Lameness Detection in Dairy Cows: Part 2. Use of Sensors to Automatically Register Changes in Locomotion or Behavior
Abstract
:Simple Summary
Abstract
1. Introduction
2. Automatic Gait and Behavior Measurements and Lameness Detection
2.1. Load Cells
2.1.1. Measurement of Walking Cows
2.1.2. Measurement of Standing Cows
2.2. Pressure-Sensitive Position Mat
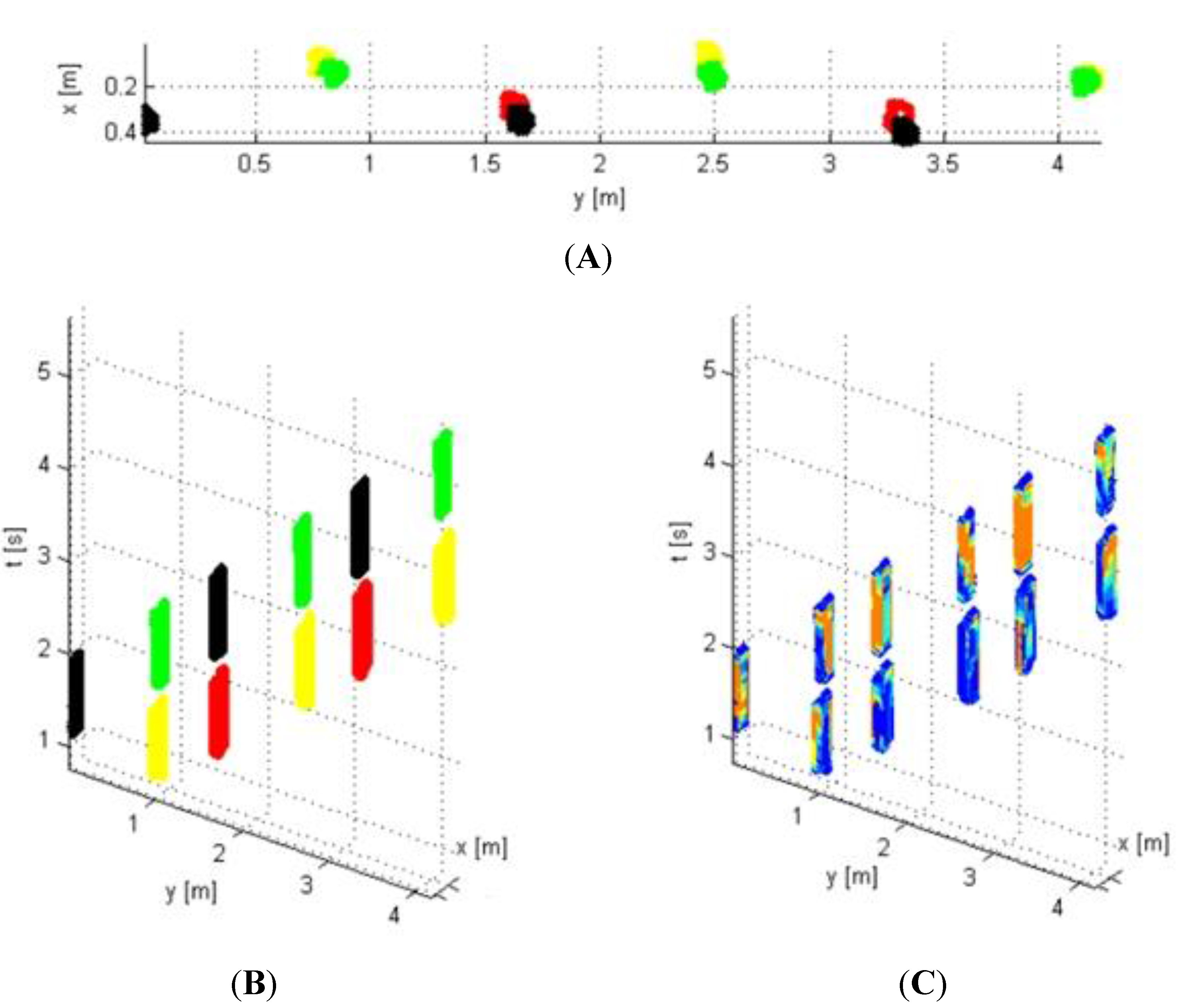
2.3. Vision Techniques
2.4. Measuring Gait and/or Activity Using Accelerometers
2.5. Measuring Lying Time Using Accelerometers
2.6. Combining Already Available Sensor Data
3. Practical Considerations in the Development of Lameness Detection Systems
| Experimental set-up | Lameness reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Sensor type | Number of cows | Normal routine | N° of variables per variable type | Automated measurements | In real- time | Person performing scoring | Number classes used | Cut-off levels for lameness |
| Rajkondawar et al. [8] | 2 floor plates with 4 load cells | 23 | no | weight variables (6) | no | no | herdsman and veterinarian | 1 to 5 | |
| Rajkondawar et al. [9] | 2 floor plates with 4 load cells | 31 | no | weight variables (5) | yes | yes | observer | 1 to 5 | classes 1 - 2 - 3 |
| Pastell and Kujala [19] | 4 balance system on floor in milking robot | 73 | yes | weight variables (26) | yes | yes | observer | 1 to 5 | non-lame: 1 and 2 + no claw lesions lame: 3 and more |
| Chapinal et al. [23] | speed | 66 | no | speed | no | no | observer | 1 to 5 | non-lame: 1 - 2 lame: 3 - 4- 5 |
| weighing platform with 4 balances | weight variables (12) | no | |||||||
| Pedometer | lying behaviour (2) | yes | |||||||
| Chapinal et al. [24] | speed | 57 | no | speed | no | no | observer | 1 to 5 | non-lame: 1 - 2 lame: 3 - 4- 5 |
| weighing platform with 4 balances | weight variables (12) | no | |||||||
| Pedometer | lying behaviour (2) | yes | |||||||
| Pastell et al. [25] | weighing platform with 4 balances | 55 | no | weight variables (16) | no | no | observer | 1 to 5 | non-lame: 1 - 2 lame: 3 - 4- 5 |
| Liu et al. [12] | StepMetrix | 346 | no | weight variables (5) and symmetry variables | yes | yes | observer | 1 to 5 | non-lame: 1 -2 (3) lame: (3) - 4 - 5 |
| Chapinal and Tucker [26] | weighing platform with 4 balances | 57 | no | steps (validate by camera observations + frequency of steps + weight shifting | no | no | observer | 1 to 5 | non-lame: 1 - 2 - 3 lame: 4 - 5 |
| Thorup et al. [16] | 2 force plates with 4 load cells | 9 | no | Full curve symmetry in 3 dimensions | no | no | observer | 1 to 5 | Non-lame: 1 lame: 2 - 5 |
| Experimental set-up | Lameness reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Sensor type | Number of cows | Normal routine | N° of variables per variable type | Automated measurements | In real- time | Person performing scoring | Number classes used | Cut-off levels for lameness |
| Maertens et al. [29] | Pressure sensitive mat | 159 | yes | Basic gait variables (20) Specific gait variables (10) | yes | yes | observer | 1 to 3 | non-lame: 1 mildly lame: 2 severely lame: 3 |
| Van Nuffel et al. [31] | Pressure sensitive mat | 40 | yes | Basic gait variables (20) Gait inconsistency variables (20) | yes | yes | observer | 1 to 3 | non-lame: 1 mildly lame: 2 severely lame: 3 |
| Van Nuffel et al. [32] | Pressure sensitive mat | 36 | yes | Basic gait variables (20) Gait inconsistency variables (20) | yes | yes | observer | 1 to 3 | non-lame: 1 mildly lame: 2 severely lame: 3 |
| Experimental set-up | Lameness reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Sensor type | Number of cows | Normal routine | N° of variables per variable type | Automated measurements | In real- time | Person performing scoring | Number classes used | Cut-off levels for lameness |
