Comparison of Intramuscular or Subcutaneous Injections vs. Castration in Pigs—Impacts on Behavior and Welfare
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Section
2.2. Study 1: Piglet Phase
2.3. Study 2: Finishing Pig Phase
2.4. Hormonal Assay
2.5. Statistical Analyses
3. Results
3.1. Study 1: Piglet Phase
3.2. Study 2: Finishing Pig Phase
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McGlone, J.J.; Nicholson, R.I.; Hellman, J.M.; Herzog, D.N. The development of pain in young pigs associated with castration and attempts to prevent castration-induced behavioral changes. J. Anim. Sci. 1993, 71, 1441–1446. [Google Scholar] [PubMed]
- Sutherland, M.A.; Davis, B.L.; Brooks, T.A.; McGlone, J.J. Physiology and behavior of pigs before and after castration: Effects of two topical anesthetics. Animal 2010, 4, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.L.; Lay, D.C., Jr.; Marchant-Forde, J.N. Castration induced pain in pigs and other livestock. Appl. Anim. Behav. Sci. 2011, 135, 214–225. [Google Scholar] [CrossRef]
- McGlone, J.J.; Hellman, J.M. Local and general anesthetic effects on behavior and performance of two- and seven-week-old castrated and uncastrated piglets. J. Anim. Sci. 1988, 66, 3049–3058. [Google Scholar] [CrossRef] [PubMed]
- Dunshea, F.R.; Colantoni, C.; Howard, K.; McCauley, I.; Jackson, P.; Long, K.A.; Lopaticki, S.; Nugent, E.A.; Simons, J.A.; Walker, J.; et al. Vaccination of boars with a GnRH vaccine (Improvac) eliminates boar taint and increases growth performance. J. Anim. Sci. 2001, 79, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, M.; Dufour, R.; Chouvet, C.; Roulet, C.; Meadus, W.; Squires, E.J. The effects of immunization against luteinizing hormone-releasing hormone on performance, sexual development, and levels of boar taint-related compounds in intact male pigs. J. Anim. Sci. 1994, 72, 14–20. [Google Scholar] [PubMed]
- Metz, C.; Hohl, K.; Waidelich, S.; Drochner, W.; Claus, R. Active immunization of boars against GnRH at an early age: Consequences for testicular function, boar taint accumulation and N-retention. Livest. Prod. Sci. 2002, 74, 147–157. [Google Scholar] [CrossRef]
- Hemsworth, P.H.; Barnett, J.L.; Hansen, C. The influence of handling by humans on the behaviour, reproduction and corticosteroids of male and female pigs. Appl. Anim. Behav. Sci. 1986, 15, 303–314. [Google Scholar] [CrossRef]
- Robert, S.; Passillé, A.; St. Pierre, N.; Pelletier, G.; Petitclerc, D.; Dubreuil, P.; Brazeau, P. Effect of the stress of injections on the serum concentration of cortisol, prolactin, and growth hormone in gilts and lactating sows. Can. J. Anim. Sci. 1989, 69, 663–672. [Google Scholar] [CrossRef]
- Mayer, M.; Rosen, F. Interaction of glucocorticoids and androgens with skeletal muscle. Metabolism 1997, 26, 937–962. [Google Scholar] [CrossRef]
- Cahill, G.F. Action of adrenal cortical steroids on carbohydrate metabolism. In The Human Adrenal Cortex; Chisty, N., Ed.; Harper & Row: New York, NY, USA, 1971; pp. 205–239. [Google Scholar]
- Sutherland, M.A.; Davis, B.L.; Brooks, T.A.; Coetzee, J.F. The physiological and behavioral response of pigs castrated with and without anesthesia or analgesia. J. Anim. Sci. 2012, 90, 2211–2221. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.; Vulin, A.; Génin, S.; Sales, P.; Prunier, A. Assessment of pain induced by castration in piglets: Behavioral and physiological responses over the subsequent 5 days. Appl. Anim. Behav. Sci. 2003, 82, 201–218. [Google Scholar] [CrossRef]
- Garcia, A.; Pirner, G.; Picinin, G.; May, M.; Guay, K.A.; Backus, B.L.; Sutherland, M.; McGlone, J.J. Effect of provision of feed and water during transport on the welfare of weaned pigs. Animals 2015, 5, 407–425. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.J.; Berry, R.J. Effects of season on the behavior of early-weaned piglets during and immediately following transport. Appl. Anim. Behav. Sci. 2006, 100, 182–192. [Google Scholar] [CrossRef]
- Lewis, N.J. Transport of early weaned pigs. Appl. Anim. Behav. Sci. 2008, 110, 128–135. [Google Scholar] [CrossRef]
