The Role of TCA Cycle Anaplerosis in Ketosis and Fatty Liver in Periparturient Dairy Cows
Abstract
:1. Introduction
2. Ketosis and Fatty Liver Disease
3. Anaplerosis and Cataplerosis of the TCA Cycle
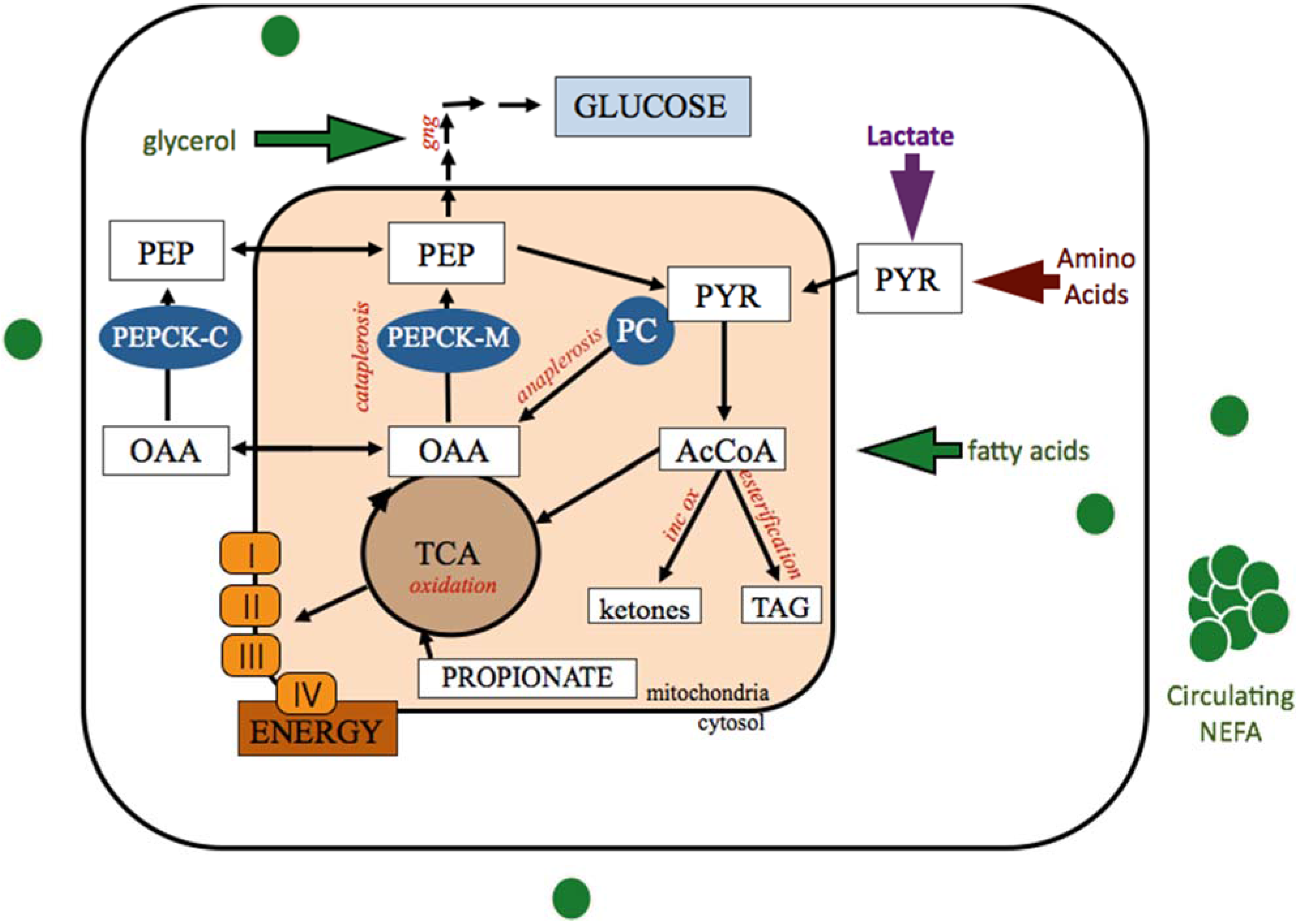
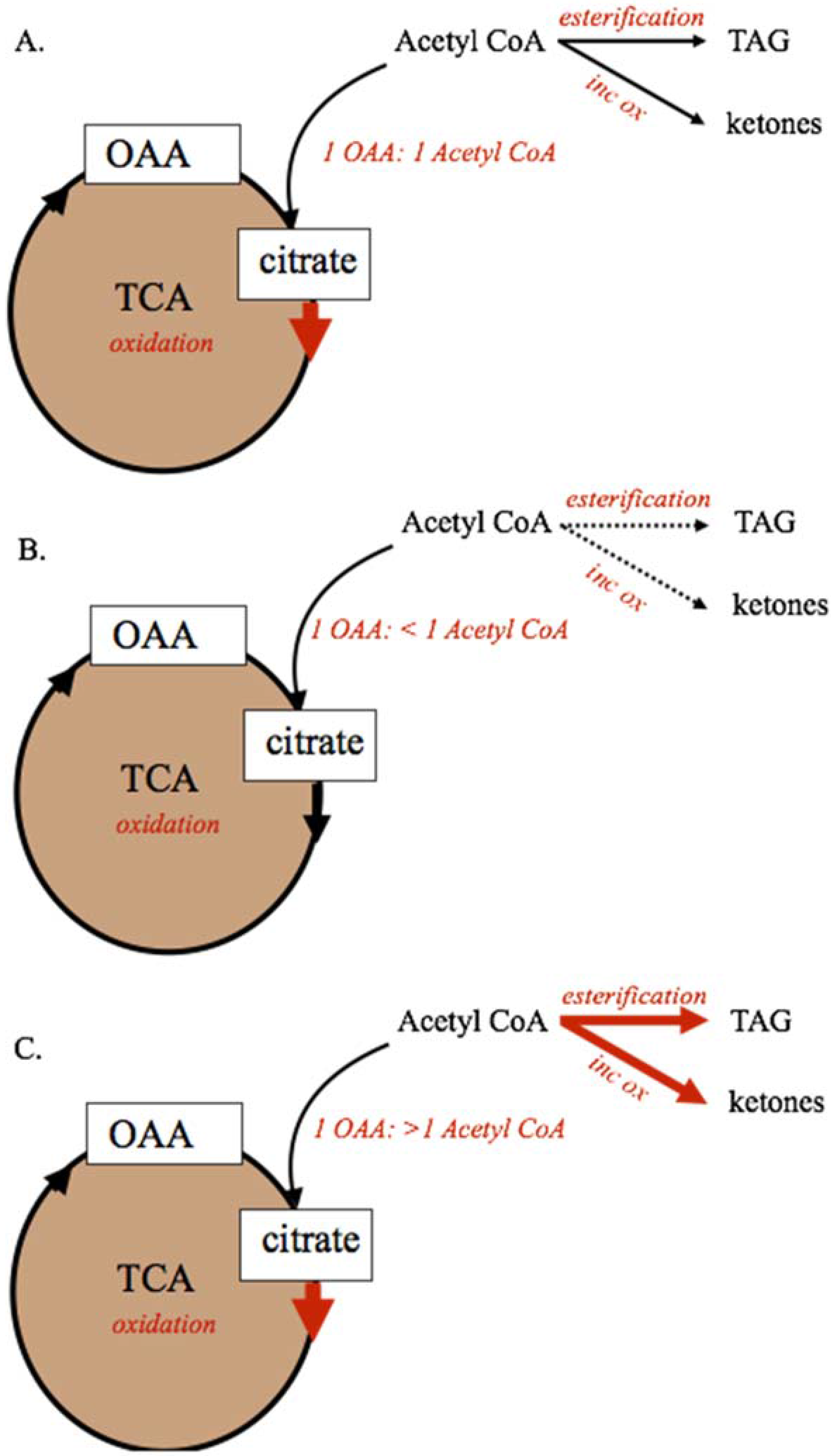
4. Conclusions and Future Directions
Conflicts of Interest
References
- Dole, V.P. A relation between non-esterified fatty acids in plasma and the metabolism of glucose. J. Clin. Investig. 1956, 35, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Gordon, R.S.; Cherkes, A. Unesterified fatty acid in human blood plasma. J. Clin. Investig. 1956, 35, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Grummer, R.R. Etiology of Lipid-Related Metabolic Disorders in Periparturient Dairy Cows. J. Dairy Sci. 1993, 76, 1–15. [Google Scholar] [CrossRef]
- Drackley, J.K. ADSA Foundation Scholar Award. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Drackley, J.K.; Veenhuizen, J.J.; Richard, M.J.; Young, J.W. Metabolic Changes in Blood and Liver of Dairy Cows During Either Feed Restriction or Administration of 1,3-Butanediol. J. Dairy Sci. 1991, 74, 4254–4264. [Google Scholar] [CrossRef]
- Drackley, J.K.; Richard, M.J.; Beitz, D.C.; Young, J.W. Metabolic changes in dairy cows with ketonemia in response to feed restriction and dietary 1,3-butanediol. J. Dairy Sci. 1992, 75, 1622–1634. [Google Scholar] [CrossRef]
- Emery, R.S.; Liesman, J.S.; Herdt, T.H. Metabolism of long chain fatty acids by ruminant liver. J. Nutr. 1992, 122, 832–837. [Google Scholar] [PubMed]
- Koltes, D.A.; Spurlock, D.M. Coordination of lipid droplet-associated proteins during the transition period of Holstein dairy cows. J. Dairy Sci. 2011, 94, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Locher, L.F.; Meyer, N.; Weber, E.M.; Rehage, J.; Meyer, U.; Dänicke, S.; Huber, K. Hormone-sensitive lipase protein expression and extent of phosphorylation in subcutaneous and retroperitoneal adipose tissues in the periparturient dairy cow. J. Dairy Sci. 2011, 94, 4514–4523. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.W. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [PubMed]
