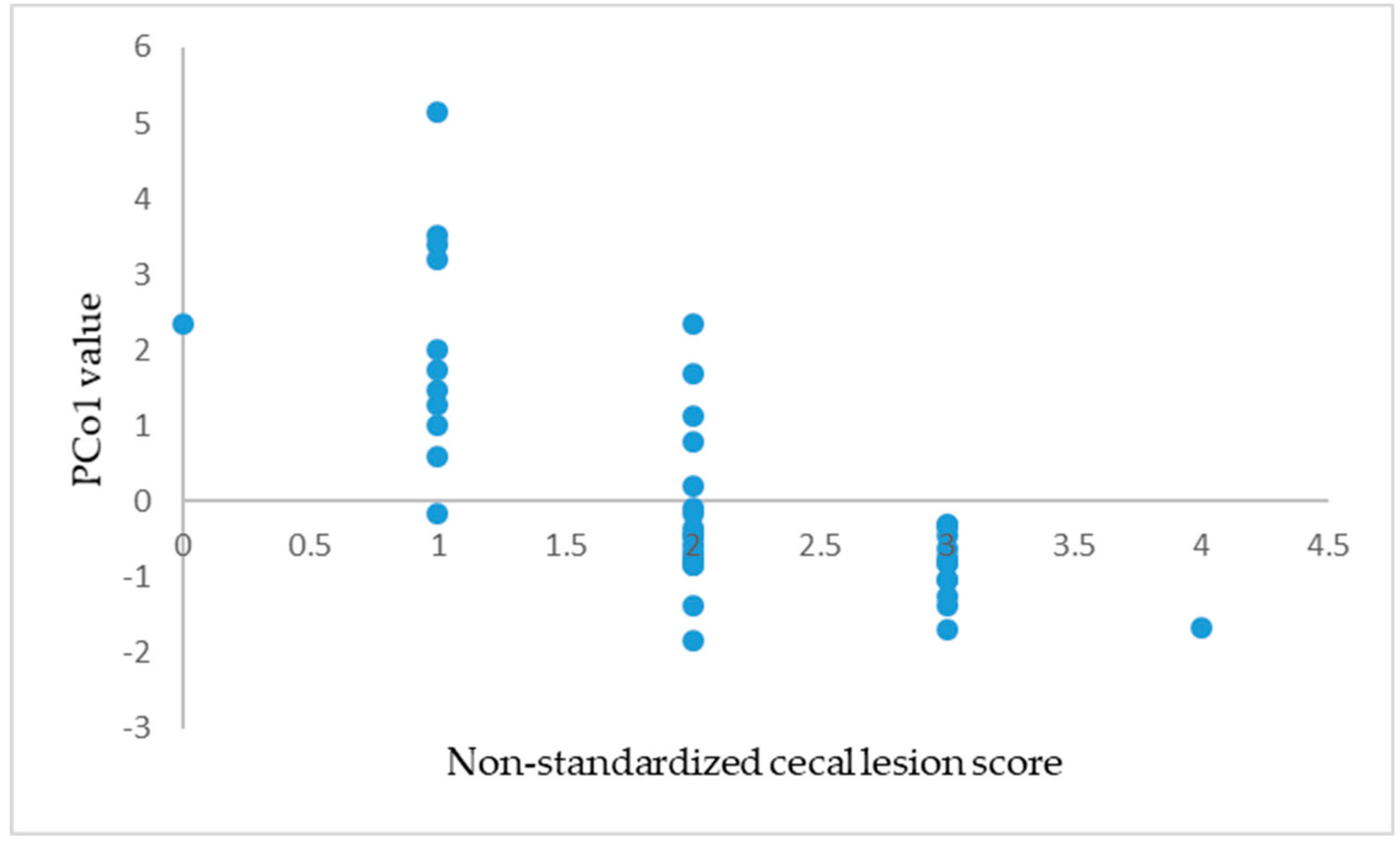
3.4. The Selection of the Optimal PCo
4. Discussion
4.1. The Selection of Resistance Parameters
4.2. The Advantage of PCA for the Selective Breeding of Coccidiosis Resistance
4.3. The Selection and Evaluation of the Optimized Model
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ojimelukwe, A.E.; Emedhem, D.E.; Agu, G.O.; Nduka, F.O.; Abah, A.E. Populations of Eimeria tenella express resistance to commonly used anticoccidial drugs in Southern Nigeria. Int. J. Vet. Sci. Med. 2018, 6, 192–200. [Google Scholar] [CrossRef]
- Vermeulen, A.N.; Schaap, D.C.; Schetters, T.P. Control of coccidiosis in chickens by vaccination. Vet. Parasitol. 2001, 100, 13–20. [Google Scholar] [CrossRef]
- Williams, R.B. Epidemiological aspects of the use of live anticoccidial vaccines for chickens. Int. J. Parasitol. 1998, 28, 1089–1098. [Google Scholar] [CrossRef]
- Suo, X.; Zhang, J.X.; Li, Z.G.; Yang, C.T.; Min, Q.R.; Xu, L.T.; Liu, Q.; Zhu, X.Q. The efficacy and economic benefits of Supercox, a live anticoccidial vaccine in a commercial trial in broiler chickens in China. Vet. Parasitol. 2006, 142, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Dalloul, R.A.; Lillehoj, H.S. Poultry coccidiosis: Recent advancements in control measures and vaccine development. Expert Rev. Vaccin. 2006, 5, 143–163. [Google Scholar] [CrossRef] [PubMed]
- McDonald, V.; Shirley, M.W. Past and future: Vaccination against Eimeria. Parasitology 2009, 136, 1477–1489. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Colwell, D.D.; Gilleard, J. Botanicals: An alternative approach for the control of avian coccidiosis. Worlds Poult. Sci. J. 2012, 68, 203–215. [Google Scholar] [CrossRef]
- Song, X.; Xu, L.; Yan, R.; Huang, X.; Li, X. Construction of Eimeria tenella multi-epitope DNA vaccines and their protective efficacies against experimental infection. Vet. Immunol. Immunopathol. 2015, 166, 79–87. [Google Scholar] [CrossRef]
- Kim, E.S.; Hong, Y.H.; Lillehoj, H.S. Genetic effects analysis of myeloid leukemia factor 2 and T cell receptor-beta on resistance to coccidiosis in chickens. Poult. Sci. 2010, 89, 20–27. [Google Scholar] [CrossRef]
- Zhu, J.J.; Lillehoj, H.S.; Allen, P.C.; Yun, C.H.; Pollock, D.; Sadjadi, M.; Emara, M.G. Analysis of disease resistance-associated parameters in broiler chickens challenged with Eimeria maxima. Poult. Sci. 2000, 79, 619–625. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, Q.P.; Wu, X.Z.; Chen, Z.G.; Chen, Y.J.; Shi, T.W.; Ye, M.Z. Studies on drug resistance in field isolates of coccidia from chickens in Shanghai. Chin. J. Vet. Parasitol. 2001, 9, 1–7. [Google Scholar]