| Song et al. [34] | digital camera | 15 | no | 1 (trackway overlap) | no | no | observer | 1 to 5 | all classes separate |
| Pluk et al. [35] | digital camera | 15/66 | no/yes | 1 (trackway overlap) | no/yes | no/no | observer | 1 to 5 | all classes separate |
| Pluk et al. [36] | digital camera combined with Gaitwise-system | 70/75 | yes/yes | 3 (touch angle, release angle, range of motion in the fetlock joint) | no/yes | no/no | observer | 1 to 3 | all classes separate |
| Poursaberi et al. [37] | digital camera | 28/66 | yes/yes | 1 (back posture) | no/no | no/no | observer | 1 to 3 | all classes separate |
| Blackie et al. [64] | leg markers, digital camera | 56 | no | 7 (stride length front and hind, tracking distance, hock flexion, max fetlock height, height of spine, head position) | no | no | observer | 1 to 5 | all classes separate (no 4 and 5 used) |
| Viazzi et al. [39] | digital camera | 98 | yes | 1 (body movement pattern) | no | no | observer | 1 to 5 | non-lame: 1 + 2 lame: 3 severely lame: 4 + 5 |
| Van Hertem et al. [43] | 3D-digital camera | 186 | yes | 1 (back posture measurement) | yes | no | observer | 1 to 5 | - all classes separate; - all classes separate with 1 level tolerance; - non-lame: 1 + 2 lame: 3 – 5 |
| Viazzi et al. [40] | digital camera and 3D-digital camera | 273 | yes | 1 back posture in 2D 1 back posture in 3D | no/yes | no/no | observer | 1 to 5 | non-lame: 1 + 2 lame: 3 - 5 |
| Experimental set-up | Lameness reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Sensor type | Number of cows | Normal routine | N° of variables per variable type | Automated measurements | In real-time | Person performing scoring | Number classes used | Cut-off levels for lameness |
| Mazrier et al. [48] | pedometer on hind leg | 400 | yes | activity (1) | yes | no | herdsman | ||
| Pastell et al. [50] | pedometers both hind legs | 6 non-lame 6 severely lame | no | activity (6) | no | no | observer | 1 to 5 | non-lame: 1 + 2 lame: 4 |
| Ito et al. [65] | pedometers | 1319 | yes | 4 (lying behaviour) | yes | no | observer | 1 to 5 | non-lame: 1 + 2 lame: 3 severely lame: 4 + (5) |
| Blackie et al. [2] | markers and video images pedometers | 25 | no yes | gait variables (7) lying behaviour (6) activity | no yes yes | no no no | observer | 1 to 5 | all (no 4 and 5 present) |
| Calderon and Cook [57] | pedometer on hind leg | 57 | yes | 3 (lying behaviour) | yes | no | observer | 1 to 4 | non-lame: 1 lame: 2 severely lame: 3 |
| Chapinal et al. [51] | accelerometers (all 4 legs + around torso) | 12/24 | no | Speed accelation variables (2) | no | no | observer | 1-5 + VAS | all |
| Alsaaod et al. [53] | pedometers on front leg | 30 | no | activity (1) 4 (lying behaviour) | yes | no | observer | 1 to 5 | non-lame: 1 + 2 lame: 3 severely lame: 4 |
| Yunta et al. [56] | pedometers | 250 | yes | 4 (lying behaviour) | yes | no | observer | 1 to 5 | non-lame: 1 lame: 3 + 4 |
| Navarro et al. [66] | pedometers | 400 | yes | standing and lying time | yes | no | observer | 1 to 5 | non-lame: 1 |
| Thorup et al. [52] | Accelerometer on 1 hind leg | 348 | yes | 13 | no | no | observer | 1 to 5 | non-lame: 1 lame: 2 - 5 |
| Experimental set-up | Lameness reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Source | Sensor type | Number of cows | Normal routine | N° of variables per variable type | Automated measurements | In real-time | Person performing scoring | Number classes used | Cut-off levels for lameness |