- Drackley, J.K.; Dann, H.M.; Douglas, G.N.; Guretzky, N. Physiological and pathological adaptations in dairy cows that may increase susceptibility to periparturient diseases and disorders. Ital. J. Anim. Sci. 2005, 4, 323–344. [Google Scholar] [CrossRef]
- Adewuyi, A.A.; Gruys, E.; van Eerdenburg, F.J.C.M. Non esterified fatty acids (NEFA) in dairy cattle. A review. Vet. Q. 2005, 27, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Palmquist, D.L. Milk Fat: Origin of fatty acids and influence of nutritional factors thereon. In Advanced Dairy Chemistry, Volume Lipids; Fox, P.F., McSweeney, P.L., Eds.; Springer: New York, NY, USA, 2006; pp. 43–92. [Google Scholar]
- Bell, A.W. Lipid metabolism in liver and selected tissues and in the whole body of ruminant animals. Prog. Lipid Res. 1979, 18, 117–164. [Google Scholar] [CrossRef]
- Reynolds, C.K.; Aikman, P.C.; Lupoli, B.; Humphries, D.J.; Beever, D.E. Splanchnic metabolism of dairy cows during the transition from late gestation through early lactation. J. Dairy Sci. 2003, 86, 1201–1217. [Google Scholar] [CrossRef]
- Lomax, M.A.; Baird, G.D. Blood flow and nutrient exchange across the liver and gut of the dairy cow. Br. J. Nutr. 1983, 49, 481–496. [Google Scholar] [CrossRef] [PubMed]
- McArt, J.A.A.; Nydam, D.V.; Ospina, P.A.; Oetzel, G.R. A field trial on the effect of propylene glycol on milk yield and resolution of ketosis in fresh cows diagnosed with subclinical ketosis. J. Dairy Sci. 2011, 94, 6011–6020. [Google Scholar] [CrossRef] [PubMed]
- Duffield, T. Subclinical ketosis in lactating dairy cattle. Vet. Clin. N. Am. Food Anim. Pract. 2000, 16, 231–253. [Google Scholar]
- Bobe, G.; Young, J.W.; Beitz, D.C. Invited review: Pathology, etiology, prevention, and treatment of fatty liver in dairy cows. J. Dairy Sci. 2004, 87, 3105–3124. [Google Scholar] [CrossRef]
- Hoyumpa, D.A.M., Jr.; Greene, H.L.; Dunn, G.D.; Schenker, S. Fatty liver: Biochemical and clinical considerations. Digest Dis. Sci. 1975, 20, 1142–1170. [Google Scholar] [CrossRef]
- Grum, D.E.; Drackley, J.K.; Younker, R.S.; LaCount, D.W.; Veenhuizen, J.J. Nutrition during the dry period and hepatic lipid metabolism of periparturient dairy cows. J. Dairy Sci. 1996, 79, 1850–1864. [Google Scholar] [CrossRef]
