Evaluation of Different Gases and Gas Combinations for On-Farm Euthanasia of Pre-Weaned Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study 1: Physiological and Behavioural Response to Gas Euthansia
2.1.1. Physiological Measures
2.1.2. Behavioural Measures
2.2. Study 2: Electrophysiological Response to Gas Euthansia
EEG and ECG Analysis
2.3. Statistical Analysis
3. Results
3.1. Study 1: Physiological and Behavioural Response to Gas Euthansia
3.1.1. Physiological Measures
3.1.2. Behavioural Measures
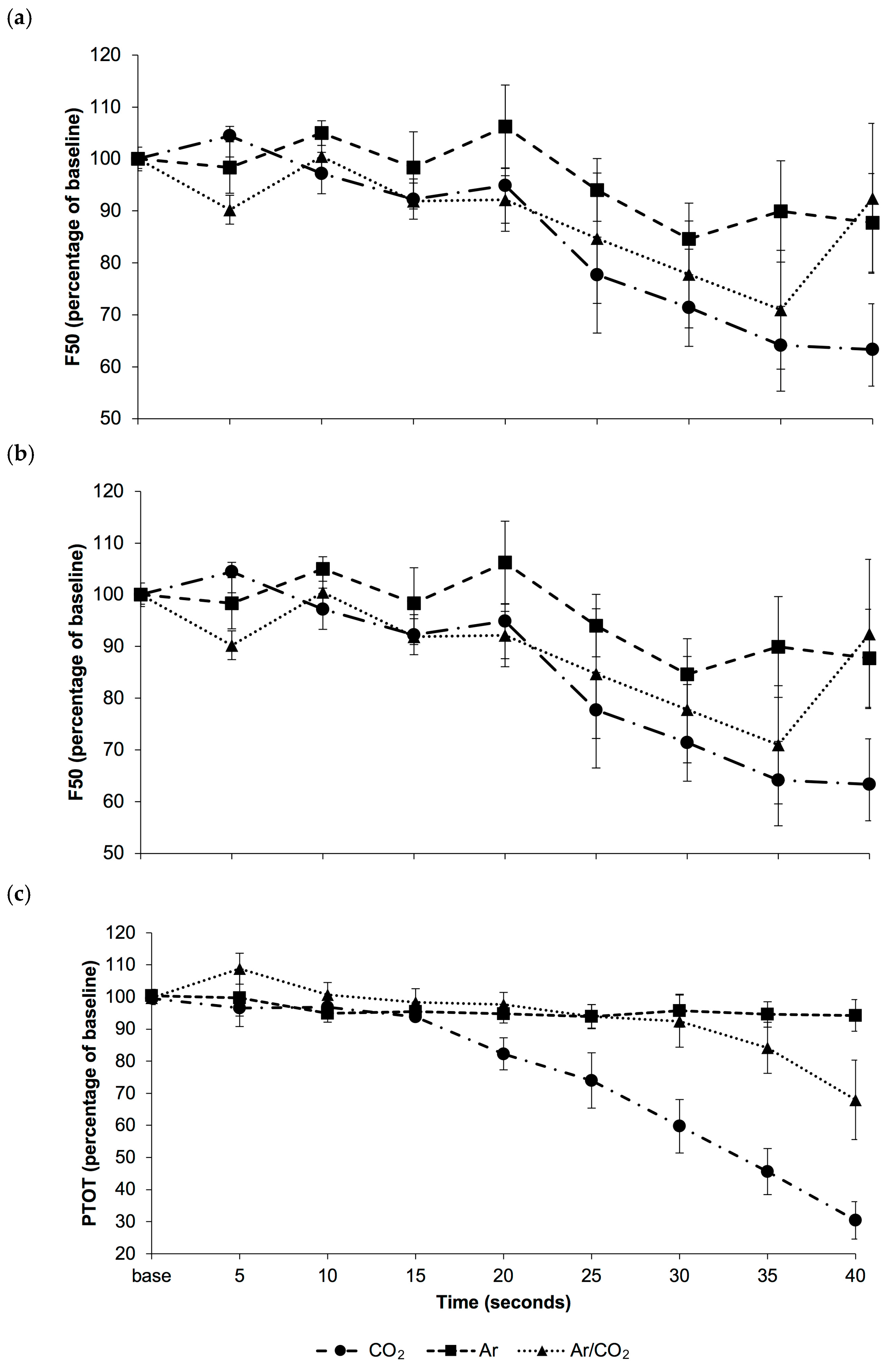
3.2. Study 2: Electrophysiological Response to Gas Euthansia
EEG Response
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Veterinary Medical Association. AVMA Guidelines for the Euthanasia of Animals: 2013 edition. Available online: https://www.avma.org/KB/Policies/Documents/euthanasia.pdf (accessed on 1 October 2014).