| De Mol et al. [59] | activity data (7) | 100 | yes | activity data (7) | yes | no | herdsmen | 1 to 5 | non-lame: 1 lame: 3 - 4 - 5 (score 2 excluded) |
| Kramer et al. [67] | milkmeters | 81 | yes | milk yield | yes | Yes | herdsman | / | Logbook lameness events |
| feeding and drinking behaviour | feeding behavior (4) | No | |||||||
| activity meters (neck) | activity | No | |||||||
| farm health records | info preliminary diseases | No | |||||||
| Miekley et al. [68] | pedometers | 653 | yes | activity data (1) | yes | no | herdsmen and veterinarian | Logbook lameness events | |
| milking data | milking data (2) | ||||||||
| feeding data | feeding data (3) | ||||||||
| Miekley et al. [69] | pedometer activity | 315 | yes | activity data (1) | yes | no | herdsmen and veterinarian | Logbook lameness events | |
| feeding patterns | feeding data (3) | ||||||||
| milking data (1) | milking data (1) | ||||||||
| feeding data (1) | feeding data (1) | ||||||||
| Kamphuis et al. [58] | weight scales | 318 lame and 3180 non-lame | yes | weight scales (1) | yes | yes | (trained) herdsmen | 1 to 5 | Logbook lameness events |
| pedometers | pedometers (1) | no | |||||||
| milk meters | milk meters (4) | yes | |||||||
| Van Hertem et al. [40] | neck activity | 44 lame and 74 non-lame | yes | neck activity (1) | yes | no | herdsmen | Logbook lameness events | |
| ruminating time | ruminating time (1) | no | |||||||
| milking data | milking datad (5) | yes | |||||||
| Garcia et al. [61] | milking data neck activity | 88 | yes | milking data (320) activity index | no | no | observer | 1 to 4 | non-lame: 1 lame: 3 + 4 (score 2 excluded) |
| Norring et al. [70] | automatic feeders milking data weight scales | 50 | yes | Feeding behavior (4) milk yield and milking frequency body weight | yes | no | observer | 1 to 5 | non-lame: 1 + 2 mildly lame: 3 severely lame: 4 + 5 |
4. Conclusions
Author Contributions
Conflicts of Interest
References and Notes
- Van Nuffel, A.; Zwertvaegher, I.; Pluym, L.; Van Weyenberg, S.; Thorup, V.M.; Pastell, M.; Sonck, B.; Saeys, W. Lameness detection in dairy cows: Part 1. How to distinguish between non-lame and lame cows based on differences in locomotion or behavior? Animals 2015. [Google Scholar] [CrossRef] [Green Version]
- Blackie, N.; Bleach, E.; Amory, J.; Scaife, J. Impact of lameness on gait characteristics and lying behaviour of zero grazed dairy cattle in early lactation. Appl. Anim. Behav. Sci. 2011, 129, 67–73. [Google Scholar] [CrossRef]
- Schlageter-Tello, A.; Bokkers, E.A.; Groot Koerkamp, P.W.; Van Hertem, T.; Viazzi, S.; Romanini, C.E.; Halachmi, I.; Bahr, C.; Berckmans, D.; Lokhorst, K. Effect of merging levels of locomotion scores for dairy cows on intra- and interrater reliability and agreement. J. Dairy Sci. 2014, 97, 5533–5542. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.C.; Green, M.J.; Huxley, J.N. Association between milk yield and seriel locomotion score assessments in UK dairy cows. J. Dairy Sci. 2010, 93, 4045–4053. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, H.; Kamphuis, C.; Steeneveld, W.; Mollenhorst, H. Sensors and clinical mastitis—The quest for the perfect alert. Sensors 2010, 10, 7991–8009. [Google Scholar] [CrossRef] [PubMed]
- Rutten, C.J.; Velthuis, A.G.J.; Steeneveld, W.; Hogeveen, H. Sensors to support health management on dairy farms. J. Dairy Sci. 2013, 96, 1928–1952. [Google Scholar] [CrossRef] [PubMed]
- Rajkondawar, P.G.; Tasch, U.; Lefcourt, A.M.; Erez, B.; Dyer, R.M.; Varner, M.A. A system for identifying lameness in dairy cattle. Appl. Eng. Agr. 2002, 18, 87–96. [Google Scholar] [CrossRef]
