Spatial Cognition and Range Use in Free-Range Laying Hens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Experiment 1: T-Maze Learning in Adult Hens
2.2.1. Animals and Housing
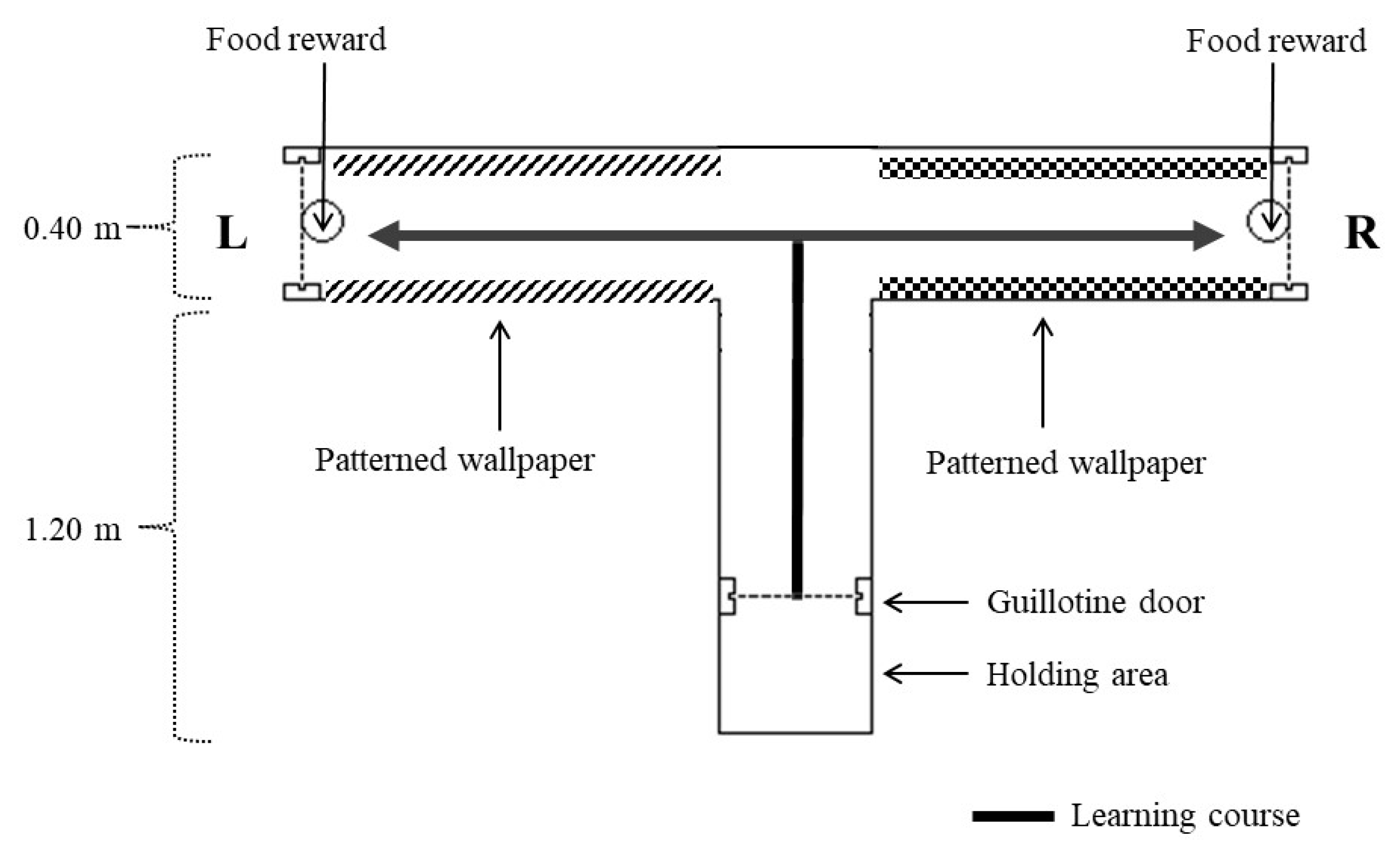
2.2.2. T-Maze Habituation
2.2.3. T-Maze Learning
2.2.4. Data and Statistical Analyses
2.3. Experiment 2: Early Enrichment and T-Maze Learning in Pullets
2.3.1. Chick Housing and Early Enrichment
2.3.2. Pullet and Layer Housing
2.3.3. Test Subjects and T-Maze Habituation
2.3.4. T-Maze Learning
2.3.5. Brain Histology
2.3.6. Telencephalon and Hippocampal Formation Size Measurements
2.3.7. Radio-Frequency Identification of Range Use
2.3.8. Data and Statistical Analyses
3. Results
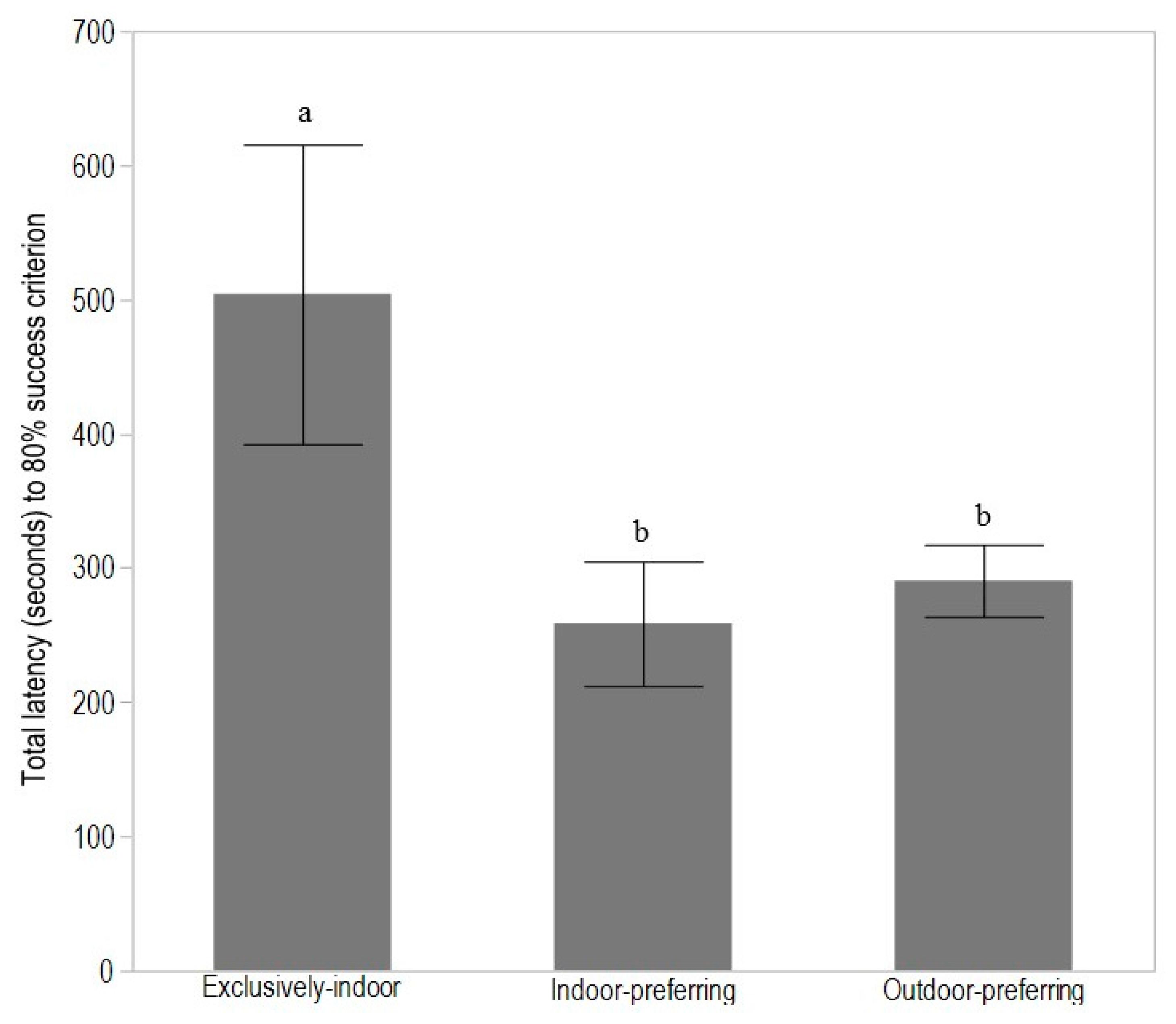
