Use of a Non-Penetrating Captive Bolt for Euthanasia of Neonate Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
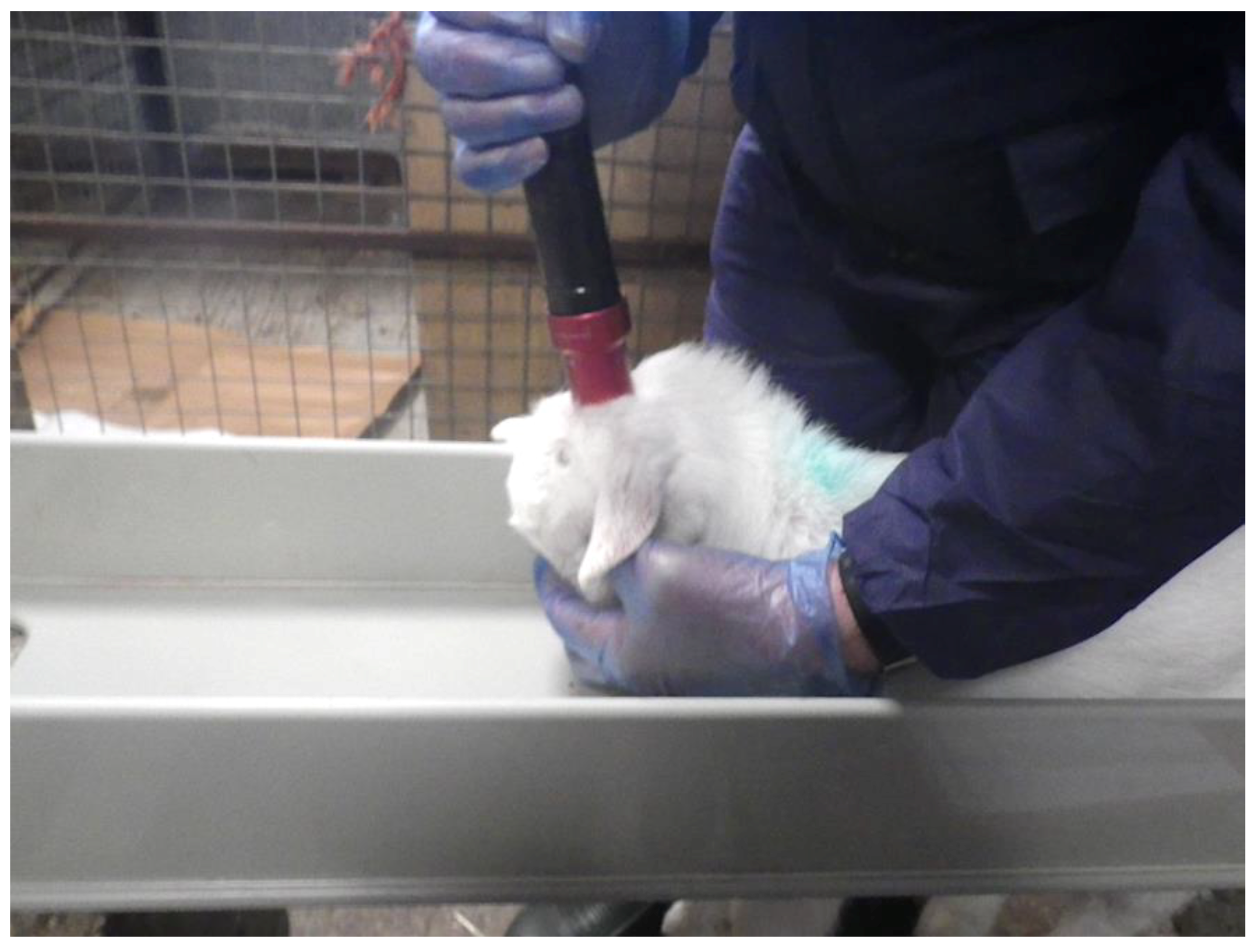
2.1. Experimental Animals
- Rhythmic breathing. This is controlled by the medulla in the brainstem and is the most reliable indicator of a stun, and if it does not return, cortical brain death (stun/kill).
- Positive corneal reflex, a brainstem reflex.
- Positive palpebral reflex, another brainstem reflex.
- Response to a painful stimulus (e.g., needle prick to the nose).