- Morehouse, N.F.; Baron, R.R. Coccidiosis: Evaluation of coccidiostats by mortality, weight gains, and fecal scores. Exp. Parasitol. 1970, 28, 25–29. [Google Scholar] [CrossRef]
- Allen, P.C.; Lillehoj, H.S. Genetic influence on nitric oxide production during Eimeria tenella infections in chickens. Avian Dis. 1998, 42, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Meister, A. Glutathione deficiency produced by inhibition of its synthesis, and its reversal; applications in research and therapy. Pharmacol. Ther. 1991, 51, 155–194. [Google Scholar] [CrossRef]
- LI, J.; Wang, H.; Stoner, G.D.; Bray, T.M. Dietary supplementation with cysteine prodrugs selectively restores tissue glutathione levels and redox status in protein-malnourished mice (1). J. Nutr. Biochem. 2002, 13, 625–633. [Google Scholar] [CrossRef]
- Georgieva, N.V.; Koinarski, V.; Gadjeva, V. Antioxidant status during the course of Eimeria tenella infection in broiler chickens. Vet. J. 2006, 172, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.S.; Choi, K.D. Recombinant chicken interferon-gamma-mediated inhibition of Eimeria tenella development in vitro and reduction of oocyst production and body weight loss following Eimeria acervulina challenge infection. Avian Dis. 1998, 42, 307–314. [Google Scholar] [CrossRef]
- Bumstead, N.; Millard, B. Genetics of resistance to coccidiosis: Response of inbred chicken lines to infection by Eimeria tenella and Eimeria maxima. Br. Poult. Sci. 1987, 28, 705–715. [Google Scholar] [CrossRef]
- Bacciu, N.; Bed’Hom, B.; Filangi, O.; Romé, H.; Gourichon, D.; Répérant, J.-M.; Le Roy, P.; Laan, M.-H.; Demeure, O. QTL detection for coccidiosis (Eimeria tenella) resistance in a Fayoumi × Leghorn F2 cross, using a medium-density SNP panel. Genet. Sel. Evol. 2014, 46, 14. [Google Scholar] [CrossRef]
- Laan, M.H.P.; Monvoisin, J.L.; Pery, P.; Hamet, N.; Thomas, M. Comparison of outbred lines of chickens for resistance to experimental infection with coccidiosis (Eimeria tenella). Poult. Sci. 1998, 77, 185–191. [Google Scholar] [CrossRef]