- Sutherland, M.A.; Backus, B.L.; McGlone, J.J. Effects of transport at weaning on the behavior, physiology and performance of pigs. Animals 2014, 4, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Torrey, S.; Devillers, N.; Lessard, M.; Farmer, C.; Widowski, T. Effect of age on the behavioral and physiological responses of piglets to tail docking and ear notching. J. Anim. Sci. 2009, 87, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Lewis, C.R.G.; McGlone, J.J. Moving finishing pigs in different group sizes: Cardiovascular responses, time, and ease of handling. Livestock Sci. 2007, 107, 86–90. [Google Scholar] [CrossRef]
- Wemelsfelder, F.; Putten, V.G. Behaviour as a Possible Indicator for Pain in Piglets; Instituut voor Veeteltkundig Onderzoek “Schoonoord”: Zeist, The Netherlands, 1985. [Google Scholar]
- Taylor, A.A.; Weary, D.M.; Lessard, M.; Braithwaite, L. Behavioural responses of piglets to castration: The effect of piglet age. Appl. Anim. Behav. Sci. 2001, 73, 35–43. [Google Scholar] [CrossRef]
- Hemsworth, P.H.; Barnett, J.L.; Campbell, R.G. A study of the relative aversiveness of a new daily injection procedure for pigs. Appl. Anim. Behav. Sci. 1996, 49, 389–401. [Google Scholar] [CrossRef]
- Leslie, E.; Hernández-Jover, M.; Newman, R.; Holyoake, P. Assessment of acute pain experienced by piglets from ear tagging, ear notching and intraperitoneal injectable transponders. Appl. Anim. Behav. Sci. 2010, 127, 86–95. [Google Scholar] [CrossRef]
- Marx, G.; Horn, T.; Thielebein, J.; Knubel, B.; von Borell, E. Analysis of pain-related vocalisation in young pigs. J. Sound Vib. 2003, 266, 687–698. [Google Scholar] [CrossRef]
- Leidig, M.S.; Hertrampf, B.; Failing, K.; Schumann, A.; Reiner, G. Pain and discomfort in male piglets during surgical castration with and without local anesthesia as determined by vocalization and defense behaviour. Appl. Anim. Behav. Sci. 2009, 116, 174–178. [Google Scholar] [CrossRef]
- Weary, D.M.; Braithwaite, L.A.; Fraser, D. Vocal response to pain in piglets. Appl. Anim. Behav. Sci. 1998, 56, 161–172. [Google Scholar] [CrossRef]
- Taylor, A.A.; Weary, D.M. Vocal responses of piglets to castration: Identifying procedural sources of pain. Appl. Anim. Behav. Sci. 2000, 70, 17–26. [Google Scholar] [CrossRef]
- Waldmann, K.H.; Otto, K.; Bollwahn, W. Piglet castration—Pain sensation and pain elimination. Dtsch Tierarztl. Wochenschr. 1994, 101, 105–109. [Google Scholar] [PubMed]
- Zankl, A.; Ritzmann, M.; Zols, S.; Heinritzi, K. Untersuchungen zur Wirksamkeit von Lokalanaesthetica bei der Kastration von männlichen Saugferkeln. Dtsch Tierarztl. Wochenschr. 2007, 114, 418–422. [Google Scholar] [PubMed]
- Von Borell, E.; Baumgartner, J.; Giersing, M.; Jäggin, N.; Prunier, A.; Tuyttens, F.A.; Edwards, S.A. Animal welfare implications of surgical castration and its alternatives in pigs. Animal 2009, 3, 1488–1496. [Google Scholar] [CrossRef] [PubMed]
- McGlone, J.J.; Salak, J.L.; Lumpkin, E.A.; Nicholson, R.I.; Gibson, M.; Norman, R.L. Shipping stress and social status effects on pig performance, plasma cortisol, natural killer cell activity, and leukocyte numbers. J. Anim. Sci. 1993, 71, 888–896. [Google Scholar] [PubMed]
- Garcia, A.; Sutherland, M.; Pirner, G.; Picinin, G.; May, M.; Backus, B.; McGlone, J. Impact of providing feed and/or water on performance, physiology, and behavior of weaned pigs during a 32-h transport. Animal 2016, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- White, R.G.; DeShazer, J.A.; Tressler, C.J.; Borcher, G.M.; Davey, S.; Waninge, A.; Parkhurst, A.M.; Milanuk, M.J.; Clemens, E.T. Vocalization and physiological response of pigs during castration with or without a local anesthetic. J. Anim. Sci. 1995, 73, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Velarde, A.; Gispert, M.; Font i Furnols, M.; Dalmau, A.; Soler, J.; Tibau, J.; Fa’ brega, E. The effect of immunocastration on the behaviour of pigs. In Proceedings of the EAAP Working Group on Production and Utilisation of Meat from Entire Male Pigs, Monells, Spain, 26–27 March 2008; pp. 32–33.