- Rajkondawar, U.; Lefcourt, A.M.; Neerchal, N.K.; Dyer, R.M.; Varner, M.A.; Erez, B.; Tasch, U. The development of an objective lameness scoring system for dairy herds: pilot study. Trans. ASABE 2002, 45, 1123–1125. [Google Scholar] [CrossRef]
- Rajkondawar, P.G.; Liu, M.; Dyer, R.M.; Neerchal, N.K.; Tasch, U.; Lefcourt, A.M.; Erez, B.; Varner, M.A. Comparison of models to identify lame cows based on gait and lesion scores, and limb movement variables. J. Dairy Sci. 2006, 89, 4267–4275. [Google Scholar] [CrossRef]
- Bicalho, R.C.; Cheong, S.H.; Cramer, G.; Guard, C.L. Association between a visual and an automated locomotion score in lactating Holstein cows. J. Dairy Sci. 2007, 90, 3294–3300. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Neerchal, N.K.; Tasch, U.; Dyer, R.M.; Rajkondawar, P.G. Enhancing the prediction accuracy of bovine lameness models through transformations of limb movement variables. J. Dairy Sci. 2009, 92, 2539–2550. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dyer, R.M.; Neerchal, N.K.; Tasch, U.; Rajkondawar, P.G. Diversity in the magnitude of hind limb unloading occurs with similar forms of lameness in dairy cows. J. Dairy Res. 2011, 78, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Schamhardt, H.C.; Vandenbogert, A.J.; Hartman, W. Measurement Techniques in Animal Locomotion Analysis. Acta Anatomica 1993, 146, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.B. Changes in limb loading with lameness for a number of friesian cattle. Brit. Vet. J. 1989, 145, 28–38. [Google Scholar] [CrossRef]
- Skjøth, F.; Thorup, V.M.; do Nascimento, O.F.; Ingvartsen, K.L.; Rasmussen, M.D.; Voigt, M. Computerized identification and classification of stance phases as made by front or hind feet of walking cows based on 3-dimensional ground reaction forces. Comput. Electr. Agr. 2013, 90, 7–13. [Google Scholar] [CrossRef]
- Thorup, V.M.; do Nascimento, O.F.; Skjøth, F.; Voight, M.; Rasmussen, M.D.; Bennedsgaard, T.W.; Ingvartsen, K.L. Changes in gait symmetry in healthy and lame dairy cows based on 3-dimensional ground reaction forces following claw trimming. J. Dairy Sci. 2014, 97, 7679–7684. [Google Scholar] [CrossRef] [PubMed]
- Pastell, M.; Hautala, M.; Poikalainen, V.; Praks, J.; Veermae, I.; Kujala, M.; Ahokas, J. Automatic observation of cow leg health using load sensors. Comput. Electr. Agric. 2008, 62, 48–53. [Google Scholar] [CrossRef]
- Pastell, M.; Takko, H.; Grohn, H.; Hautala, M.; Poikalainen, V.; Praks, J.; Veermae, I.; Kujala, M.; Ahokas, J. Assessing cows’ welfare: Weighing the cow in a milking robot. Biosyst. Eng. 2006, 93, 81–87. [Google Scholar] [CrossRef]
- Pastell, M.E.; Kujala, M. A probabilistic neural network model for lameness detection. J. Dairy Sci. 2007, 90, 2283–2292. [Google Scholar] [CrossRef] [PubMed]
- Pastell, M.; Madsen, H. Application of CUSUM charts to detect lameness in a milking robot. Expert Syst. Appl. 2008, 35, 2032–2040. [Google Scholar] [CrossRef]
- Kujala, M.; Pastell, M.; Soveri, T. Use of force sensors to detect and analyse lameness in dairy cows. Vet. Rec. 2008, 162, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Neveux, S.; Weary, D.M.; Rushen, J.; von Keyserlingk, M.A.G.; de Passille, A.M. Hoof discomfort changes how dairy cattle distribute their body weight. J. Dairy Sci. 2006, 89, 2503–2509. [Google Scholar] [CrossRef]