- Chevillion, P.; Mircovich, C.; Dubroca, S.; Fleho, J.Y. Comparison of different pig euthanasia methods available to farmers. Proc. Int. Soc. Anim. Hyg. 2004, 45–46. [Google Scholar]
- Widowski, T.M.; Elgie, R.H.; Lawlis, P. Assessing the effectiveness of a non-penetrating captive bolt for euthanasia of newborn piglets. Proc. Allen D. Leman Swine Conf. 2008, 107–111. [Google Scholar]
- Casey-Trott, T.M.; Millman, S.T.; Turner, P.V.; Nykamp, S.G.; Widowski, T.M. Effectiveness of a nonpentrating captive bolt for euthanasia of piglets less than 3 d of age. J. Anim. Sci. 2013, 91, 5477–5484. [Google Scholar] [CrossRef] [PubMed]
- Casey-Trott, T.M.; Millman, S.T.; Turner, P.V.; Nykamp, S.G.; Lawlis, P.C.; Widowski, T.M. Effectiveness of a non-penetrating captive bolt for euthanasia of 3–9 kg pigs. J. Anim. Sci. 2014, 92, 5166–5174. [Google Scholar] [CrossRef] [PubMed]
- Grist, A.; Murrell, J.C.; McKinstry, J.L.; Knowles, T.G.; Wotton, S.B. Humane euthanasia of neonates I: Validation of the effectiveness of the Zephyr EXL non-penetrating captive-bolt euthanasia system on neonate piglets up to 10.9 kg live-weight. Anim. Welf. 2017, 26, 111–120. [Google Scholar] [CrossRef]
- Finnie, J.W.; Manavis, J.; Summersides, G.E.; Blumbergs, P.C. Brain damage in pigs produced by impact with a nonpenetrating captive bolt pistol. Aust. Vet. J. 2003, 81, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Whiting, T.L.; Marion, C.R. Perpetration-induced traumatic stress—A risk for veterinarians involved in the destruction of healthy animals. Can. Vet. J. 2011, 52, 794–795. [Google Scholar] [PubMed]
- Matthis, J.S. Selected Employee Attributes and Perceptions Regarding Methods and Animal Welfare Concerns Associated with Swine Euthanasia. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2004. [Google Scholar]
- McKeegan, D.E.F.; McIntyre, J.A.; Demmers, T.; Lowe, J.C.; Wathes, C.; van den Broek, P.; Coenen, A.M.L.; Gentle, M. Physiological and behavioural responses of broilers to controlled atmosphere stunning: Implications for welfare. Anim. Welf. 2007, 16, 409–426. [Google Scholar]
- Rault, J.-L.; McMunn, K.A.; Marchant-Forde, J.N.; Lay, D.C., Jr. Gas alternatives to carbon dioxide for euthanasia: A piglet perspective. J. Anim. Sci. 2013, 91, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Sadler, L.J.; Hagen, C.D.; Wang, C.; Widowski, T.M.; Johnson, A.K.; Millman, S.T. Effects of flow rate and gas mixture on the welfare of weaned and neonate pigs during gas euthanasia. J. Anim. Sci. 2014, 92, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Velarde, A.; Cruz, J.; Gispert, M.; Carrion, D.; Ruiz de la Torre, J.L.; Diestre, A.; Manteca, X. Aversion to carbon dioxide stunning in pigs: Effect of carbon dioxide concentration and halothane genotype. Anim. Welf. 2007, 16, 513–522. [Google Scholar]
