Finding the Balance: Fertility Control for the Management of Fragmented Populations of a Threatened Rock-Wallaby Species
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
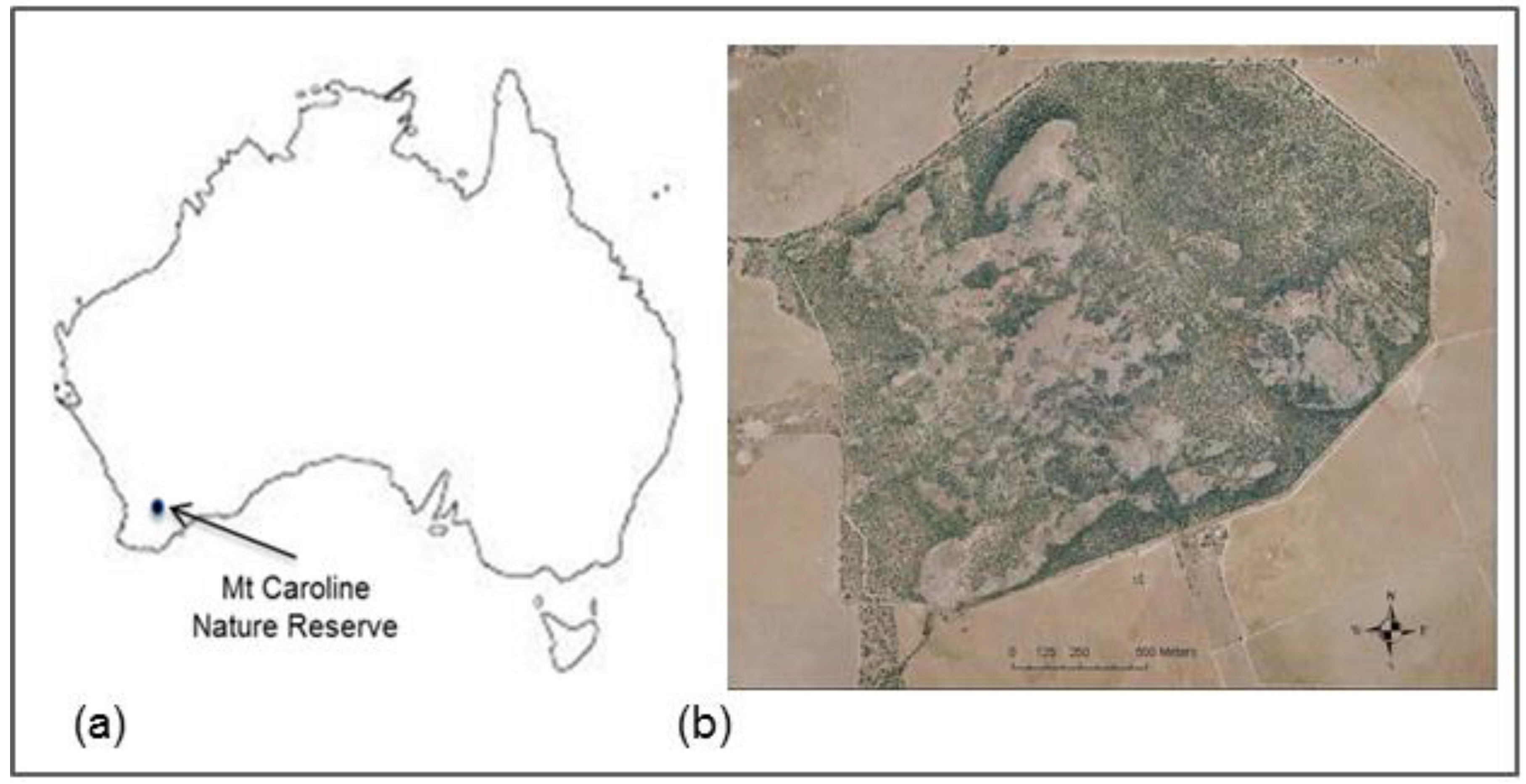
2.1. Study Site
2.2. Trapping Protocol, Animal Handling and Data Collection
2.3. Deslorelin Implantation
2.4. Blood Sampling and GnRH Challenges
2.5. LH Assays
2.6. Statistical Analyses
3. Results
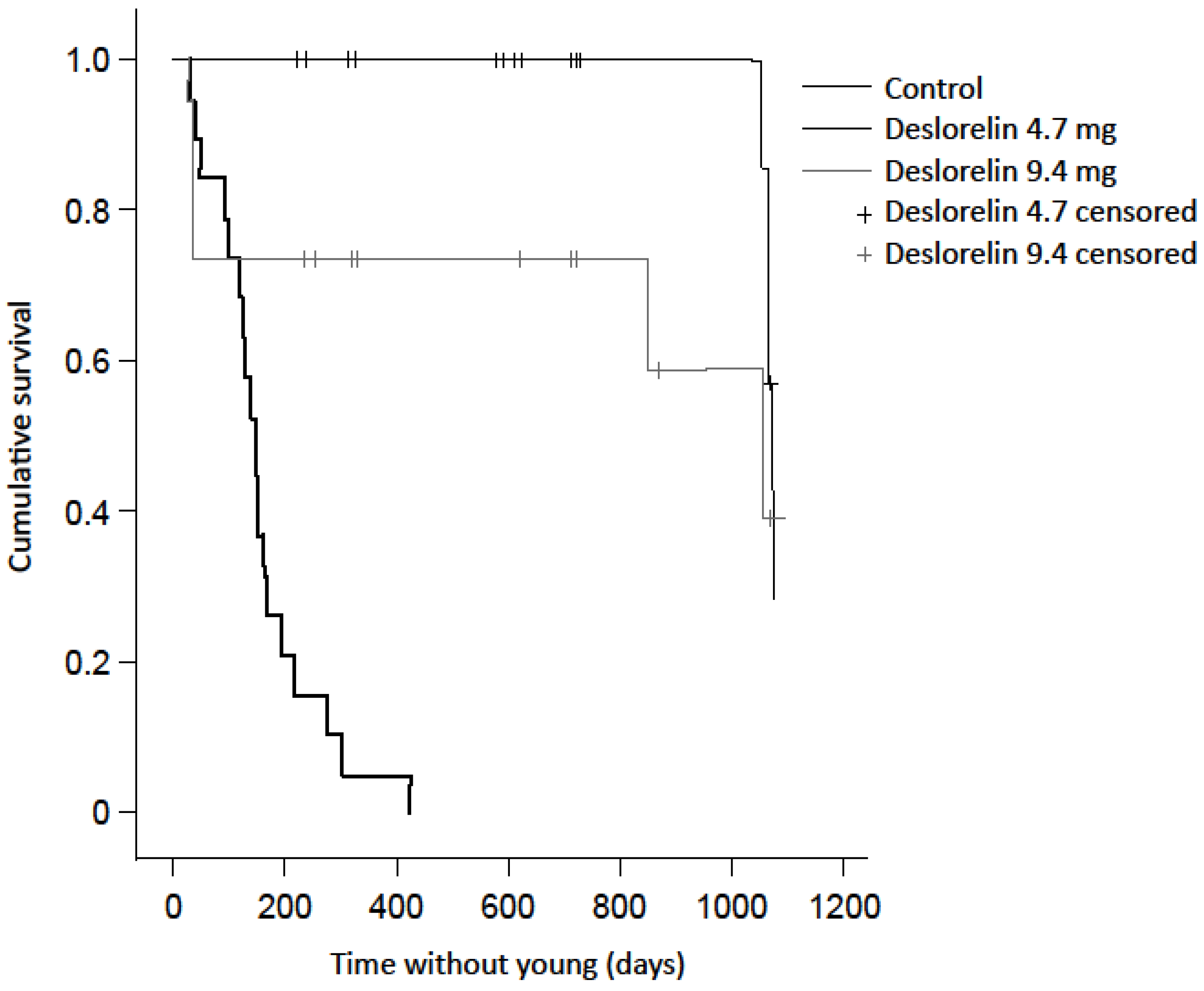
3.1. Births
| Treatment | Placebo | One Implant | Two Implants |
|---|---|---|---|
| Recaptured with new young during study | 19 (86.4%) | 4 (18.2%) | 7 (35 %) * |
| Not recaptured following implantation | 3 (13.6%) | 2 (9.1%) | 1 (5 %) |
| Recorded in final year without young | n/a | 8 (36.4%) | 5 (25 %) |
| Not recaptured in final year | n/a | 8 (36.4%) | 7 (35 %) |
3.2. Luteinising Hormone Concentrations

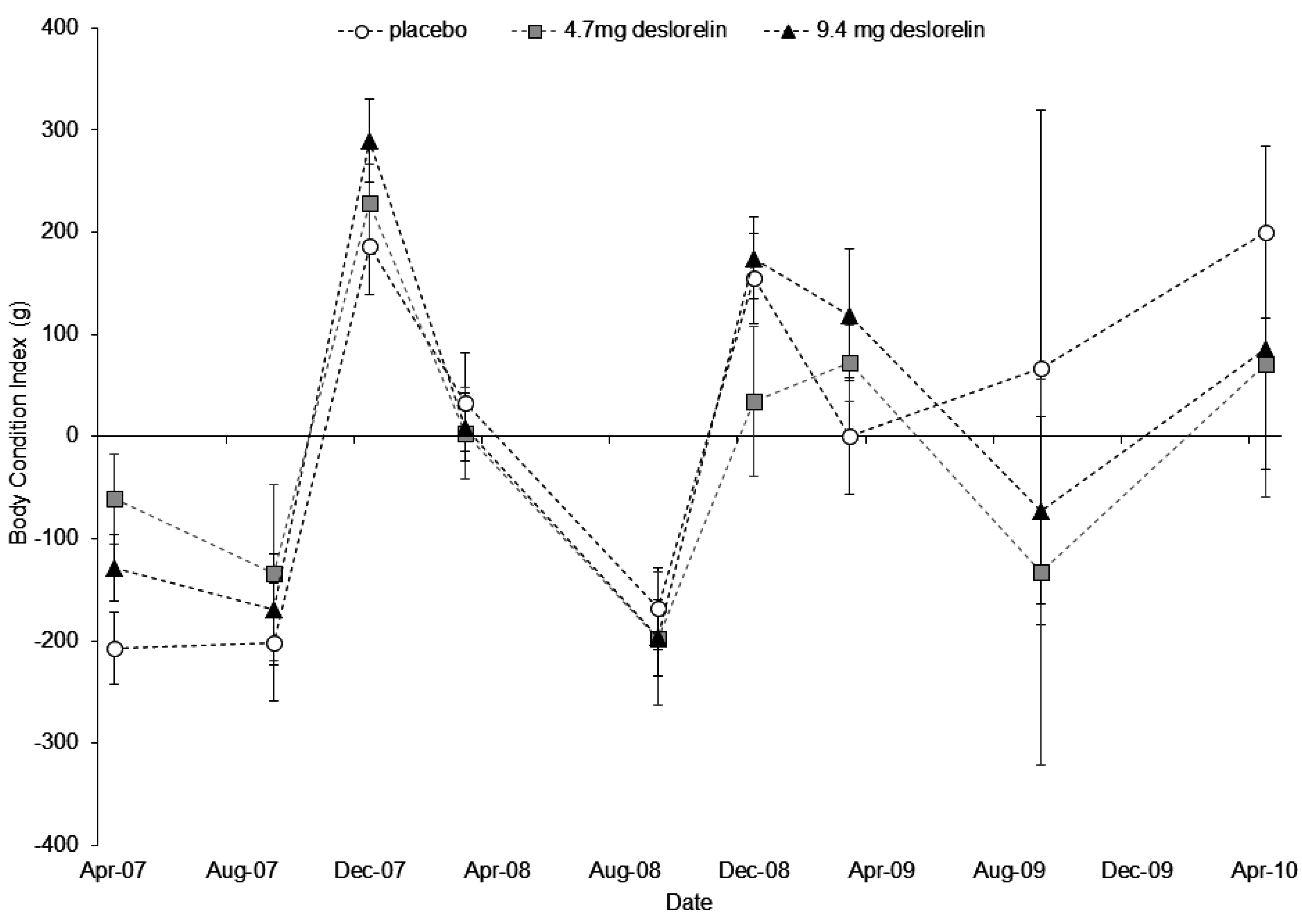
3.3. Body Condition
4. Discussion
4.1. Efficacy of Deslorelin Implants
4.2. Potential Risks of Deslorelin Implants
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wilson, M.E.; Coulson, G.; Shaw, G.; Renfree, M.B. Deslorelin implants in free-ranging female eastern grey kangaroos (Macropus giganteus): Mechanism of action and contraceptive efficacy. Wildlife Res. 2013, 40, 403–412. [Google Scholar] [CrossRef]