- Chapinal, N.; de Passille, A.M.; Rushen, J.; Wagner, S. Effect of analgesia during hoof trimming on gait, weight distribution, and activity of dairy cattle. J. Dairy Sci. 2010, 93, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Chapinal, N.; de Passille, A.M.; Rushen, J.; Wagner, S. Automated methods for detecting lameness and measuring analgesia in dairy cattle. J. Dairy Sci. 2010, 93, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Pastell, M.; Hanninen, L.; de Pasille, A.M.; Rushen, J. Measures of weight distribution of dairy cows to detect lameness and the presence of hoof lesions. J. Dairy Sci. 2010, 93, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Chapinal, N.; Tucker, C.B. Validation of an automated method to count steps while cows stand on a weighing platform and its application as a measure to detect lameness. J. Dairy Sci. 2012, 95, 6523–6528. [Google Scholar] [CrossRef] [PubMed]
- van der Tol, P.P.J.; Metz, J.H.M.; Noordhuizen-Stassen, E.N.; Back, W.; Braam, C.R.; Weijs, W.A. The pressure distribution under the bovine claw during square standing on a flat substrate. J. Dairy Sci. 2002, 85, 1476–1481. [Google Scholar] [CrossRef]
- van der Tol, P.P.J.; Metz, J.H.M.; Noordhuizen-Stassen, E.N.; Back, W.; Braam, C.R.; Weijs, W.A. The vertical ground reaction force and the pressure distribution on the claws of dairy cows while walking on a flat substrate. J. Dairy Sci. 2003, 86, 2875–2883. [Google Scholar] [CrossRef]
- Maertens, W.; Vangeyte, J.; Baert, J.; Jantuan, A.; Mertens, K.C.; De Campeneere, S.; Pluk, A.; Opsomer, G.; Van Weyenberg, S.; Van Nuffel, A. Development of a real time cow gait tracking and analysing tool to assess lameness using a pressure sensitive walkway: The GAITWISE system. Biosyst. Eng. 2011, 110, 29–39. [Google Scholar] [CrossRef]
- Van Nuffel, A.; Sprenger, M.; Tuyttens, F.; Maertens, W. Can people see gait irregularities? Anim. Welf. 2009, 19, 433–439. [Google Scholar]
- Van Nuffel, A.; Vangeyte, J.; Mertens, K.C.; Pluym, L.; De Campeneere, S.; Saeys, W.; Opsomer, G.; Van Weyenberg, S. Exploration of measurement variation of gait variables for early lameness detection in cattle using the GAITWISE. Livestock Sci. 2013, 156, 88–95. [Google Scholar] [CrossRef]
- Van Nuffel, A. Development of an automated detection system for lameness in cattle: The Gaitwise system. Ph.D. Thesis, Katholic University of Leuven, Leuven, Belgium, 2014; p. 209. [Google Scholar]
- Flower, F.C.; Sanderson, D.J.; Weary, D.M. Hoof pathologies influence kinematic measures of dairy cow gait. J. Dairy Sci. 2005, 88, 3166–3173. [Google Scholar] [CrossRef]
- Song, X.Y.; Leroy, T.; Vranken, E.; Maertens, W.; Sonck, B.; Berckmans, D. Automatic detection of lameness in dairy cattle—Vision-based trackway analysis in cow's locomotion. Comput. Electr. Agric. 2008, 64, 39–44. [Google Scholar] [CrossRef]
- Pluk, A.; Bahr, C.; Leroy, T.; Poursaberi, A.; Song, X.; Vranken, E.; Maertens, W.; Van Nuffel, A.; Berckmans, D. Evaluation of step overlap as an automatic measure in dairy cow locomotion. Trans. ASABE 2010, 53, 1305–1312. [Google Scholar] [CrossRef]
- Pluk, A.; Bahr, C.; Poursaberi, A.; Maertens, W.; Van Nuffel, A.; Berckmans, D. Automatic measurement of touch and release angles of the fetlock joint for lameness detection in dairy cattle using vision techniques. J. Dairy Sci. 2012, 95, 1738–1748. [Google Scholar] [CrossRef] [PubMed]
- Poursaberi, A.; Bahr, C.; Pluk, A.; Van Nuffel, A.; Berckmans, D. Real-time automatic lameness detection based on back posture extraction in dairy cattle: Shape analysis of cow with image processing techniques. Comput. Electr. Agric. 2010, 74, 110–119. [Google Scholar] [CrossRef]
- Poursaberi, A.; Bahr, C.; Pluk, A.; Berckmans, D.; Veermäe, I.; Kokin, E.; Pokalainen, V. Online lameness detection in dairy cattle using Body Movement Pattern (BMP). In Proceedings of the 11th International Conference on Intelligent Systems Designs and Applications, Cordoba, Spain, 22–24 November 2011; pp. 732–736.