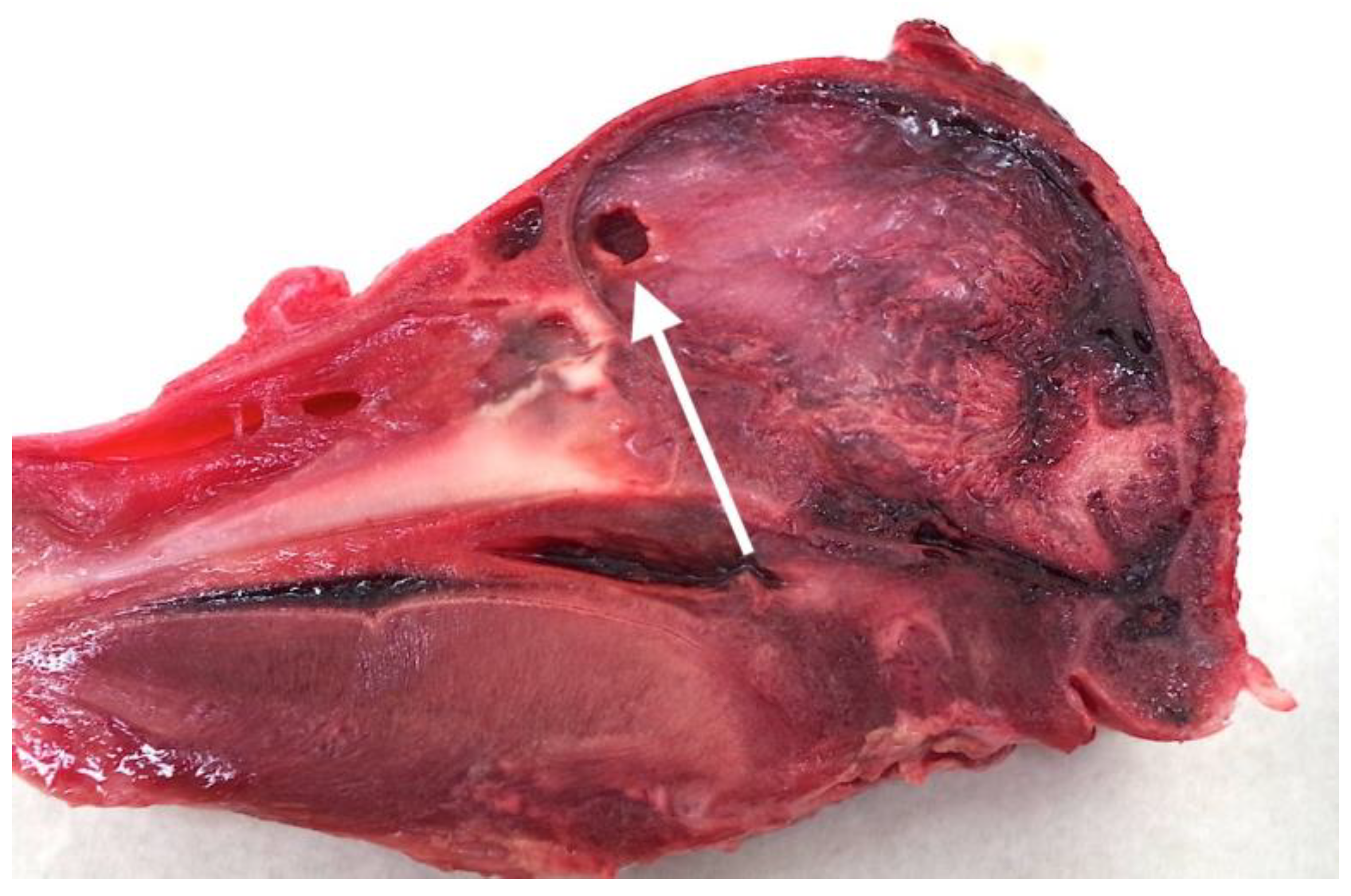
2.2. Post Mortem Examination of Heads
2.3. Statistical Analysis
3. Results
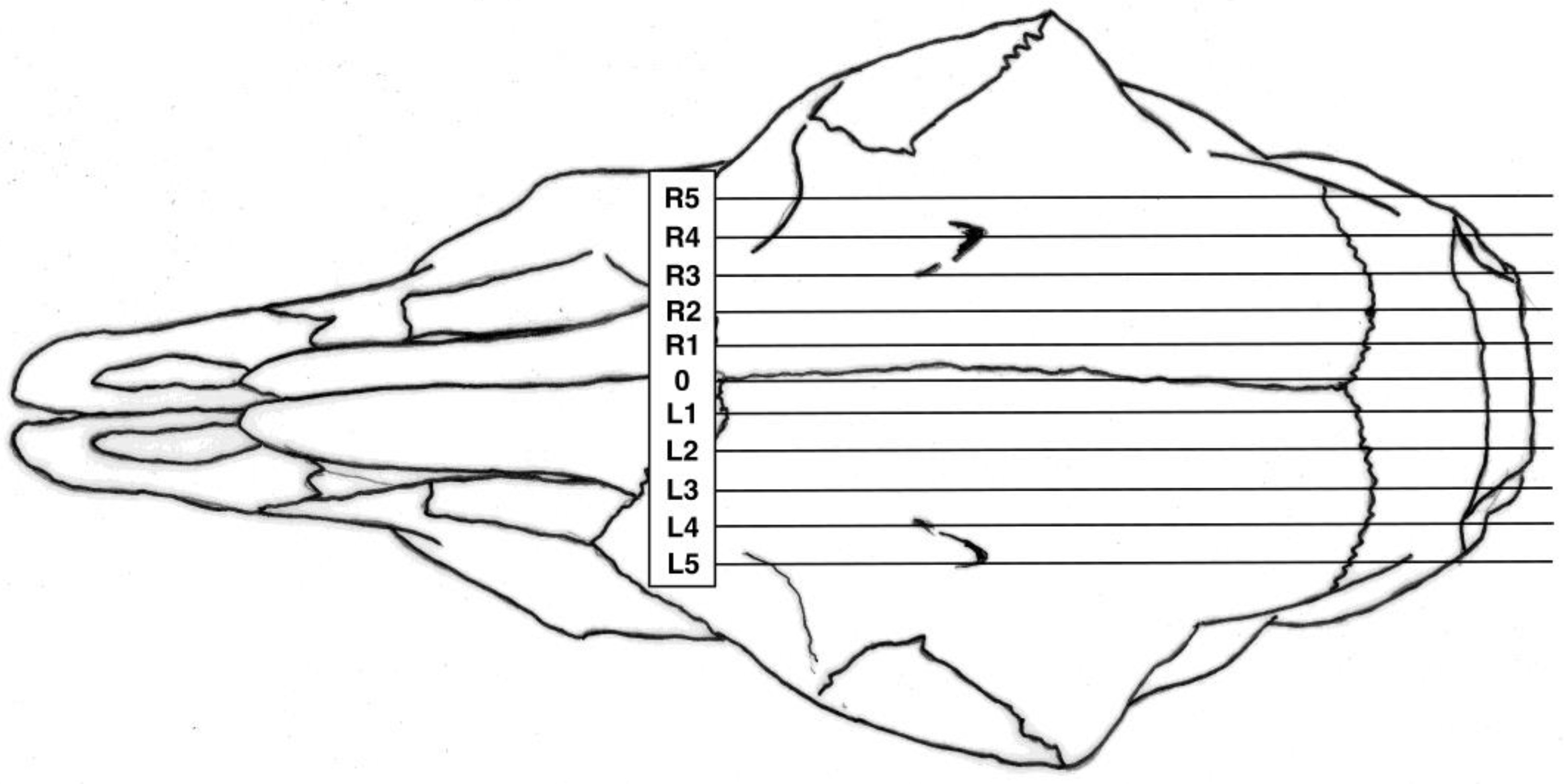
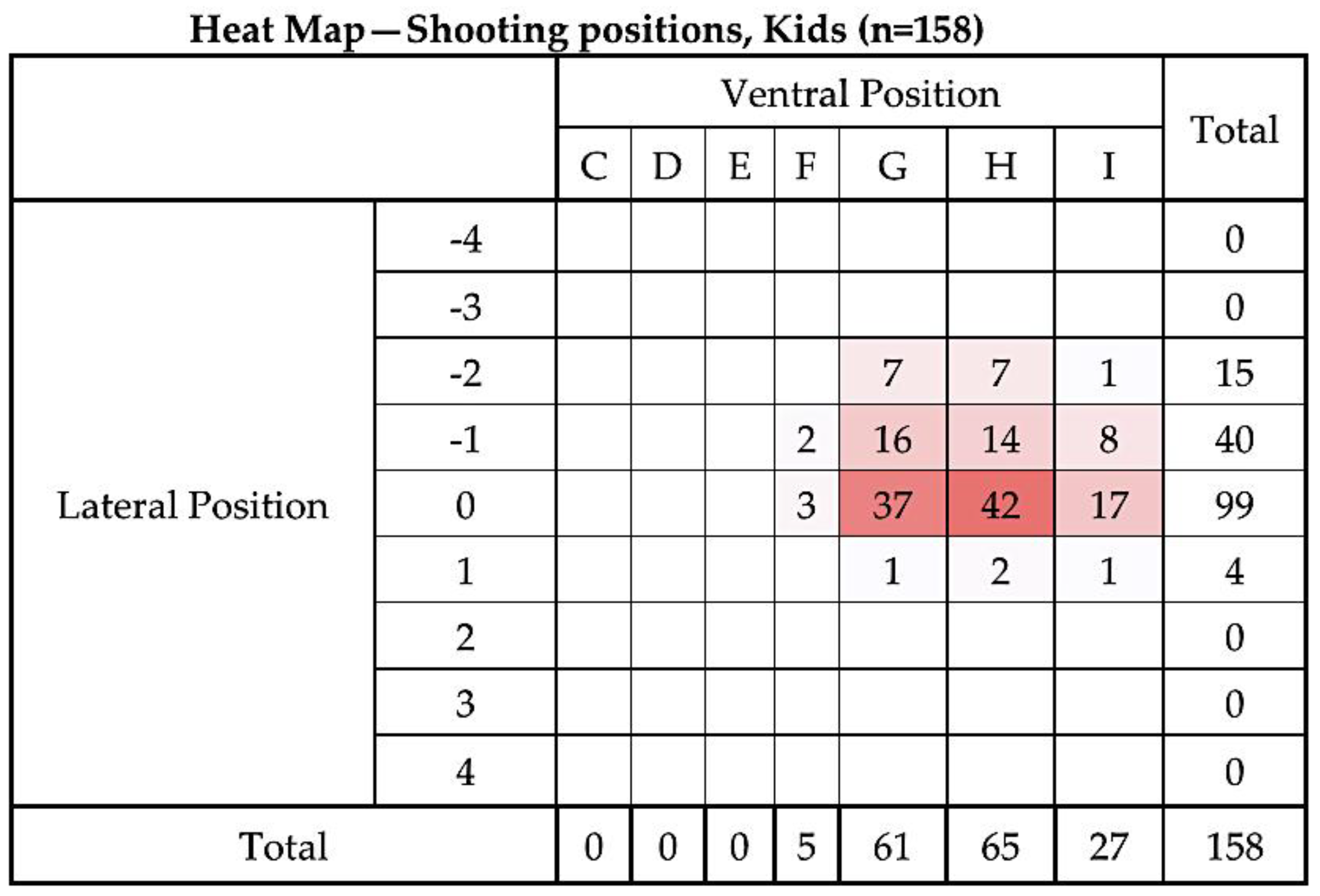
3.1. Shot Position
3.2. Factors Related to ‘Time to Loss of Movement’
3.3. Factors Related to ‘Movement Score’
4. Discussion
4.1. Animal Movement Post Shot
4.2. Shooting Position
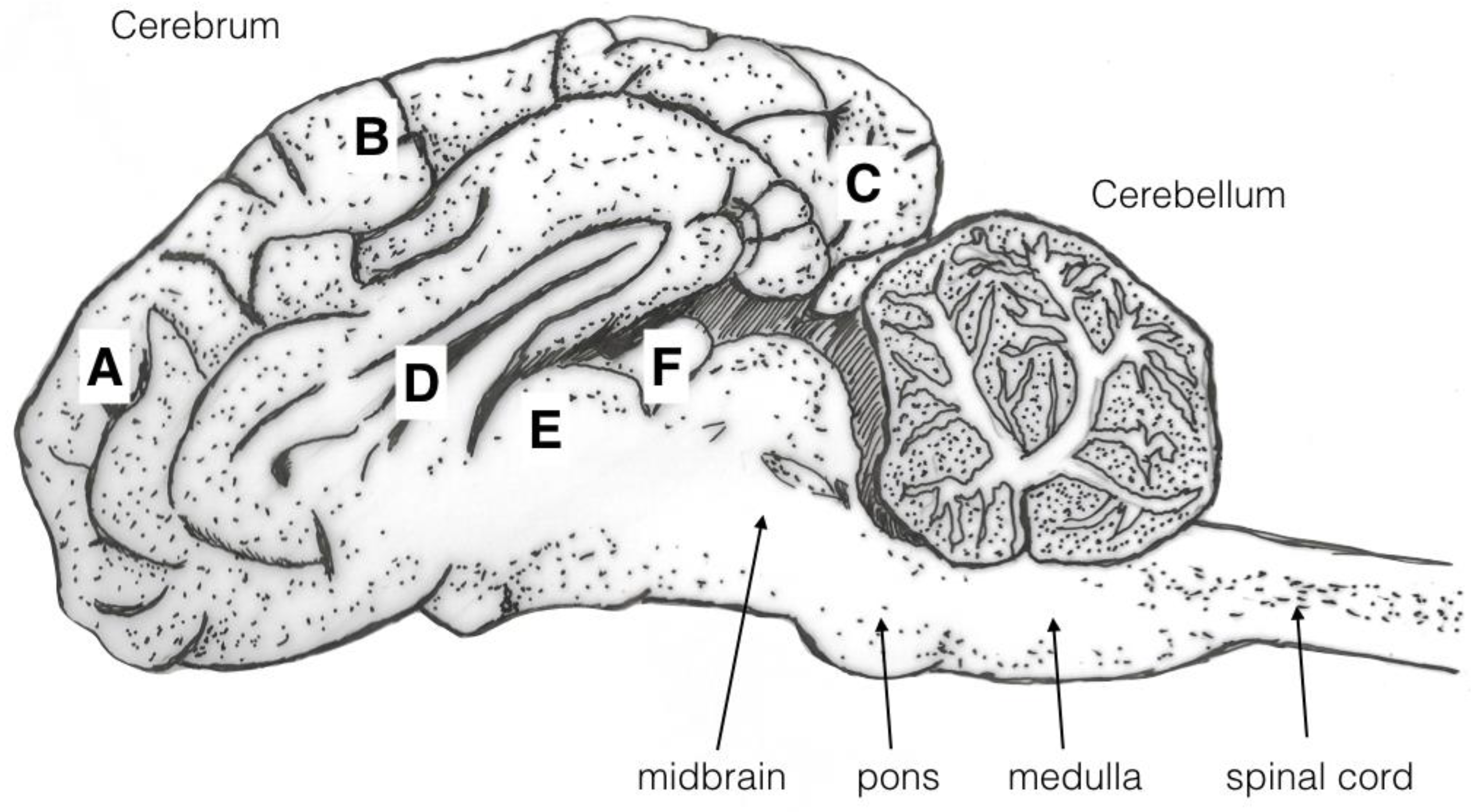