- Bayliss, P. The population dynamics of red and western grey kangaroos in arid new South Wales, Australia. J. Anim. Ecol. 1985, 54, 111–125. [Google Scholar] [CrossRef]
- Cowan, P.E.; Tyndale-Biscoe, C.H. Australian and New Zealand mammal species considered to be pests or problems. Reprod. Fert. Develop. 1997, 9, 27–36. [Google Scholar] [CrossRef]
- Rutberg, A.T.; Naugle, R.E. Population-level effects of immunocontraception in white-tailed deer (Odocoileus virginianus). Wildlife Res. 2008, 35, 494–501. [Google Scholar] [CrossRef]
- Cheal, D. A park with a kangaroo problem. Oryx 1986, 20, 95–99. [Google Scholar] [CrossRef]
- Arnold, G.; Steven, D.; Weeldenburg, J. The use of surrounding farmland by western gray kangaroos living in a remnant of wandoo woodland and their impact on crop production. Wildlife Res. 1989, 16, 85–93. [Google Scholar] [CrossRef]
- Neave, H.; Tanton, M. The effects of grazing by kangaroos and rabbits on the vegetation and the habitat of other fauna in the Tidbinbilla Nature Reserve, Australian Capital Territory. Wildlife Res. 1989, 16, 337–351. [Google Scholar] [CrossRef]
- Coulson, G. Road-kills of macropds on a section of highway in central victoria. Wildlife Res. 1982, 9, 21–26. [Google Scholar] [CrossRef]