- Viazzi, S.; Bahr, C.; Schlageter-Tello, A.; Van Hertem, T.; Romanini, C.E.B.; Pluk, A.; Halachmi, I.; Lokhorst, C.; Berckmans, D. Analysis of individual classification of lameness using automatic measurement of back posture in dairy cattle. J. Dairy Sci. 2013, 96, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Van Hertem, T.; Maltz, E.; Antler, A.; Romanini, C.E.B.; Viazzi, S.; Bahr, C.; Schlageter-Tello, A.; Lokhorst, C.; Berckmans, D.; Halachmi, I. Lameness detection based on multivariate continuous sensing of milk yield, rumination, and neck activity. J. Dairy Sci. 2013, 96, 4286–4298. [Google Scholar] [CrossRef] [PubMed]
- Poursaberi, A.; Bahr, C.; Pluk, A.; Maertens, W.; Veermäe, I.; Kokin, E.; Praks, J.; Pokalainen, V.; Pastell, M.; Ahokas, J.; Van Nuffel, A.; Vangeyte, J.; Sonck, B.; Berckmans, D. Image based separation of dairy cows for automatic lameness detection with a real time vision system. In Proceedings of ASABE Annual International Meeting, St Joseph, NE, USA, 21–24 June 2009; p. 10.
- Viazzi, S.; Bahr, C.; Van Hertem, T.; Schlageter-Tello, A.; Romanini, C.E.B.; Halachmi, I.; Lokhorst, C.; Berckmans, D. Comparison of a three-dimensional and two-dimensional camera system for automated measurements of back posture in dairy cows. Comput. Electr. Agric. 2014, 100, 139–147. [Google Scholar] [CrossRef]
- Van Hertem, T.; Viazzi, S.; Steensels, M.; Maltz, E.; Antler, A.; Alchanatis, V.; Schlageter-Tello, A.; Lokhorst, C.; Romanini, C.E.B.; Bahr, C.; Berckmans, D.; Halachmi, I. Automatic lameness detection based on consecutive 3D-video recordings. Biosyst. Eng. 2014, 119, 108–116. [Google Scholar] [CrossRef]
- Eddy, A.L.; Van Hoogmoed, L.M.; Snyder, J.R. The role of thermography in the management of equine lameness. Vet. J. 2001, 162, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, A.; Plaizier, J.C.; Einarson, M.S.; Berry, R.J.; Scott, S.L.; Kennedy, A.D. Short communication: Infrared thremography and visual examination of hooves of dairy cows in two stages of lactation. J. Dairy Sci. 2005, 88, 1749–1753. [Google Scholar] [CrossRef]
- Alsaaod, M.; Buscher, W. Detection of hoof lesions using digital infrared thermography in dairy cows. J. Dairy Sci. 2012, 95, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.E.; Leach, K.A.; Main, D.C.J.; Whay, H.R. An investigation into the use of infrared thermography (IRT) as a rapid diagnostic tool for foot lesions in dairy cattle. Vet. J. 2012, 193, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Mazrier, H.; Tal, S.; Aizinbud, E.; Bargai, U. A field investigation of the use of the pedometer for the early detection of lameness in cattle. Can. Vet. J. 2006, 47, 883–886. [Google Scholar] [PubMed]
- Steensels, M.; Bahr, C.; Berckmans, D.; Halachmi, I.; Antler, A.; Maltz, E. Lying patterns of high producing healthy dairy cows after calving in commercial herds as affected by age, environmental conditions and production. Appl. Anim. Behav. Sci. 2012, 136, 88–95. [Google Scholar] [CrossRef]
- Pastell, M.; Tiusanen, J.; Hakojarvi, M.; Hanninen, L. A wireless accelerometer system with wavelet analysis for assessing lameness in cattle. Biosyst. Eng. 2009, 104, 545–551. [Google Scholar] [CrossRef]
- Chapinal, N.; de Passille, A.M.; Pastell, M.; Hanninen, L.; Munksgaard, L.; Rushen, J. Measurement of acceleration while walking as an automated method for gait assessment in dairy cattle. J. Dairy Sci. 2011, 94, 2895–2901. [Google Scholar] [CrossRef] [PubMed]
- Thorup, V.M.; Munksgaard, L.; Robert, P.-E.; Erhard, H.W.; Thomsen, P.T.; Friggens, N.C. Lameness detection via leg-mounted accelerometers on dairy cows on four commercial farms. Animal 2015. [Google Scholar] [CrossRef] [PubMed]