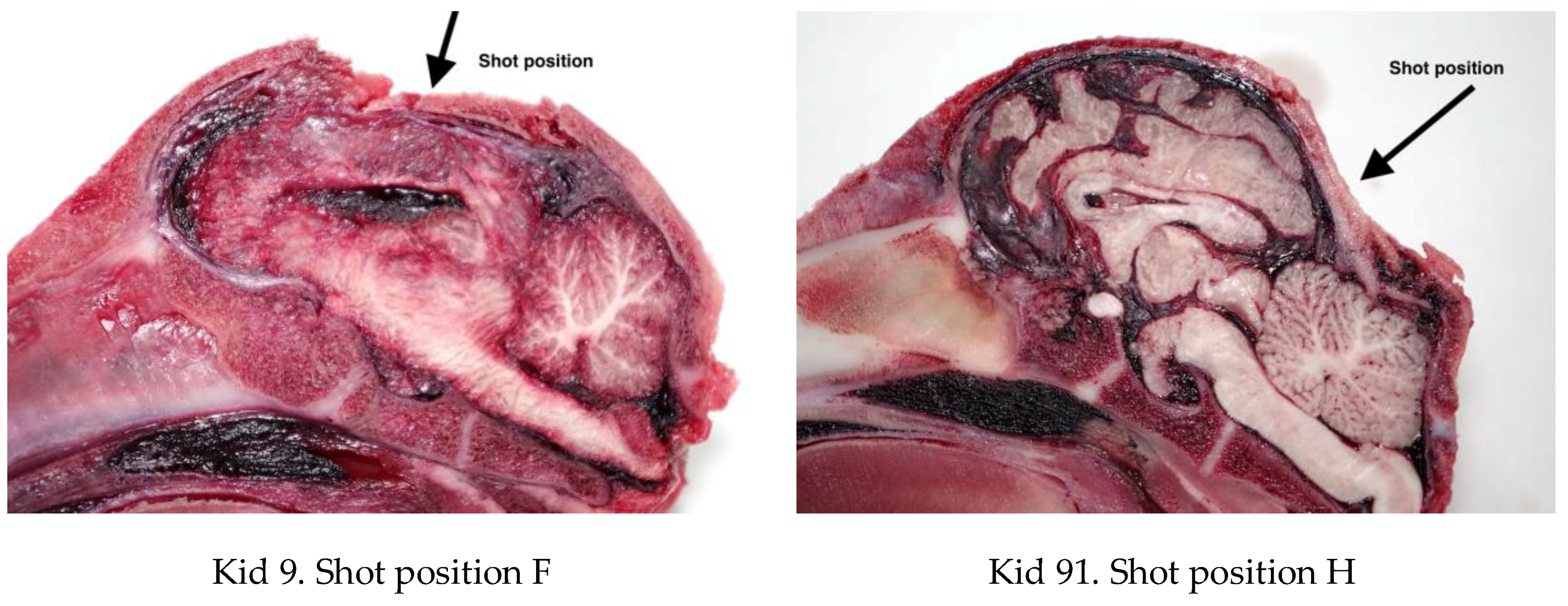
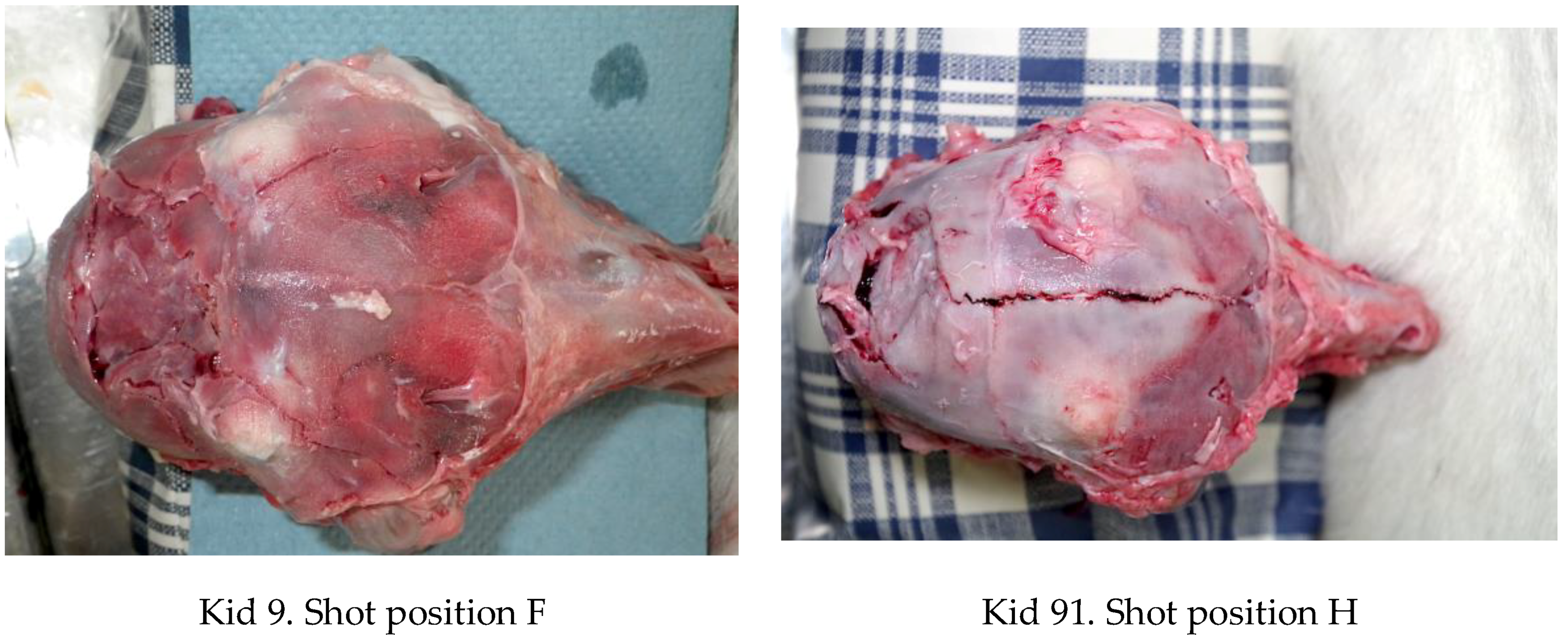
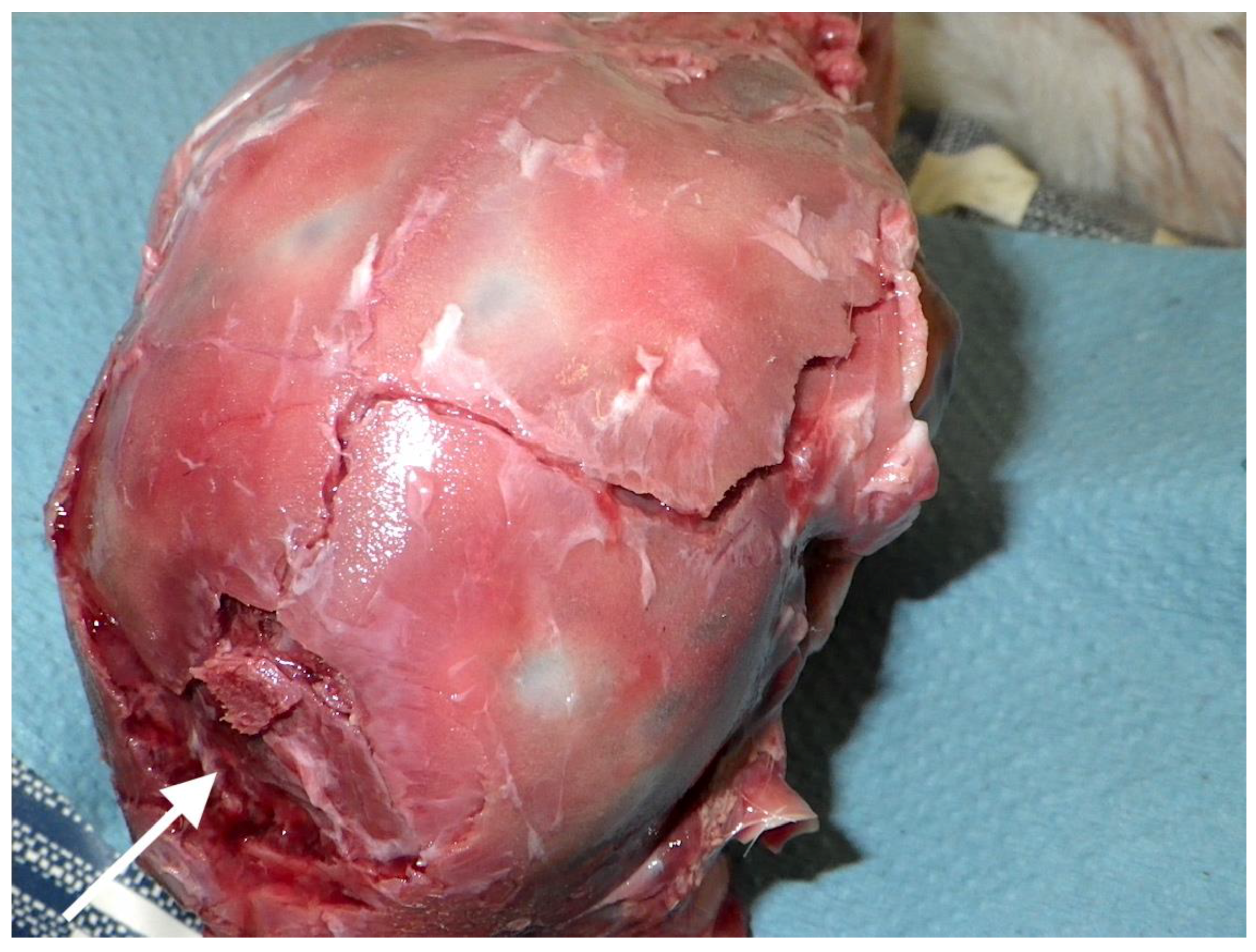
4.3. Brain Damage
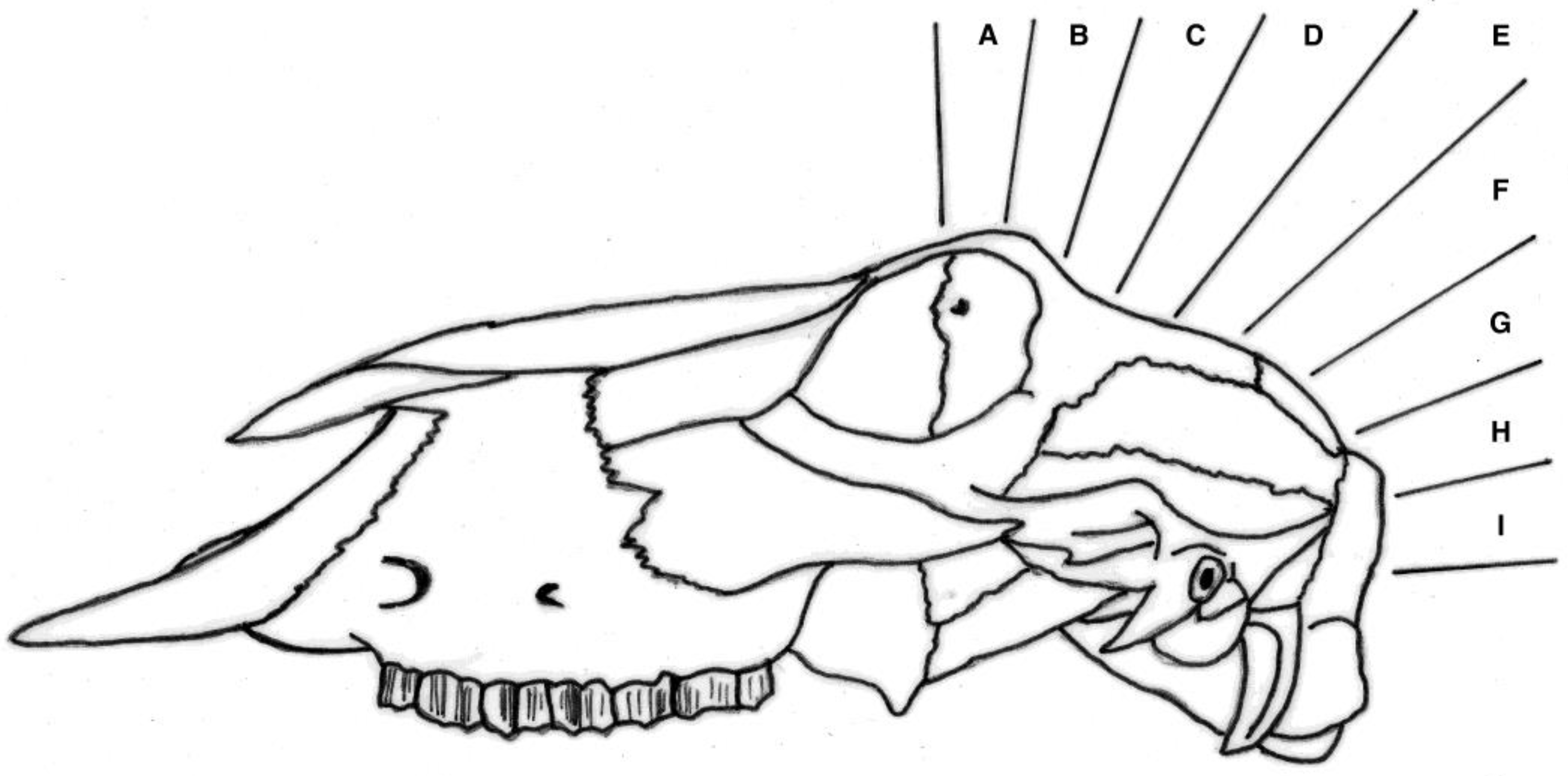
4.4. Fracture Pattern
4.5. Agonal Breathing
4.6. Pronecephaly
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Farm Animal Welfare Council (FAWC UK) 2007 Report on Stockmanship and Farm Animal Welfare. Available