- Drackley, J.K.; Beaulieu, A.D.; Elliott, J.P. Responses of milk fat composition to dietary fat or nonstructural carbohydrates in Holstein and Jersey cows. J. Dairy Sci. 2001, 84, 1231–1237. [Google Scholar] [CrossRef]
- Kornberg, H.L. The role and control of the glyoxylate cycle in Escherichia coli. Biochem. J. 1966, 99, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef] [PubMed]
- Aschenbach, J.R.; Kristensen, N.B.; Donkin, S.S.; Hammon, H.M.; Penner, G.B. Gluconeogenesis in dairy cows: The secret of making sweet milk from sour dough. IUBMB Life 2010, 62, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Jitrapakdee, S.; Vidal-Puig, A.; Wallace, J.C. Anaplerotic roles of pyruvate carboxylase in mammalian tissues. Cell. Mol. Life Sci. 2006, 63, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Jitrapakdee, S.; St Maurice, M.; Rayment, I.; Cleland, W.W.; Wallace, J.C.; Attwood, P.V. Structure, mechanism and regulation of pyruvate carboxylase. Biochem. J. 2008, 413, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Schäff, C.; Börner, S.; Hacke, S.; Kautzsch, U.; Albrecht, D.; Hammon, H.M.; Röntgen, M.; Kuhla, B. Increased Anaplerosis, TCA Cycling, and Oxidative Phosphorylation in the Liver of Dairy Cows with Intensive Body Fat Mobilization during Early Lactation. J. Proteome Res. 2012, 11, 5503–5514. [Google Scholar] [CrossRef] [PubMed]
- Baird, G.D.; Heitzman, R.J.; Hibbitt, K.G. Effects of starvation on intermediary metabolism in the lactating cow. A comparison with metabolic changes occurring during bovine ketosis. Biochem. J. 1972, 128, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Van Knegsel, A.T.M.; van den Brand, H.; Dijkstra, J.; Tamminga, S.; Kemp, B. Effect of dietary energy source on energy balance, production, metabolic disorders and reproduction in lactating dairy cattle. Reprod. Nutr. Dev. 2005, 45, 665–688. [Google Scholar] [CrossRef] [PubMed]
- Bequette, B.J.; Sunny, N.E.; El-Kadi, S.W.; Owens, S.L. Application of stable isotopes and mass isotopomer distribution analysis to the study of intermediary metabolism of nutrients. J. Anim. Sci. 2006, 84, E50–E59. [Google Scholar] [PubMed]