- Hewett, T.A.; Kovacs, M.S.; Artwohl, J.E.; Bennett, B.T. A comparison of euthanasia methods in rats, using carbon dioxide in prefilled and fixed flow-rate filled chambers. Lab. Anim. Sci. 1993, 43, 579–582. [Google Scholar] [PubMed]
- Niel, L.; Weary, D.M. Behavioural responses of rats to gradual-fill carbon dioxide euthanasia and reduced oxygen concentrations. Appl. Anim. Behav. Sci. 2006, 100, 295–308. [Google Scholar] [CrossRef]
- Pritchett, K.; Corrow, D.; Stockwell, J.; Smith, A. Euthanasia of neonatal mice with carbon dioxide. Comp. Med. 2005, 55, 275–281. [Google Scholar] [PubMed]
- Pritchett-Corning, K.R. Euthanasia of neonatal rats with carbon dioxide. J. Am. Assoc. Lab. Anim. Sci. 2009, 48, 23–27. [Google Scholar] [PubMed]
- Liotti, M.; Brannan, S.; Egan, G.; Shade, R.; Madden, L.; Abplanalp, B.; Robillard, R.; Lancaster, J.; Zamarripa, F.E.; Fox, P.T.; et al. Brain responses associated with consciousness of breathlessness (air hunger). Proc. Natl. Acad. Sci. USA 2001, 98, 2035–2040. [Google Scholar] [CrossRef] [PubMed]
- Lansing, R.W.; Gracely, R.H.; Banzett, R.B. The multiple dimensions of dyspnea: Review and hypothesis. Respir. Physiol. Neurobiol. 2009, 167, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, N.J.; Mellor, D.J. Introducing breathlessness as a significant animal welfare issue. N. Z. Vet. J. 2015, 63, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Anton, F.; Euchner, I.; Handwerker, H.O. Psychophysical examination of pain induced by defined CO2 pulses applied to the nasal mucosa. Pain 1992, 49, 53–60. [Google Scholar] [CrossRef]
- Danneman, P.J.; Stein, S.; Walshaw, S.O. Humane and practical implications of using carbon dioxide mixed with oxygen for anaesthesia or euthanasia of rats. Lab. Anim. Sci. 1997, 47, 376–385. [Google Scholar] [PubMed]
- Lambooij, E.; Gerritzen, M.A.; Engal, B.; Hillebrand, S.J.W.; Lankhaar, J.; Pieterse, C. Behavioural responses during exposure of broiler chickens to different gas mixtures. Appl. Anim. Behav. Sci. 1999, 62, 255–265. [Google Scholar] [CrossRef]
- Raj, A.B.M.; Gregory, N.G. Welfare implications of the gas stunning of pigs 2. Stress of induction of anaesthesia. Anim. Welf. 1996, 5, 71–78. [Google Scholar]
- Sutherland, M.A.; Bryer, P.J.; Backus, B.L. The effect of age and method of gas delivery on carbon dioxide euthanasia of pigs. Anim. Welf. 2017, 26, 293–299. [Google Scholar] [CrossRef]
- Freed, D.L.J. CO2 euthanasia. Nature 1983, 304, 482. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.B.M.; Gregory, N.G. Welfare implications of the gas stunning of pigs 1. Determination of aversion to the initial inhalation of carbon dioxide or argon. Anim. Welf. 1995, 4, 273–280. [Google Scholar]
- Thurauf, N.; Friedel, I.; Hummel, C.; Kobal, G. The mucosal potential elicited by noxious chemical stimuli with CO2 in rats: Is it a peripheral nociceptive event? Neurosci. Lett. 1991, 128, 297–300. [Google Scholar] [CrossRef]