- Guay, K.; Salgado, G.; Thompson, G.; Backus, B.; Sapkota, A.; Chaya, W.; McGlone, J.J. Behavior and handling of physically and immunologically castrated market pigs on farm and going to market. J. Anim. Sci. 2013, 91, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- Mark, A.; Carlsson, R.M.; Granström, M. Subcutaneous versus intramuscular injection for booster DT vaccination of adolescents. Vaccine 1999, 17, 2067–2072. [Google Scholar] [CrossRef]
- Sluka, K.A.; Kalra, A.; Moore, S.A. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve 2001, 24, 37–46. [Google Scholar] [CrossRef]
- Reyes, L.; Tinworth, K.D.; Li, K.M.; Yau, D.F.; Waters, K.A. Observer-blinded comparison of two nonopioid analgesics for postoperative pain in piglets. Pharmacol. Biochem. Behav. 2002, 73, 521–528. [Google Scholar] [CrossRef]
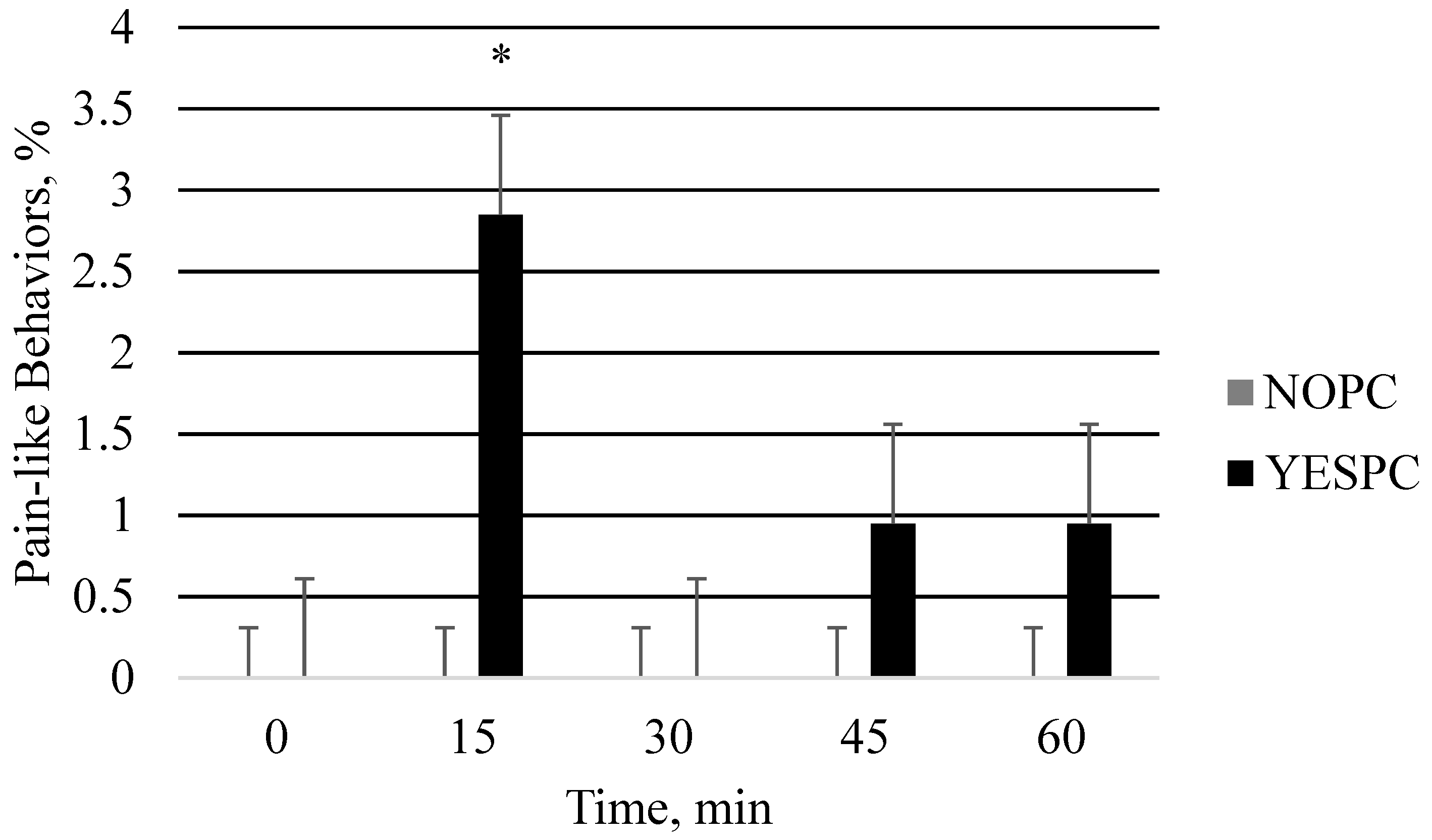
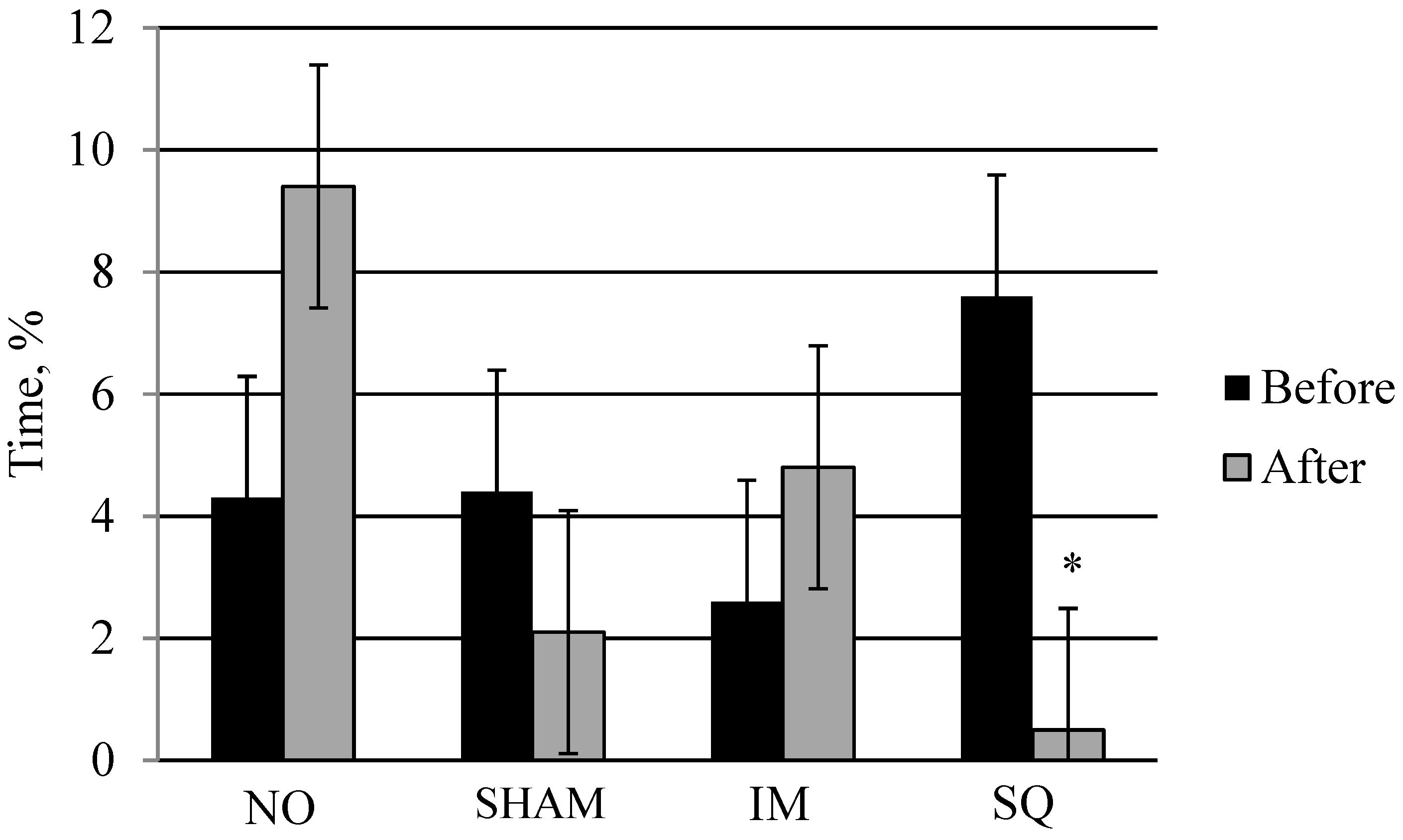