3.1. Experiment 1: T-Maze Learning in Adult Hens
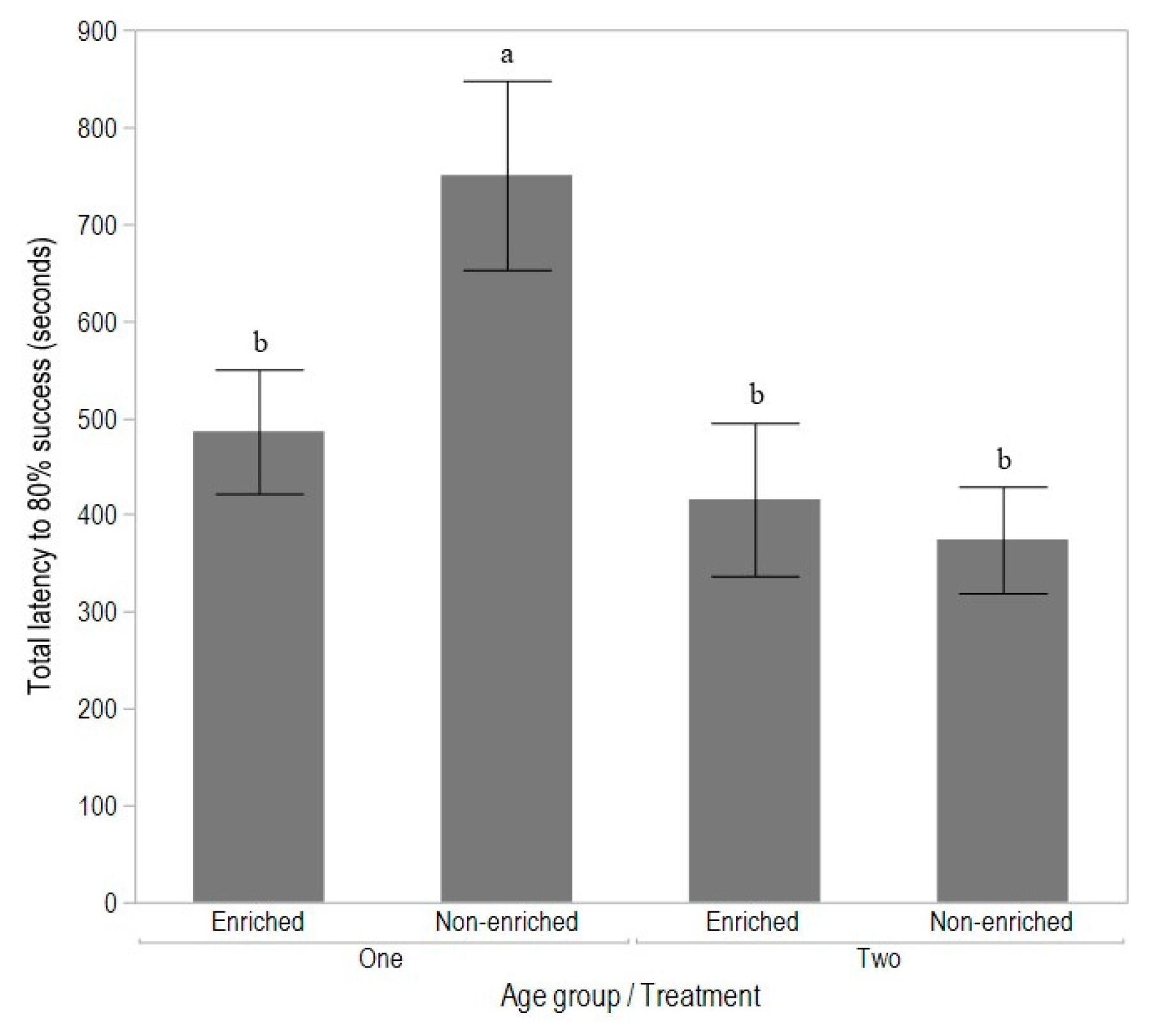
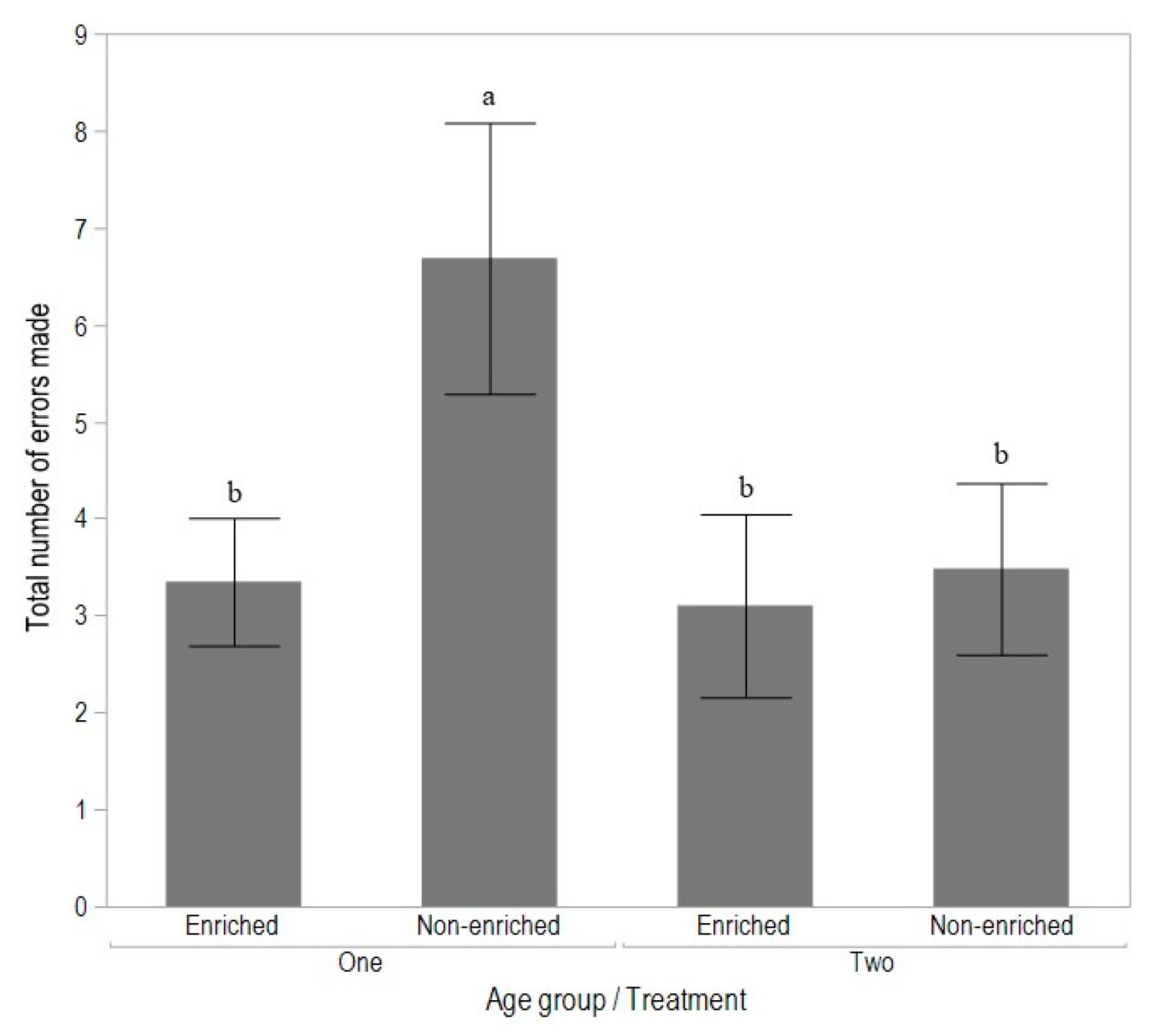
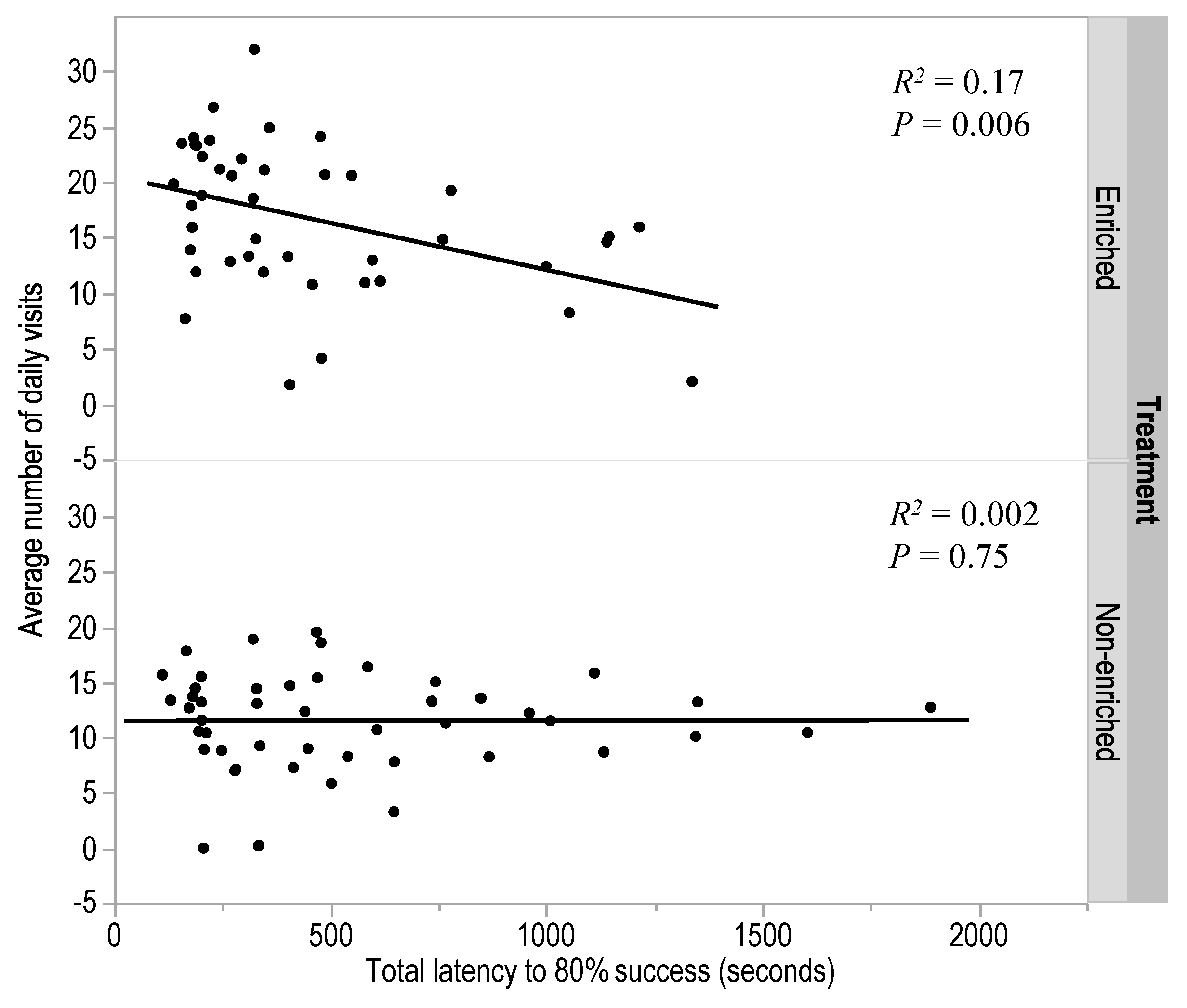
3.2. Experiment 2: Early Enrichment and T-Maze Learning in Pullets
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Knierim, U. Animal welfare aspects of outdoor runs for laying hens: A review. NJAS Wagening. J. Life Sci. 2006, 54, 133–145. [Google Scholar] [CrossRef]
- Schröder, M.J.A.; McEachern, M.G. Consumer value conflicts surrounding ethical food purchase decisions: A focus on animal welfare. Int. J. Consum. Stud. 2004, 28, 168–177. [Google Scholar] [CrossRef]