- Tribe, A.; Hanger, J.; McDonald, I.J.; Loader, J.; Nottidge, B.J.; McKee, J.J.; Phillips, C.J.C. A reproductive management program for an urban population of eastern grey kangaroos (Macropus giganteus). Animals 2014, 4, 562–582. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.W.; Herbert, C.A. Genetics, biotechnology and population management of over-abundant mammalian wildlife in australasia. Reprod. Fert. Develop. 2001, 13, 451–458. [Google Scholar] [CrossRef]
- Herbert, C.A.; Trigg, T.E.; Cooper, D.W. Fertility control in female eastern grey kangaroos using the GnRH agonist deslorelin—Effects on reproduction. Wildlife Res. 2006, 33, 41–46. [Google Scholar] [CrossRef]
- Coulson, G.; Nave, C.D.; Shaw, G.; Renfree, M.B. Long-term efficacy of levonorgestrel implants for fertility control of eastern grey kangaroos (Macropus giganteus). Wildlife Res. 2008, 35, 520–524. [Google Scholar] [CrossRef]
- Herbert, C.A.; Trigg, T.E.; Renfree, M.B.; Shaw, G.; Eckery, D.C.; Cooper, D.W. Long-term effects of deslorelin implants on reproduction in the female tammar wallaby (Macropus eugenii). Reproduction 2005, 129, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Lohr, C.A.; Mills, H.; Robertson, H.; Bencini, R. Deslorelin implants control fertility in urban brushtail possums (Trichosurus vulpecula) without negatively influencing their body-condition index. Wildlife Res. 2009, 36, 324–332. [Google Scholar] [CrossRef]