- White, H.M.; Koser, S.L.; Donkin, S.S. Gluconeogenic enzymes are differentially regulated by fatty acid cocktails in Madin-Darby bovine kidney cells. J. Dairy Sci. 2012, 95, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- White, H.M.; Donkin, S.S.; Lucy, M.C.; Grala, T.M.; Roche, J.R. Short communication: Genetic differences between New Zealand and North American dairy cows alter milk production and gluconeogenic enzyme expression1. J. Dairy Sci. 2011, 95, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, R.B.; Cecava, M.J.; Donkin, S.S. Changes in mRNA expression for gluconeogenic enzymes in liver of dairy cattle during the transition to lactation. J. Dairy Sci. 2000, 83, 1228–1236. [Google Scholar] [CrossRef]
- White, H.M.; Koser, S.L.; Donkin, S.S. Bovine pyruvate carboxylase 5′ untranslated region variant expression during transition to lactation and feed restriction in dairy cows. J. Anim. Sci. 2011, 89, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- White, H.M.; Carvalho, E.R.; Donkin, S.S. Regulation of hepatic gluconeogenic enzymes by dietary glycerol in transition dairy cows. J. Dairy Sci. 2010, 88 (Suppl. S2), 719. [Google Scholar]
- Loor, J.J.; Dann, H.M.; Guretzky, N.A.J.; Everts, R.E.; Oliveira, R.; Green, C.A.; Litherland, N.B.; Rodriguez-Zas, S.L.; Lewin, H.A.; Drackley, J.K. Plane of nutrition prepartum alters hepatic gene expression and function in dairy cows as assessed by longitudinal transcript and metabolic profiling. Physiol. Genom. 2006, 27, 29–41. [Google Scholar] [CrossRef] [PubMed]
- White, H.M.; Koser, S.L.; Donkin, S.S. Characterization of bovine pyruvate carboxylase promoter 1 responsiveness to serum from control and feed-restricted cows. J. Anim. Sci. 2011, 89, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- White, H.M.; Koser, S.L.; Donkin, S.S. Differential regulation of bovine pyruvate carboxylase promoters by fatty acids and peroxisome proliferator-activated receptor-α agonist. J. Dairy Sci. 2011, 94, 3428–3436. [Google Scholar] [CrossRef] [PubMed]
- Grum, D.E.; Drackley, J.K.; Hansen, L.R.; Cremin, J.D. Production, digestion, and hepatic lipid metabolism of dairy cows fed increased energy from fat or concentrate. J. Dairy Sci. 1996, 79, 1836–1849. [Google Scholar] [CrossRef]