- Campbell, D.L.M.; Hinch, G.N.; Dyall, T.; Warin, L.; Little, B.; Lee, C. Outdoor stocking density in free-range laying hens: Radio-frequency identification of impacts on range use. Animal 2017, 11, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Gebhardt-Henrich, S.G.; Toscano, M.J.; Fröhlich, E.K.F. Use of outdoor ranges by laying hens in different sized flocks. Appl. Anim. Behav. Sci. 2014, 155, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Richards, G.J.; Wilkins, L.J.; Knowles, T.G.; Booth, F.; Toscano, M.J.; Nicol, C.J.; Brown, S.N. Continuous monitoring of pop hole usage by commercially housed free-range hens throughout the production cycle. Vet. Rec. 2011, 169, 338. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.L.M.; Hinch, G.N.; Downing, J.A.; Lee, C. Fear and coping styles of outdoor-preferring, moderate-outdoor and indoor-preferring free-range laying hens. Appl. Anim. Behav. Sci. 2016, 185, 73–77. [Google Scholar] [CrossRef]
- Hartcher, K.M.; Hickey, K.A.; Hemsworth, P.H.; Cronin, G.M.; Wilkinson, S.J.; Singh, M. Relationships between range access as monitored by radio frequency identification technology, fearfulness, and plumage damage in free-range laying hens. Animal 2016, 10, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.E.; Lee, C.; Ferguson, D.; Dyall, T.; Belson, S.; Lea, J.; Hinch, G. Personality traits of high, low and non-users of a free range area in laying hens. In Proceedings of the 48th Congress of the International Society for Applied Ethology, Vitoria-Gasteiz, Spain, 29 July–2 August 2014; Volume 48, p. 89. [Google Scholar]