- Peppel, P.; Anton, F. Responses of rat medullary dorsal horn neurons following intranasal noxious chemical stimulation: Effects of stimulus intensity, duration, and interstimulus interval. J. Neurophysiol. 1993, 70, 2260–2275. [Google Scholar] [CrossRef] [PubMed]
- Sadler, L.J.; Karriker, L.A.; Schwartz, K.J.; Johnson, A.K.; Widowski, T.M.; Wang, C.; Sutherland, M.A.; Millman, S.T. Are severely depressed suckling pigs resistant to gas euthanasia? Anim. Welf. 2014, 3, 145–155. [Google Scholar] [CrossRef]
- Gibson, T.J.; Johnson, C.B.; Murrell, J.C.; Mitchinson, S.L.; Stafford, K.J.; Mellor, D.J. Amelioration of electroencephalographic responses to slaughter by non-penetrative captive-bolt stunning after ventral-neck incision in halothane-anaesthetised calves. N. Z. Vet. J. 2009, 57, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, A.; Rodriguez, P.; Llonch, P.; Velarde, A. Stunning pigs with different gas mixtures: Aversion in pigs. Anim. Welf. 2010, 19, 325–333. [Google Scholar]
- Rodriguez, P.; Dalmau, A.; Ruiz de la Torre, J.L.; Manteca, X.; Jensen, E.W.; Rodriguez, B.E.; Litvan, H.; Velarde, A. Assessment of unconsciousness during carbon dioxide stunning in pigs. Anim. Welf. 2008, 17, 341–349. [Google Scholar]
- Llonch, P.; Dalmau, A.; Rodriguez, P.; Manteca, X.; Velarde, A. Aversion to nitrogen and carbon dioxide mixtures for stunning pigs. Anim. Welf. 2012, 21, 33–39. [Google Scholar] [CrossRef]
- Noonan, G.J.; Rand, J.S.; Priest, J.; Ainscow, J.; Blackshaw, J.K. Behavioural observations of piglets undergoing tail docking, teeth clipping and ear notching. Appl. Anim. Behav. Sci. 1994, 39, 201–213. [Google Scholar] [CrossRef]
- Prunier, A.; Mounier, A.; Hay, M. Effects of castration, tooth resection, or tail docking on plasma metabolites and stress hormones in young pigs. J. Anim. Sci. 2005, 83, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.A.; Berg, E.L.; Strauch, T.A.; Roberts, M.P.; Kattesh, H.G. Hormonal profiles, behavioural responses, and short-term growth performance after castration of pigs at three, six, nine, or twelve days of age. J. Anim. Sci. 2006, 84, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, M.A.; Bryer, P.J.; Krebs, N.; McGlone, J.J. Tail docking in pigs: Acute physiological and behavioural responses. Animal 2008, 2, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R.E.; Whitley, J.T.; Morrow, W.E.M.; Stikeleather, L.F.; Baird, C.L.; Rice, J.M.; Halbert, B.V.; Styles, D.K.; Whisnant, C.S. Effect of physical and inhaled euthanasia methods on hormonal measures of stress in pigs. J. Swine Health Prod. 2013, 21, 261–269. [Google Scholar]
- Blackmore, D.K.; Newhook, J.C. Electroencephalographic studies of stunning and slaughter of sheep and calves‚ Part 3: The duration of insensibility induced by electrical stunning in sheep and calves. Meat Sci. 1982, 7, 19–28. [Google Scholar] [CrossRef]