- Alsaaod, M.; Romer, C.; Kleinmanns, J.; Hendriksen, K.; Rose-Meierhofer, S.; Plumer, L.; Buscher, W. Electronic detection of lameness in dairy cows through measuring pedometric activity and lying behavior. Appl. Anim. Behav. Sci. 2012, 142, 134–141. [Google Scholar] [CrossRef]
- Munksgaard, L.; Reenen, C.G.; Boyce, R. Automatic monitoring of lying, standing and walking behavior in dairy cattle. J. Anim. Sci. 2006, 84 (Suppl.), 304. [Google Scholar]
- O'Driscoll, K.; Boyle, L.; Hanlon, A. A brief note on the validation of a system for recording lying behaviour in dairy cows. Appl. Anim. Behav. Sci. 2008, 111, 195–200. [Google Scholar] [CrossRef]
- Yunta, C.; Guasch, I.; Bach, A. Lying behavior of lactating dairy cows is influenced by lameness especially around feeding time. J. Dairy Sci. 2012, 95, 6546–6549. [Google Scholar] [CrossRef] [PubMed]
- Calderon, D.F.; Cook, N.B. The effect of lameness on the resting behavior and metabolic status of dairy cattle during the transition period in a freestall-housed dairy herd. J. Dairy Sci. 2011, 94, 2883–2894. [Google Scholar] [CrossRef] [PubMed]
- Kamphuis, C.; Frank, E.; Burke, J.K.; Verkerk, G.A.; Jago, J.G. Applying additive logistic regression to data derived from sensors monitoring behavioral and physiological characteristics of dairy cows to detect lameness. J. Dairy Sci. 2013, 96, 7043–7053. [Google Scholar] [CrossRef] [PubMed]
- de Mol, R.M.; Andre, G.; Bleumer, E.J.B.; van der Werf, J.T.N.; de Haas, Y.; van Reenen, C.G. Applicability of day-to-day variation in behavior for the automated detection of lameness in dairy cows. J. Dairy Sci. 2013, 96, 3703–3712. [Google Scholar] [CrossRef] [PubMed]
- Liberati, P.; Zappavigna, P. Improving the automated monitoring of dairy cows by integrating various data acquisition systems. Comput. Electr. Agric. 2009, 68, 62–67. [Google Scholar] [CrossRef]
- Garcia, E.; Klaas, I.; Amigo, J.M.; Bro, R.; Enevoldsen, C. Lameness detection challenges in automated milking systems addressed with partial least squares discriminant analysis. J. Dairy Sci. 2014, 97, 7476–7486. [Google Scholar] [CrossRef] [PubMed]
- Espejo, L.A.; Endres, M.I.; Salfer, J.A. Prevalence of lameness in high-producing Holstein cows housed in freestall barns in Minnesota. J. Dairy Sci. 2006, 89, 3052–3058. [Google Scholar] [CrossRef]
- Leach, K.A.; Whay, H.R.; Maggs, C.M.; Barker, Z.E.; Paul, E.S.; Bell, A.K.; Main, D.C.J. Working towards a reduction in cattle lameness: 1. Understanding barriers to lameness control on dairy farms. Res. Vet. Sci. 2010, 89, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Blackie, N.; Bleach, E.; Amory, J.; Scaife, J. Association between locomotion score and kinematic measures in dairy cows with a varying hoof lesion type. J. Dairy Sci. 2013, 96, 3564–3572. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; von Keyserlingk, M.A.G.; LeBlanc, S.J.; Weary, D.M. Lying behavior as an indicator of lameness in dairy cows. J. Dairy Sci. 2010, 93, 3553–3560. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Green, L.E.; Tadich, N. Effect of lameness and lesion specific causes of lameness on time budgets of dairy cows at pasture and when housed. Vet. J. 2013, 197, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Kramer, E.; Cavero, D.; Stamer, E.; Krieter, J. Mastitis and lameness detection in dairy cows by application of fuzzy logic. Livestock Sci. 2009, 125, 92–96. [Google Scholar] [CrossRef]