online: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/325176/FAWC_report_on_stockmanship_and_farm_animal_welfare.pdf (accessed on 6 January 2018).
- Mort, M.; Convery, I.; Baxter, J.; Bailey, C. Animal disease and human trauma: The psychosocial implications of the 2001 UK foot and mouth disease disaster. J. Appl. Anim. Welf. 2008, 11, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Whiting, T.L.; Marion, C.R. Perpetration-induced traumatic stress—A risk for veterinarians involved in the destruction of healthy animals. Can. Vet. J. 2011, 52, 794–796. [Google Scholar] [PubMed]
- Matthis, J.S. Selected Employee Attributes and Perceptions Regarding Methods and Animal Welfare Concerns Associated with Swine Euthanasia. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2004. [Google Scholar]
- Accles & Shelvoke Ltd., Unit 5a, Maybrook Business Park, Maybrook Road, Sutton Coldfield, B76 1AL. Available online: http://www.acclesandshelvoke.co.uk/media/downloads/2017/August/user-manuals/CASH-Small-Animal-Tool-JUN-2017-V3.2.pdf (accessed on 12 August 2017).
- Sutherland, M. Evaluation of a non-penetrating captive bolt to euthanase neonatal goat kids. In Proceedings of the HSA International Symposium, Zagreb, Croatia, 16–17 July 2015. [Google Scholar]
- Sutherland, M.A.; Watson, T.J.; Johnson, C.B.; Millman, S.T. Evaluation of the efficacy of a non-penetrating captive bolt to euthanase neonatal goats up to 48 hours of age. Anim. Welf. 2016, 25, 471–479. [Google Scholar] [CrossRef]
- Bock Industries, Inc. (BI), 156 Bock Lane, Philipsburg, PA 16866, USA. Available online: http://www.bock-industries.com (accessed on 16 August 2017).