- Mayberry, C. Management of Isolated Populations of Western Grey Kangaroos (Macropus Fuliginosus Ocydromus) through Fertility Control, in South-Western Australia. Ph.D. Thesis, The University of Western Australia, Crawley, Australia, 2010. [Google Scholar]
- Eymann, J.; Herbert, C.A.; Thomson, B.P.; Trigg, T.E.; Cooper, D.W.; Eckery, D.C. Effects of deslorelin implants on reproduction in the common brushtail possum (Trichosurus vulpecula). Reprod. Fert. Develop. 2007, 19, 899–909. [Google Scholar] [CrossRef]
- Herbert, C.A.; Eckery, D.C.; Trigg, T.E.; Cooper, D.W. Chronic treatment of female tammar wallabies with deslorelin implants during pouch life: Effects on reproductive maturation. Reprod. Fert. Develop. 2013, 25, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Herbert, C.A.; Trigg, T.E.; Cooper, D.W. Effect of deslorelin implants on follicular development, parturition and post-partum oestrus in the tammar wallaby (Macropus eugenii). Reproduction 2004, 127, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.B.; Stuckey, B.G.A.; Tjondronegoro, S.; Keogh, E.J. The Need for More Precise Assessment of the Biological Activity of GnRH Agonists, Proceedings of the Thirteenth International Congress on Animal Reproduction Sydney, Sydney, Australia, 30 June 4–July 1996; pp. 3–12.