- Miekley, B.; Traulsen, I.; Krieter, J. Detection of mastitis and lameness in dairy cows using wavelet analysis. Livestock Sci. 2012, 148, 227–236. [Google Scholar] [CrossRef]
- Miekley, B.; Stamer, E.; Traulsen, I.; Krieter, J. Implementation of multivariate cumulative sum control charts in mastitis and lameness monitoring. J. Dairy Sci. 2013, 96, 5723–5733. [Google Scholar] [CrossRef] [PubMed]
- Norring, M.; Häggman, J.; Simojoki, H.; Tamminen, P.; Winckler, C.; Pastell, M. Lameness impairs feeding behaviour of dairy cows. J. Dairy Sci. 2014, 97, 4317–4321. [Google Scholar] [CrossRef] [PubMed]
- Weary, D.; Huzzley, J.M.; von Keyserlinck, M.A.G. Board invited review: Using behavior to predict and identify ill health in animals. J. Anim. Sci. 2009, 87, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Alawneh, J.I.; Laven, R.A.; Stevenson, M.A. The effect of lameness on the fertility of dairy cattle in a seasonally breeding pasture-based system. J. Dairy Sci. 2011, 94, 5487–5493. [Google Scholar] [CrossRef] [PubMed]
- Van Hertem, T.; Steensels, M.; Viazzi, S.; Bahr, C.; Romanini, C.E.B.; Lokhorst, C.; Schlageter-Tello, A.; Maltz, E.; Halachmi, I.; Berckmans, D. Effect if cow traffic on an implemented automatic 3D vision monitor for dairy cow locomotion. In Proceedings of the 65th Annual Meeting of the European Federation of Animal Science, Copenhagen, Denmark, 25–29 August 2014.
- Clarkson, M.J.; Downham, D.Y.; Faull, W.B.; Hughes, J.W.; Manson, F.J.; Merritt, J.B.; Murray, R.D.; Russell, W.B.; Sutherst, J.E.; Ward, W.R. Incidence and prevalence of lameness in dairy cattle. Vet. Rec. 1996, 138, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, A. Lameness in Dairy Cattle: Are Activity Levels, Hoof Lesions and Lameness Correlated? Faculty of Land and Food Systems. Ph.D. Thesis, The University of British Colombia, Vancouver, Canada, 2001; p. 32. [Google Scholar]
- Bruijnis, M.; Hogeveen, H.; Garforth, C.; Stassen, E. Dairy farmers’ attitudes and intentions towards improving dairy cow foot health. Livestock Sci. 2013, 155, 103–113. [Google Scholar] [CrossRef]
- Mollenhorst, H.; Rijkaart, L.J.; Hogeveen, H. Mastitis alert preferences of farmers milking with automatic milking systems. J. Dairy Sci. 2012, 95, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Bahr, C.; Van Nuffel, A.; Van Weyenberg, S.; Berckmans, D. The ease of movement—How automatic gait and posture analysis can contribute to early lameness detection in dairy cattle. In Livestock Housing 22. Modern Management to Ensure Optimal Health and Welfare of Farm Animals; Aland, A., Banhazi, T., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2014; pp. 419–430. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Nuffel, A.; Zwertvaegher, I.; Van Weyenberg, S.; Pastell, M.; Thorup, V.M.; Bahr, C.; Sonck, B.; Saeys, W. Lameness Detection in Dairy Cows: Part 2. Use of Sensors to Automatically Register Changes in Locomotion or Behavior. Animals 2015, 5, 861-885. https://doi.org/10.3390/ani5030388
Van Nuffel A, Zwertvaegher I, Van Weyenberg S, Pastell M, Thorup VM, Bahr C, Sonck B, Saeys W. Lameness Detection in Dairy Cows: Part 2. Use of Sensors to Automatically Register Changes in Locomotion or Behavior. Animals. 2015; 5(3):861-885. https://doi.org/10.3390/ani5030388
Chicago/Turabian StyleVan Nuffel, Annelies, Ingrid Zwertvaegher, Stephanie Van Weyenberg, Matti Pastell, Vivi M. Thorup, Claudia Bahr, Bart Sonck, and Wouter Saeys. 2015. "Lameness Detection in Dairy Cows: Part 2. Use of Sensors to Automatically Register Changes in Locomotion or Behavior" Animals 5, no. 3: 861-885. https://doi.org/10.3390/ani5030388