- European Union. Council Regulation (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing; Official Journal of the European Union L303/1-30; European Union: Brussels, Belgium, 2009. [Google Scholar]
- Grist, A.; Lines, J.A.; Knowles, T.G.; Wotton, S.B. The use of a non-penetrating captive bolt for the Euthanasia for neonate piglets. Animals 2018, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Grist, A.; Knowles, T.G.; Wotton, S.B. Humane Euthanasia of Neonates II: Field study of the effectiveness of the Zephyr EXL Non-Penetrating Captive Bolt system for euthanasia of new-born and weaned piglets. Anim. Welf. 2018. under review. [Google Scholar]
- Hewitt, L. The Development of a Novel Device for Humanely Dispatching Casualty Poultry. Ph.D. Thesis, University of Bristol, Bristol, UK, 2000. [Google Scholar]
- Sharp, T.M.; McLeod, S.R.; Leggett, K.E.A.; Gibson, T.J. Evaluation of a spring-powered captive bolt gun for killing kangaroo pouch young. Wildl. Res. 2014, 41, 623–632. [Google Scholar] [CrossRef]
- Grist, A.; Murrell, J.; McKinstry, J.; Knowles, T.G.; Wotton, S.B. Humane Euthanasia of Neonates I: Validation of the Effectiveness of the Zephyr EXL Non-Penetrating Captive Bolt Euthanasia System on Neonate Piglets up to 10.9 kg Liveweight. Anim. Welf. 2017, 26, 111–120. [Google Scholar] [CrossRef]
- Altman, D.G.; Machin, D.; Bryant, T.N.; Gardner, M.J. Statistics with Confidence, 2nd ed.; BMJ Books: London, UK, 2000; ISBN 0727913751. [Google Scholar]
- Kinney, C. Heat Maps. Registration #75263259; United States Patent and Trademark Office: Alexandria, VA, USA, 1998. [Google Scholar]
- Gregory, N.G. The Assessment of Welfare and the Scientific Aspects. In Proceedings of the World Congress on Alternatives and Animal Use in the Life Sciences, Baltimore, MD, USA, 26 September 1993. [Google Scholar]
- Blackmore, D.K. Differences in behavior between sheep and cattle during slaughter. Res. Vet. Sci. 1984, 37, 223–226. [Google Scholar] [PubMed]
- Tidswell, S.J.; Blackmore, D.K.; Newhook, J.C. Slaughter methods: Electroencephalographs (EEG) studies on spinal cord section, decapitation and gross trauma of the brain in lambs. N. Z. Vet. J. 1987, 35, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Terlouw, C.E.M.; Bourguet, C.; Deiss, V.; Mallet, C. Origins of movements following stunning and during bleeding in cattle. Meat Sci. 2015, 110, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Terlouw, C.E.M.; Bourguet, C.; Deiss, V. Consciousness, unconsciousness and death in the context of slaughter. Part I. Neurobiological mechanisms underlying stunning and killing. Meat Sci. 2016, 118, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Terlouw, C.E.M.; Bourguet, C.; Deiss, V. Consciousness, unconsciousness and death in the context of slaughter. Part II Evaluation Methods Meat Sci. 2016, 118, 147–156. [Google Scholar] [PubMed]
- Summers, B.A.; Cummings, J.F.; de Lahunta, A. Veterinary Neuropathology; Mosby: St. Louis, MO, USA, 1995; ISBN 0801650631. [Google Scholar]



| Score | Descriptor | Description |
|---|---|---|
| 0 | No activity | Very little movement |
| 1 | Mild activity | Some mild uncontrolled physical movement of limbs |
| 2 | Moderate activity | Considerable uncontrolled physical movement of the limbs |
| 3 | Severe | Gross uncontrolled physical movement |
| Parameter | B | S.E. | t | Sig. |
|---|---|---|---|---|
| Intercept | 41.695 | 30.529 | 1.366 | 0.174 |
| Dead weight (kg) | −0.052 | 3.094 | −0.017 | 0.986 |
| Total damage score | −1.296 | 3.054 | −0.424 | 0.672 |
| Total haemorrhage score | −2.054 | 2.081 | −0.987 | 0.325 |
| Lateral position score | −1.978 | 3.895 | −0.508 | 0.612 |
| Ventral position score | 7.960 | 3.648 | 2.182 | 0.031 |
| Parameter | B | S.E. | t | Sig. |
|---|---|---|---|---|
| Intercept | 1.447 | 0.474 | 3.049 | 0.003 |
| Dead weight (kg) | 0.404 | 0.048 | 8.411 | ≤0.001 |
| Total damage score | −0.015 | 0.047 | −0.313 | 0.755 |
| Total haemorrhage score | 0.008 | 0.032 | 0.239 | 0.811 |
| Lateral position score | 0.083 | 0.061 | 1.373 | 0.172 |
| Ventral position score | −0.098 | 0.057 | −1.737 | 0.084 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grist, A.; Lines, J.A.; Knowles, T.G.; Mason, C.W.; Wotton, S.B. Use of a Non-Penetrating Captive Bolt for Euthanasia of Neonate Goats. Animals 2018, 8, 58. https://doi.org/10.3390/ani8040058
Grist A, Lines JA, Knowles TG, Mason CW, Wotton SB. Use of a Non-Penetrating Captive Bolt for Euthanasia of Neonate Goats. Animals. 2018; 8(4):58. https://doi.org/10.3390/ani8040058
Chicago/Turabian StyleGrist, Andrew, Jeff A. Lines, Toby G. Knowles, Charles W. Mason, and Stephen B. Wotton. 2018. "Use of a Non-Penetrating Captive Bolt for Euthanasia of Neonate Goats" Animals 8, no. 4: 58. https://doi.org/10.3390/ani8040058