- Herbert, C.A.; Trigg, T.E.; Renfree, M.B.; Shaw, G.; Eckery, D.C.; Cooper, D.W. Effects of a gonadotropin-releasing hormone agonist implant on reproduction in a male marsupial, Macropus eugenii. Biol. Reprod. 2004, 70, 1836–1842. [Google Scholar] [CrossRef] [PubMed]
- Tuyttens, F.A.M.; Macdonald, D.W. Fertility control: An option for non-lethal control of wild carnivores? Anim. Welfare 1998, 7, 339–364. [Google Scholar]
- Ramsey, D. Effects of fertility control on behavior and disease transmission in brushtail possums. J. Wildlife Manag. 2007, 71, 109–116. [Google Scholar] [CrossRef]
- Eldridge, M.D.B.; Close, R.L. Chromosomes and evolution in rock-wallabies, Petrogale (Marsupialia: Macropodidae). Aust. Mammal. 1997, 19, 123–135. [Google Scholar]
- Burbidge, A.A.; McKenzie, N.L.; Brennan, K.E.C.; Woinarski, J.C.Z.; Dickman, C.R.; Baynes, A.; Gordon, G.; Menkhorst, P.W.; Robinson, A.C. Conservation status and biogeography of Australia’s terrestrial mammals. Aust. J. Zool. 2009, 56, 411–422. [Google Scholar] [CrossRef]
- IUCN. IUCN Red List of Threatened Species. Aviable online: http://www.Iucnredlist.Org. (accessed on 26 July 2015).
- Pearson, D.J.; Kinnear, J.E. A review of the distribution, status and conservation of rock-wallabies in Western Australia. Aust. Mammal. 1997, 19, 137–152. [Google Scholar]
- Eldridge, M.D.B.; Pearson, D.J. Black-footed rock-wallaby. In The Mammals of Australia, 3rd ed.; van Dyck, S., Strahan, R., Eds.; Reed New Holland: Bowling Green, KY, USA, 2008; pp. 376–380. [Google Scholar]
- Kinnear, J.E.; Onus, M.L.; Bromilow, R.N. Fox control and rock wallaby population dynamics. Aus. Wildlife Res. 1988, 15, 435–450. [Google Scholar] [CrossRef]
- Kinnear, J.E.; Krebs, C.J.; Pentland, C.; Orell, P.; Holme, C.; Karvinen, R. Predator-baiting experiments for the conservation of rock-wallabies in western Australia: A 25-year review with recent advances. Wildlife Res. 2010, 37, 57–67. [Google Scholar] [CrossRef]
- Kinnear, J.E.; Onus, M.L.; Sumner, N.R. Fox control and rock wallaby population dynamics—An update. Wildlife Res. 1998, 25, 81–88. [Google Scholar] [CrossRef]
- Willers, N.; Mawson, P.; Morris, K.; Bencini, R. Biology and population dynamics of the black-flanked rock-wallaby (Petrogale lateralis lateralis) in the central wheatbelt of Western Australia. Aust. Mammal. 2011, 33, 117–127. [Google Scholar] [CrossRef]
- Bertschinger, H.J.; Guimarães, M.A.B.V.; Trigg, T.E.; Human, A. The use of deslorelin implants for the long-term contraception of lionesses and tigers. Wildlife Res. 2008, 35, 525–530. [Google Scholar] [CrossRef]
- Willers, N.; Butler, R.; Mayberry, C.; Mawson, P. Bronchogenic carcinoma in a male rock wallaby (Petrogale lateralis lateralis). Aust. Mammal. 2010, 32, 205–206. [Google Scholar] [CrossRef]
- NHMRC. Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, 7th ed.Australian Government: Canberra, Australian, 2004; pp. 1–84.