- Lillehoj, H.S. Influence of inoculation dose, inoculation schedule, chicken age, and host genetics on disease susceptibility and development of resistance to Eimeria tenella infection. Avian Dis. 1988, 32, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Lillehoj, H.S.; Ruff, M.D. Comparison of disease susceptibility and subclass-specific antibody response in SC and FP chickens experimentally inoculated with Eimeria tenella, E. acervulina, or E. maxima. Avian Dis. 1987, 31, 112–119. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Poultry: Ninth Revised Edition; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Johnson, J.; Reid, W.M. Anticoccidial drugs: Lesion scoring techniques in battery and floor-pen experiments with chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- Swaggerty, C.L.; Pevzner, I.Y.; Kogut, M.H. Selection for pro-inflammatory mediators produces chickens more resistant to Eimeria tenella. Poult. Sci. 2015, 94, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Q.; Kanu, S.; Xiang, F.Y.; Xiao, S.M.; Zhang, L.; Chen, H.W.; Ye, H.J. Isolation and selection of ionophore-tolerant Eimeria precocious lines: E. tenella, E. maxima and E. acervulina. Vet. Parasitol. 2004, 119, 261–276. [Google Scholar] [CrossRef] [PubMed]
- McDougald, L.R.; Fuller, L.; Solis, J. Drug-sensitivity of 99 isolates of coccidia from broiler farms. Avian Dis. 1986, 30, 690–694. [Google Scholar] [CrossRef]
- Williams, R.B.; Catchpole, J. A new protocol for a challenge test to assess the efficacy of live anticoccidial vaccines for chickens. Vaccine 2000, 18, 1178–1185. [Google Scholar] [CrossRef]
- Li, J.; Gu, W.; Tao, J.; Liu, Z. The effects of S-nitroso-glutathione on the activities of some isoenzymes in Eimeria tenella oocysts. Vet. Parasitol. 2009, 162, 236–240. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Y.; Dai, G.; An, T.; Sun, M.; Zhang, G.; Xie, K.; Wang, J. Selecting Eimeria tenella-resistance markers by comparing resistant and susceptible groups of Jinghai Yellow chickens. Turk. J. Vet. Anim. Sci. 2016, 40, 764–768. [Google Scholar] [CrossRef]
- Pearson, K. LIII. on lines and planes of closest fit to systems of points in space. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1901, 2, 559–572. [Google Scholar] [CrossRef]
- Lemistre, M.; Balageas, D. Structural health monitoring system based on diffracted Lamb wave analysis by multiresolution processing. Smart Mater. Struct. 2001, 10, 504–511. [Google Scholar] [CrossRef]
- Lam, K.C.; Hu, T.S.; Ng, S.T. Using the principal component analysis method as a tool in contractor pre-qualification. Constr. Manag. Econ. 2005, 23, 673–684. [Google Scholar] [CrossRef]