- Johnson, C.B.; Wilson, P.; Woodbury, M.; Caulkett, N. Comparison of analgesic techniques for antler removal in halothane-anaesthetised red deer (Cervus elaphus): Electroencephalographic responses. Vet. Anaesth. Anal. 2005, 32, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Murrell, J.C.; Johnson, C.B. Neurophysiological techniques to assess pain in animals. J. Vet. Pharmacol. Therapy 2006, 29, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Gibson, T.J.; Johnson, C.B.; Stafford, K.J.; Mitchinson, S.L.; Mellor, D.J. Validation of the acute electroencephalographic responses of calves to noxious stimulus with scoop dehorning. N. Z. Vet. J. 2007, 55, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Kongara, K.; Chambers, J.P.; Johnson, C.B. Electroencephalographic responses of tramadol, parecoxib and morphine to acute noxious electrical stimulation in anaesthetised dogs. Res. Vet. Sci. 2010, 88, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Diesch, T.J.; Mellor, D.J.; Johnson, C.B.; Lentle, R.G. Electroencephalographic responses to tail clamping in anaesthetized rat pups. Lab. Anim. 2009, 43, 224–231. [Google Scholar] [CrossRef] [PubMed]
| Behaviour | Definition | Variables Recorded |
|---|---|---|
| Convulsions | Involuntary contraction of skeletal muscles, may be tonic 1, clonic 1 or both; includes paddling 1 motion of the limbs | Latency to onset, frequency and total duration of bouts 2 |
| Escape attempt | Backing away or purposeful and vigorous butting of head, snout or shoulder into the chamber lid or walls or raising of forelegs against chamber walls | Latency to onset, frequency, duration |
| Gasping | Low frequency (≤4/min), very deep breathing through a wide open mouth, accompanied by large abdominal movements and stretching of the neck | Latency to onset |
| Head shaking | Vigorous, rapid, purposeful movement of head from side to side (at least 2 consecutive movements) | Frequency |
| Laboured breathing | Increase in rate and/or depth of ventilation compared with baseline (prior to transfer to chamber) | Latency to onset, total duration, intensity 3 |
| Loss of coordination | Loss of balance, stumbling, or diminished muscle control | Latency to onset |
| Loss of posture | Animal collapses into a recumbent position, with no evidence of postural control, and does not regain posture, or show further evidence of awareness | Latency to onset |
| Respiratory arrest | Permanent cessation of respiratory movement (minimum of 60 s without a breath) | Latency to onset |
| Sneezing/Coughing | Animal sneezed or coughed | Frequency |
| Elimination | Evacuation of the bladder or bowels | Frequency |
| Vocalisation | Piglet emits an audible squeal or grunt | Type (squeal or grunt), number and total duration of bouts 2 |
| Variable | CO2 | Ar | Ar/CO2 | p-Value |
|---|---|---|---|---|
| Latency to first escape attempt (s) | 3.9 ± 2.2 a (min 0, max 6.5) | 19 ± 2.9 b (min 11.5, max 29) | 8.0 ± 2.2 a (min 4, max 12) | 0.007 |