| Behavior | NO | SHAM | IM | SQ | PC | SE | p-Value |
|---|---|---|---|---|---|---|---|
| Lying with contact | 60.4 | 60.8 | 61.2 | 57.4 | 53.9 | 2.17 | 0.15 |
| Lying without contact | 5.0 | 3.2 | 2.0 | 1.9 | 8.8 a | 1.80 | 0.09 |
| Sitting | 1.4 | 2.5 | 2.4 | 2.9 | 2.1 | 0.69 | 0.57 |
| Standing | 7.9 | 8.3 | 11.0 | 9.4 | 10.2 | 1.46 | 0.72 |
| Walking | 8.5 | 7.5 | 7.6 | 6.7 | 7.8 | 1.80 | 0.88 |
| Nursing/Eating | 16.9 | 17.6 | 15.7 | 21.8 | 16.4 | 2.39 | 0.46 |
| Exhibiting pain | 0.0 | 0.0 | 0.0 | 0.0 | 0.60 b | 0.18 | 0.07 |
| Cortisol (ng/mL) | 75.8 | 43.5 | 55.5 | 53.7 | 46.1 | 12.3 | 0.34 |
| Behavior | NO | SHAM | IM | SQ | SE | p-Value TRT | p-Value TRT × PER |
|---|---|---|---|---|---|---|---|
| Lying | 77.7 a | 87.6 b | 80.0 a | 80.7 a | 2.26 | 0.05 | 0.68 |
| Eating | 6.9 | 3.1 | 3.7 | 4.1 | 1.69 | 0.38 | 0.03 * |
| Sitting | 0.55 | 0.13 | 0.96 | 1.6 | 0.49 | 0.21 | 0.71 |