- Ye, Z.S. The improvement method of existing problems in principal component analysis. Statistics. Inform. Forum. 2004, 2, 29–31. [Google Scholar]
- Fu, R. Discussion of integrated evaluation models of the principal component analysis. Syst. Eng. Theory Pract. 2001, 21, 68–74. [Google Scholar]
| Traits (g) | Control Group (n = 22) | Infected Group (n = 44) | t-Value | Probability |
|---|---|---|---|---|
| IBW * (0 dPI) | 215.79 ± 6.02 | 215.42 ± 4.69 | ||
| IBW (3 dPI) | 242.91 ± 6.65 | 241.98 ± 5.45 | ||
| IBW (5 dPI) | 252.15 ± 6.89 | 240.96 ± 5.43 | ||
| IBW (8 dPI) | 266.88 ± 8.12 | 255.69 ± 5.65 | ||
| BWG ** 0–3 | 27.13 ± 1.21 | 26.55 ± 0.75 | 0.155 | 0.877 |
| BWG3–5 | 9.25 ± 0.44 | −1.03 ± 0.10 | 3.062 | 0.004 ** |
| BWG5–8 | 14.75 ± 2.33 | 14.72 ± 2.81 | 0.032 | 0.995 |
| BWG0–8 | 51.00 ± 2.41 | 40.30 ± 3.07 | 1.970 | 0.054 |
| Resistance Traits | Control Group (n = 22) | Infected Group (n = 44) | t-Value | Probability |
|---|---|---|---|---|
| Nitric oxide (NO) (µmol/L) | 55.49 ± 1.23 | 54.10 ± 0.80 | 0.853 | 0.398 |
| catalase (CAT) (U/L) | 54.26 ± 2.32 | 63.28 ± 1.66 | 2.744 | 0.008 ** |
| superoxide dismutase (SOD) (U/L) | 106.87 ± 3.29 | 120.60 ± 2.85 | 2.558 | 0.014 * |
| glutathione peroxidase (GSH-Px) (U/L) | 396.31 ± 11.88 | 455.72 ± 11.01 | 2.902 | 0.005 ** |
| malondialdehyde aldehyde (MDA) (mmol/L) | 4.75 ± 0.20 | 5.86 ± 0.15 | 3.789 | 0.001 ** |
| γ-interferon (IFN-γ) (ng/L) | 38.88 ± 1.08 | 35.89 ± 0.69 | 2.100 | 0.041 * |
| β-carotene (β-C) (μmol/L) | 60.56 ± 5.53 | 65.49 ± 4.04 | 0.620 | 0.538 |
| interleukin-2 (IL-2) (ng/L) | 35.31 ± 1.14 | 33.05 ± 0.87 | 1.335 | 0.188 |
| interleukin-16 (IL-16) (ng/L) | 47.88 ± 2.39 | 52.84 ± 1.19 | 1.842 | 0.071 |
| interleukin-17 (IL-17) (ng/L) | 40.78 ± 0.96 | 38.70 ± 1.22 | 0.960 | 0.341 |
| Cecal lesion score | 0.00 ± 0.00 | 1.98 ± 0.11 | 10.975 | 0.000 ** |
| No. of PCo | Eigenvalue (λi) | Contribution Rate (%) | Cumulative Rate (%) |
|---|---|---|---|
| PCo 1 | 1.497 | 24.956 | 24.956 |
| PCo 2 | 1.194 | 19.905 | 44.861 |
| PCo 3 | 1.123 | 18.714 | 63.575 |
| PCo 4 | 0.963 | 16.058 | 79.633 |
| PCo 5 | 0.740 | 12.335 | 91.968 |
| PCo 6 | 0.482 | 8.032 | 100.000 |
| Standardized Traits | Eigenvectors of Principal Components | |||||
|---|---|---|---|---|---|---|
| BWG3–5 (zx1) | −0.636 | 0.296 | 0.177 | 0.417 | 0.498 | 0.234 |
| CAT (zx2) | 0.311 | −0.478 | 0.069 | 0.792 | −0.004 | −0.207 |
| SOD (zx3) | 0.801 | 0.277 | −0.183 | 0.187 | −0.005 | 0.461 |