- Krause, E.T.; Naguib, M.; Trillmich, F.; Schrader, L. The effects of short term enrichment on learning in chickens from a laying strain. Appl. Anim. Behav. Sci. 2006, 101, 318–327. [Google Scholar] [CrossRef]
- Tahamtani, F.M.; Nordgreen, J.; Nordquist, R.E.; Janczak, A.M. Early life in a barren environment adversely affects spatial cognition in laying hens (Gallus gallus domesticus). Front. Vet. Sci. 2015, 2, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patzke, N.; Ocklenburg, S.; van der Staay, F.J.; Güntürkün, O.; Manns, M. Consequences of different housing conditions on brain morphology in laying hens. J. Chem. Neuroanat. 2009, 37, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Tahamtani, F.M.; Nordgreen, J.; Brantsæter, M.; Østby, G.C.; Nordquist, R.E.; Janczak, A.M. Does early environmental complexity influence tyrosine hydroxylase in the chicken hippocampus and ‘prefrontal’ caudolateral nidopallium? Front. Vet. Sci. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.L.M.; Hinch, G.N.; Downing, J.A.; Lee, C. Early enrichment in free-range laying hens: Effects on ranging behaviour, welfare and response to stressors. Animal 2017. [Google Scholar] [CrossRef] [PubMed]
- National Health and Medical Research Council. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 7th ed.; Commonwealth Government of Australia: Canberra, Australia, 2004.