- Jones, M.; Taggart, D.; Temple-Smith, P. Age determination and growth in wild Petrogale lateralis pearsoni and captive Petrogale lateralis “Macdonnell ranges race”. Aust. J. Zool. 2004, 52, 447–461. [Google Scholar] [CrossRef]
- Scheele, F.; Hompes, P.G.A.; Lambalk, C.B.; Schoute, E.; Broekmans, F.J.; Schoemaker, J. The GnRH challenge test: A quantitative measure of pituitary desensitization during gnrh agonist administration. Clin. Endocrinol. 1996, 44, 581–586. [Google Scholar] [CrossRef]
- Matson, P.; Mayberry, C.; Willers, N.; Blackberry, M.A.; Martin, G.B. The measurement of luteinising hormone in the western grey kangaroo (Macropus fuliginosus ocydromus) and the black-flanked rock wallaby (Petrogale lateralis lateralis). Aust. Mammal. 2009, 31, 61–63. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Amer. Statist. Assn. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. The logrank test. Brit. Med. J. 2004. [Google Scholar] [CrossRef] [PubMed]
- Robert, K.A.; Schwanz, L.E. Monitoring the health status using free-ranging tammar wallabies using hematology, serum biochemistry and parasite loads. J. Wildlife Manag. 2013, 77, 1232–1243. [Google Scholar] [CrossRef]
- Wayne, A.F.; Ward, C.G.; Rooney, J.F.; Vellios, C.V.; Lindenmayer, D.B. The life history of Trichosurus vulpecula hypoleucus (Phalangeridae) in the jarrah forest of South-Western Australia. Aust. J. Zool. 2005, 53, 265–278. [Google Scholar] [CrossRef]
- Johnson, P.M. Reproduction in the plain rock wallaby, Petrogale penicillata inornata Gould, in captivity, with age estimation of the pouch young. Aust. Wildlife Res. 1979, 6, 1–4. [Google Scholar] [CrossRef]
- Poole, W.E.; Merchant, J.C.; Carpenter, S.M.; Calaby, J.H. Reproduction, growth and age determination in the yellow-footed rock wallaby Petrogale xanthopus Gray, in captivity. Aust. Wildlife Res. 1985, 12, 127–136. [Google Scholar] [CrossRef]
- Johnson, P.M.; Delean, S.C. Reproduction in the proserpine rock wallaby, Petrogale persephone Maynes (Marsupialia: Macropodidae), in captivity, with age estimation and development of pouch young. Wildlife Res. 1999, 26, 631–639. [Google Scholar] [CrossRef]
- Johnson, P.M.; Delean, S. Reproduction of the purple-necked rock wallaby, Petrogale purpureicollis Le Souef (Marsupialia: Macropodidae) in captivity, with age estimation and development of the pouch young. Wildlife Res. 2002, 29, 463–468. [Google Scholar] [CrossRef]
- Taggart, D.A.; Schultz, D.; White, C.; Whitehead, P.; Underwood, G.; Phillips, K. Cross-fostering, growth and reproductive studies in the brush-tailed rock wallaby, Petrogale penicillata (Marsupialia: Macropodidae): Efforts to accelerate breeding in a threatened marsupial species. Aust. J. Zool. 2005, 53, 313–323. [Google Scholar] [CrossRef]
- Junaidi, A.; Williamson, P.E.; Martin, G.B.; Blackberry, M.A.; Cummins, J.M.; Trigg, T.E. Dose-response studies for pituitary and testicular function in male dogs treated with the gnrh superagonist, deslorelin. Reprod. Domest. Anim. 2009, 44, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Herbert, C.A.; Trigg, T.E. Applications of GnRH in the control and management of fertility in female animals. Anim. Reprod. Sci. 2005, 88, 141–153. [Google Scholar] [CrossRef] [PubMed]
- D’Occhio, M.J.; Aspden, W.J.; Whyte, T.R. Controlled, reversible suppression of estrous cycles in beef heifers and cows using agonists of gonadotropin-releasing hormone. J. Anim. Sci. 1996, 74, 218–225. [Google Scholar] [PubMed]
- Turner, N.C. Sustainable production of crops and pastures under drought in a mediterranean environment. Ann. Appl. Biol. 2004, 144, 139–147. [Google Scholar] [CrossRef]
- Gélin, U.; Wilson, M.E.; Coulson, G.; Festa-Bianchet, M. Experimental manipulation of female reproduction demonstrates its fitness costs in kangaroos. J. Anim. Ecol. 2015, 84, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Willers, N.; Berry, O.; Roberts, J.D. Fine-scale genetic structure and the design of optimal fertility control for an overabundant mammal. Conserv. Genet. 2014, 15, 1053–1062. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Willers, N.; Martin, G.B.; Matson, P.; Mawson, P.R.; Morris, K.; Bencini, R. Finding the Balance: Fertility Control for the Management of Fragmented Populations of a Threatened Rock-Wallaby Species. Animals 2015, 5, 1329-1344. https://doi.org/10.3390/ani5040414
Willers N, Martin GB, Matson P, Mawson PR, Morris K, Bencini R. Finding the Balance: Fertility Control for the Management of Fragmented Populations of a Threatened Rock-Wallaby Species. Animals. 2015; 5(4):1329-1344. https://doi.org/10.3390/ani5040414
Chicago/Turabian StyleWillers, Nicole, Graeme B. Martin, Phill Matson, Peter R. Mawson, Keith Morris, and Roberta Bencini. 2015. "Finding the Balance: Fertility Control for the Management of Fragmented Populations of a Threatened Rock-Wallaby Species" Animals 5, no. 4: 1329-1344. https://doi.org/10.3390/ani5040414