| Standing | 6.0 | 3.3 | 4.5 | 6.1 | 1.13 | 0.14 | 0.81 |
| Walking | 6.6 | 3.9 | 7.0 | 5.6 | 1.31 | 0.30 | 0.56 |
| Drinking | 1.9 | 0.7 | 3.2 | 2.6 | 0.6 | 0.11 | 0.62 |
| Cortisol, ng/mL | 5.4 | 10.5 | 5.1 | 13.1 | 3.10 | 0.19 | -- |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGlone, J.; Guay, K.; Garcia, A. Comparison of Intramuscular or Subcutaneous Injections vs. Castration in Pigs—Impacts on Behavior and Welfare. Animals 2016, 6, 52. https://doi.org/10.3390/ani6090052
McGlone J, Guay K, Garcia A. Comparison of Intramuscular or Subcutaneous Injections vs. Castration in Pigs—Impacts on Behavior and Welfare. Animals. 2016; 6(9):52. https://doi.org/10.3390/ani6090052
Chicago/Turabian StyleMcGlone, John, Kimberly Guay, and Arlene Garcia. 2016. "Comparison of Intramuscular or Subcutaneous Injections vs. Castration in Pigs—Impacts on Behavior and Welfare" Animals 6, no. 9: 52. https://doi.org/10.3390/ani6090052