- Campbell, D.L.M.; Hinch, G.N.; Downing, J.A.; Lee, C. Outdoor stocking density in free-range laying hens: Effects on behaviour and welfare. Animal 2017, 11, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Primary Industries Standing Committee. Model Code of Practice for the Welfare of Animals—Domestic Poultry, 4th ed.; CSIRO Publishing: Collingwood, Victoria, Australia, 2002. [Google Scholar]
- Packard, M.G.; McGaugh, J.L. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol. Learn. Mem. 1996, 65, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Hy-Line® International. Hy-Line® Brown Management Guide, Commercial Layers; Hy-Line® International: West Des Moines, IA, USA, 2014. [Google Scholar]
- Puelles, L.; Paxinos, G.; Watson, C.; Martinez, S.; Martinez-de-la-Torre, M. The Chick Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Dixon, L.M.; Sparks, N.H.C.; Rutherford, K.M.D. Early experiences matter: A review of the effects of prenatal environment on offspring characteristics in poultry. Poult. Sci. 2015, 95, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Hernandez, C.E.; Lee, C.; Hinch, G.; Cowieson, A.J. Wanderers versus stay at home: Who has the better guts? In Proceedings of the 27th Australian Poultry Science Symposium, Sydney, Australia, 14–17 February 2016; Volume 27, pp. 78–81. [Google Scholar]
- Lazic, M.; Schneider, S.M.M.; Lickliter, R. Enriched rearing facilitates spatial exploration in Northern Bobwhite (Colinus virginianus) neonates. Dev. Psychobiol. 2007, 49, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, M.A.; Sage, R.; Madden, J.R. Multiple behavioural, morphological and cognitive developmental changes arise from a single alteration to early life spatial environment, resulting in fitness consequences for released pheasants. R. Soc. Open Sci. 2016, 3, 160008. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Waddington, D. Modification of fear in domestic chicks, Gallus gallus domesticus, via regular handling and early environmental enrichment. Anim. Behav. 1992, 43, 1021–1033. [Google Scholar] [CrossRef]
- Regolin, L.; Vallortigara, G.; Zanforlin, M. Detour behaviour in the domestic chick: Searching for a disappearing pretty or a disappearing social partner. Anim. Behav. 1995, 50, 203–211. [Google Scholar] [CrossRef]
- De Haas, E.N.; Lee, C.; Rodenburg, B.T. Learning and judgement can be affected by fearfulness in laying hens. Front. Vet. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Asymmetry of brain and behavior in animals: Its development, function, and human relevance. Genesis 2014, 52, 555–571. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Behavioural transitions in early posthatching life. In The Development of Brain and Behaviour in the Chicken; CAB International: Wallingford, UK, 1995; pp. 157–183. [Google Scholar]
- Raine, R.E.E.; Chittka, L. The correlation of learning speed and natural foraging success in bumble-bees. Proc. R. Soc. Lond. B 2008, 275, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Guilette, L.M.M.; Reddon, A.R.R.; Hurd, P.L.L.; Sturdy, C.B. Exploration of a novel space is associated with individual differences in learning speed in black-capped chickadees. Poecile atricapillus. Behav. Proc. 2009, 82, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Shaw, R.C.C.; Boogert, N.J.J.; Clayton, N.S.S.; Burns, K.C. Wild psychometrics: Evidence for ‘general’ cognitive performance in wild New Zealand robins, Petroica longipes. Anim. Behav. 2015, 109, 101–111. [Google Scholar] [CrossRef]
- Clayton, N.S.; Krebs, J.R. Memory in food-storing birds: From behaviour to brain. Curr. Opin. Neurobiol. 1995, 5, 149–154. [Google Scholar] [CrossRef]
- Reiner, A.; Yamamoto, K.; Karten, H.J. Organization and evolution of the avian forebrain. Anat. Rec. 2005, 287, 1080–1102. [Google Scholar] [CrossRef] [PubMed]
- Freire, R.; Cheng, H.-W. Experience-dependent changes in the hippocampus of domestic chicks: A model for spatial memory. Eur. J. Neurosci. 2004, 20, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Robertson, B.A.; Rathbone, L.; Cirillo, G.; D’Eath, R.B.; Bateson, M.; Boswell, T.; Wilson, P.W.; Dunn, I.C.; Smulders, T.V. Food restriction reduces neurogenesis in the avian hippocampal formation. PLoS ONE 2017, 12, e0189158. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, D.L.M.; Talk, A.C.; Loh, Z.A.; Dyall, T.R.; Lee, C. Spatial Cognition and Range Use in Free-Range Laying Hens. Animals 2018, 8, 26. https://doi.org/10.3390/ani8020026
Campbell DLM, Talk AC, Loh ZA, Dyall TR, Lee C. Spatial Cognition and Range Use in Free-Range Laying Hens. Animals. 2018; 8(2):26. https://doi.org/10.3390/ani8020026
Chicago/Turabian StyleCampbell, Dana L.M., Andrew C. Talk, Ziyang A. Loh, Tim R. Dyall, and Caroline Lee. 2018. "Spatial Cognition and Range Use in Free-Range Laying Hens" Animals 8, no. 2: 26. https://doi.org/10.3390/ani8020026