- Loor, J.J.; Everts, R.E.; Bionaz, M.; Dann, H.M.; Morin, D.E.; Oliveira, R.; Rodriguez-Zas, S.L.; Drackley, J.K.; Lewin, H.A. Nutrition-induced ketosis alters metabolic and signaling gene networks in liver of periparturient dairy cows. Physiol. Genom. 2007, 32, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Karcher, E.L.; Pickett, M.M.; Varga, G.A.; Donkin, S.S. Effect of dietary carbohydrate and monensin on expression of gluconeogenic enzymes in liver of transition dairy cows. J. Anim. Sci. 2007, 85, 690–699. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, E.R.; Schmelz-Roberts, N.S.; White, H.M.; Doane, P.H.; Donkin, S.S. Replacing corn with glycerol in diets for transition dairy cows. J. Dairy Sci. 2011, 94, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of Body Condition Score on Relationships Between Metabolic Status and Oxidative Stress in Periparturient Dairy Cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef]
- Turk, R.; Juretić, D.; Gereš, D.; Svetina, A.; Turk, N.; Flegar-Meštrić, Z. Influence of oxidative stress and metabolic adaptation on PON1 activity and MDA level in transition dairy cows. Anim. Reprod. Sci. 2008, 108, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Hernández, J.; Benedito, J.L.; Castillo, C. The importance of the oxidative status of dairy cattle in the periparturient period: Revisiting antioxidant supplementation. J. Anim. Physiol. Anim. Nutr. (Berl.) 2014. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, L.M.; Aitken, S.L. Impact of oxidative stress on the health and immune function of dairy cattle. Vet. Immunol. Immunopathol. 2009, 128, 104–109. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, H.M. The Role of TCA Cycle Anaplerosis in Ketosis and Fatty Liver in Periparturient Dairy Cows. Animals 2015, 5, 793-802. https://doi.org/10.3390/ani5030384
White HM. The Role of TCA Cycle Anaplerosis in Ketosis and Fatty Liver in Periparturient Dairy Cows. Animals. 2015; 5(3):793-802. https://doi.org/10.3390/ani5030384
Chicago/Turabian StyleWhite, Heather M. 2015. "The Role of TCA Cycle Anaplerosis in Ketosis and Fatty Liver in Periparturient Dairy Cows" Animals 5, no. 3: 793-802. https://doi.org/10.3390/ani5030384