| Number of escape attempts 1 | 2.4 ± 0.35 a (min 1, max 4) | 0.6 ± 0.35 b (min 0, max 1) | 1.2 ± 0.35 ab (min 1, max 2) | 0.010 |
| Duration of escape attempts (s) | 7.2 ± 0.64 a (min 6, max 10) | 1.3 ± 0.64 b (min 0, max 3.5) | 2.8 ± 0.64 a (min 2, max 4) | <0.001 |
| Number of squeals 1 | 0 ± 0.0 | 0.6 ± 2.4 (min 0, max 1) | 0.6 ± 2.4 (min 0, max 1) | 0.088 |
| Duration of squeals (s) 1 | 0 ± 0.0 | 1.9 ± 0.61 (min 0, max 4.5) | 0.4 ± 0.61 (min 0, max 1.0) | 0.114 |
| Number of grunts 1 | 0 ± 0.0 a | 1.4 ± 0.39 b (min 0 , max 4) | 0 ± 0.0 a | 0.04 |
| Duration of grunts (s) 1 | 0 ± 0.0 a | 5.4 ± 2.8 b (min 0, max 10) | 0 ± 0.0 a | 0.003 |
| Latency to laboured breathing (s) | 5.8 ± 1.1 a (min 4, max 7) | 21.2 ± 1.1 b (min 16, max 27) | 4.6 ± 1.1 a (min 4, max 6) | <0.001 |
| Duration of laboured breathing 1 | 8.6 ± 1.3 a (min 6, max 11) | 3.0 ± 1.3 b (min 0, max 9) | 6.8 ± 1.3 ab (min 4, max 9) | 0.028 |
| Intensity of laboured breathing (s) 1 | 2.0 ± 0.18 a (min 2, max 2) | 0.4 ± 0.18 b (min 0, max 1) | 1.8 ± 0.18 a (min 1, max 2) | 0.003 |
| Latency to loss of coordination (s) | 9.5 ± 2.4 (min 5, max 13) | 16.5 ± 2.4 (min 10, max 30) | 8.5 ± 2.4 (min 5, max 13.5) | 0.066 |
| Latency to loss of posture 1 | 14 .4 ± 1.6 a (min 13, max 16) | 20.8 ± 1.6 b (min 16, max 30) | 11.4 ± 1.6 a (min 10, max 14) | 0.004 |
| Latency to first convulsion (s) 2 | 14.4 ± 1.6 a (min 13, max 16) | 20.8 ± 1.6 b (min 16, max 30) | 11.4 ± 1.6 a (min 10, max 14) | 0.004 |
| Number of convulsive bouts 2 | 4.6 ± 1.1 a (min 2, max 7) | 12.2 ± 1.1 b (min 8, max 15) | 5.4 ±1.1 a (min 2, max 9) | <0.001 |
| Duration of convulsions (s) 2 | 20.3 ± 5.3 a (min 12, max 32) | 71.6 ± 5.3 b (min 55, max 93) | 36.7 ± 5.3 a (min 28, max 50) | <0.001 |
| Latency to gasping (s) 2 | 33.2 ± 4.9 a (min 20, max 46) | 84.2 ± 20 b (min 48, max 151) | 30.2 ± 5.7 a (min 19, max 51) | 0.013 |
| Respiratory arrest (s) 2 | 113 ± 15.3 a (min 98, max 134) | 331 ± 15.3 b (min 293, max 415) | 225 ± 15.3 c (min 181, max 252) | <0.001 |
| EEG Classification | CO2 | Ar | Ar/CO2 | p-Value |
|---|---|---|---|---|
| Transitional EEG | 32.9 ± 2.92 a | 61.1 ± 4.53 b | 48.9 ± 7.73 ab | 0.03 |
| Isoelectric EEG | 46.2 ± 2.10 a | 69.0 ± 5.02 b | 67.1 ± 6.30 ab | 0.011 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kells, N.; Beausoleil, N.; Johnson, C.; Sutherland, M. Evaluation of Different Gases and Gas Combinations for On-Farm Euthanasia of Pre-Weaned Pigs. Animals 2018, 8, 40. https://doi.org/10.3390/ani8030040
Kells N, Beausoleil N, Johnson C, Sutherland M. Evaluation of Different Gases and Gas Combinations for On-Farm Euthanasia of Pre-Weaned Pigs. Animals. 2018; 8(3):40. https://doi.org/10.3390/ani8030040
Chicago/Turabian StyleKells, Nikki, Ngaio Beausoleil, Craig Johnson, and Mhairi Sutherland. 2018. "Evaluation of Different Gases and Gas Combinations for On-Farm Euthanasia of Pre-Weaned Pigs" Animals 8, no. 3: 40. https://doi.org/10.3390/ani8030040