| GSH-Px (zx4) | −0.046 | −0.349 | 0.857 | −0.082 | −0.246 | 0.272 |
| MDA (zx5) | −0.076 | 0.779 | 0.250 | 0.290 | −0.461 | −0.166 |
| IFN-γ (zx6) | 0.588 | 0.269 | 0.505 | −0.192 | 0.469 | −0.266 |
| No. of PCo | Expression Functions of Each Principal Component Model |
|---|---|
| PCo 1 | |
| PCo 2 | |
| PCo 3 | |
| PCo 4 | |
| PCo 5 | |
| PCo 6 | |
| Comprehensive PCo |
| Standardized Traits | PCo 1 | PCo 2 | PCo 3 | PCo 4 | PCo 5 | PCo 6 | Comprehensive PCo |
|---|---|---|---|---|---|---|---|
| Cecal lesion score ▲ | −0.539 ** | 0.075 | 0.036 | 0.036 | −0.138 | −0.029 | −0.368 |
| BWG0–3 | 0.510 ** | −0.200 | −0.024 | −0.151 | −0.291 | −0.032 | 0.142 |
| BWG3–5 | −0.635 ** | 0.237 | 0.191 | 0.415 ** | 0.468 ** | 0.205 | −0.021 |
| BWG5–8 | 0.426 ** | −0.037 | −0.176 | −0.193 | −0.115 | −0.034 | 0.022 |
| BWG0–8 | 0.340 * | 0.096 | −0.014 | −0.048 | 0.156 | 0.072 | 0.097 |
| NO | 0.512 ** | −0.011 | 0.302 | −0.240 | 0.006 | 0.016 | 0.261 |
| CAT | 0.326 * | −0.464 ** | 0.012 | 0.756 ** | −0.001 | −0.185 | −0.225 |
| SOD | 0.776 ** | 0.171 | −0.192 | 0.247 | 0.061 | 0.459 ** | 0.552 ** |
| GSH-Px | −0.047 | −0.371 * | 0.784 ** | −0.153 | −0.281 | 0.267 | 0.332 * |
| MDA | −0.076 | 0.779 ** | 0.250 | 0.290 | −0.461 ** | −0.166 | 0.109 |
| IFN-γ | 0.521 ** | 0.222 | 0.513 ** | −0.196 | 0.445 ** | −0.353 * | −0.065 |
| β-C | −0.001 | −0.089 | 0.013 | 0.163 | 0.263 | −0.209 | −0.223 |
| IL-2 | −0.065 | 0.049 | −0.069 | 0.004 | −0.069 | −0.274 | −0.302 |
| IL-16 | −0.107 | −0.189 | 0.007 | 0.160 | −0.153 | 0.074 | −0.086 |
| IL-17 | 0.218 | −0.127 | −0.039 | −0.292 | −0.154 | −0.150 | −0.011 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zou, W.; Yu, H.; Wang, X.; Dai, G.; Sun, M.; Zhang, G.; Zhang, T.; Shi, H.; Xie, K.; Wang, J. Establishing a Model for Evaluating Chicken Coccidiosis Resistance Based on Principal Component Analysis. Animals 2019, 9, 926. https://doi.org/10.3390/ani9110926
Zou W, Yu H, Wang X, Dai G, Sun M, Zhang G, Zhang T, Shi H, Xie K, Wang J. Establishing a Model for Evaluating Chicken Coccidiosis Resistance Based on Principal Component Analysis. Animals. 2019; 9(11):926. https://doi.org/10.3390/ani9110926
Chicago/Turabian StyleZou, Wenbin, Hailiang Yu, Xiaohui Wang, Guojun Dai, Mingming Sun, Genxi Zhang, Tao Zhang, Huiqiang Shi, Kaizhou Xie, and Jinyu Wang. 2019. "Establishing a Model for Evaluating Chicken Coccidiosis Resistance Based on Principal Component Analysis" Animals 9, no. 11: 926. https://doi.org/10.3390/ani9110926




