A Critical Look at Biomedical Journals’ Policies on Animal Research by Use of a Novel Tool: The EXEMPLAR Scale
Abstract
:Simple Summary
Abstract
1. Introduction
| EXEMPLAR Scale | Score | |
|---|---|---|
| A—Reporting of regulatory compliance | ||
| Authors must attest of prior ethical approval of animal studies, or an analogous project evaluation process involving harm-benefit appraisal, (e.g., by providing documental evidence or ethics review board process reference) and include a statement of compliance with relevant legislation and national, international or institutional guidelines on animal care and use. | 5 | |
| Authors must declare: | Ethical approval of studies or analogous evaluation process by competent authority, institutional animal care and use committee, animal welfare body or equivalent. | 2 |
| Compliance with relevant national, international or institutional guidelines on animal care and use. | 1 | |
| Compliance with relevant legislation on the use of animals. | 1 | |
| B—Quality of research and reporting of results | ||
| Journal refers authors to relevant guidelines on the quality of reporting of animal studies (e.g., ARRIVE guidelines, Gold Standard Publication Checklist, or other) | 5 | |
| Authors must include information regarding: | Animals (e.g., sex, age, genotype, background, supplier, acclimatization period, etc.) | 1 |
| Experimental conditions (e.g., housing, lighting, temperature, feeding regime, environmental enrichment, etc.) | 1 | |
| Experimental design and statistics | 1 | |
| Experimental procedures and outcomes | 1 | |
| C—Animal Welfare and ethics | ||
| Journal demands that the methods described are coherent with best principles and practice on the ethical treatment of animals in research, e.g., by demanding strict compliance with relevant guidelines on animal care and use, the 3Rs for replacement, reduction and refinement or the journal's own policies. | 5 | |
| Authors must: | state the rationale for choosing the animal model(s) used | 1 |
| report the impact of the experiment on animal health and wellbeing | 1 | |
| describe any measures to minimize harm (e.g., anesthetics, analgesics, humane endpoints) and/or improve the wellbeing of animals (e.g., husbandry adaptations for more vulnerable animals) | 1 | |
| justify the necessity of any unrelieved pain, suffering or distress inflicted | 1 | |
| D—Criteria for the exclusion of papers | ||
| Meeting journal standards on animal ethics care and welfare is indispensable for manuscript acceptance and/or publication. Studies raising serious concerns over animal welfare, or presenting significant discrepancies between the approved protocol and methods described may be reported to the institution or committee responsible for ethical approval. | 5 | |
| Studies raising serious ethical concerns (e.g., serious neglect regarding animal welfare or unjustifiable suffering considering the value of the experiment) may be rejected by editors or reviewers. | 3 | |
| Journal states specific procedure(s) that will not be accepted for publication (e.g., use of muscle relaxants or paralytic drugs alone for surgery, severe lesion/trauma without anesthesia, or death as an endpoint) | 1 | |
2. Experimental Section
2.1. The Exemplar Scale
2.2. Journals Search
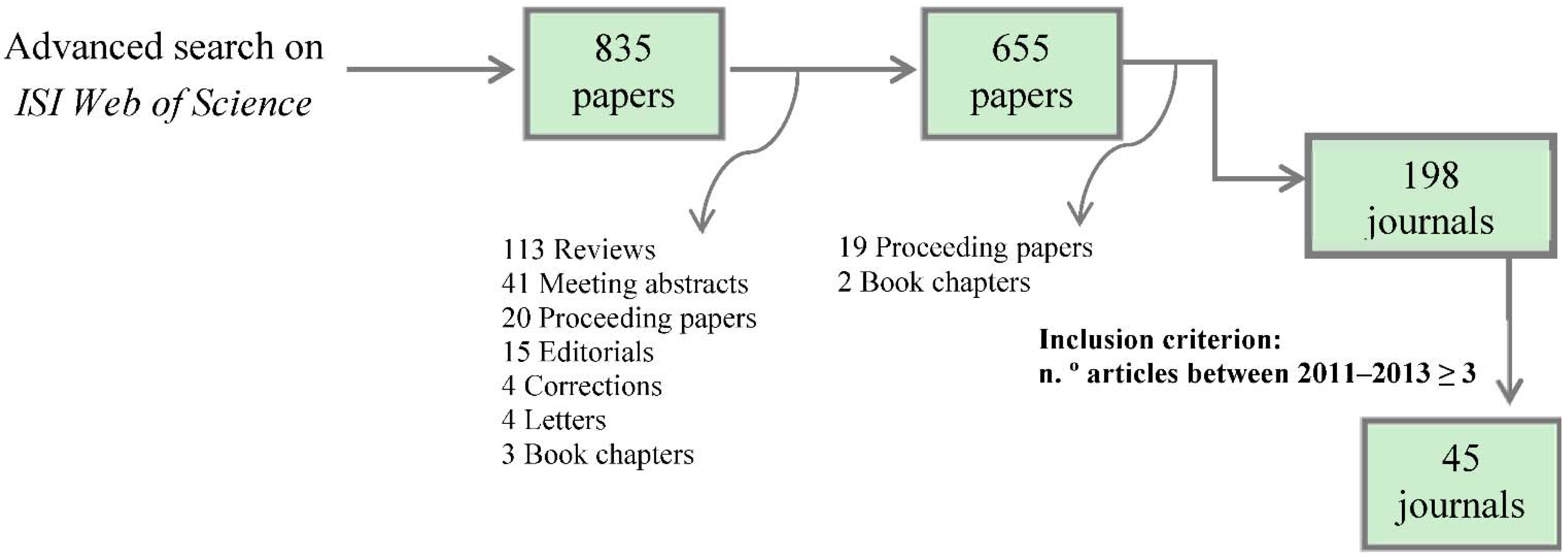
2.3. Data Collection
2.4. Statistical Analysis
3. Results and Discussion
3.1. Sample Characterization

3.2. EXEMPLAR Score
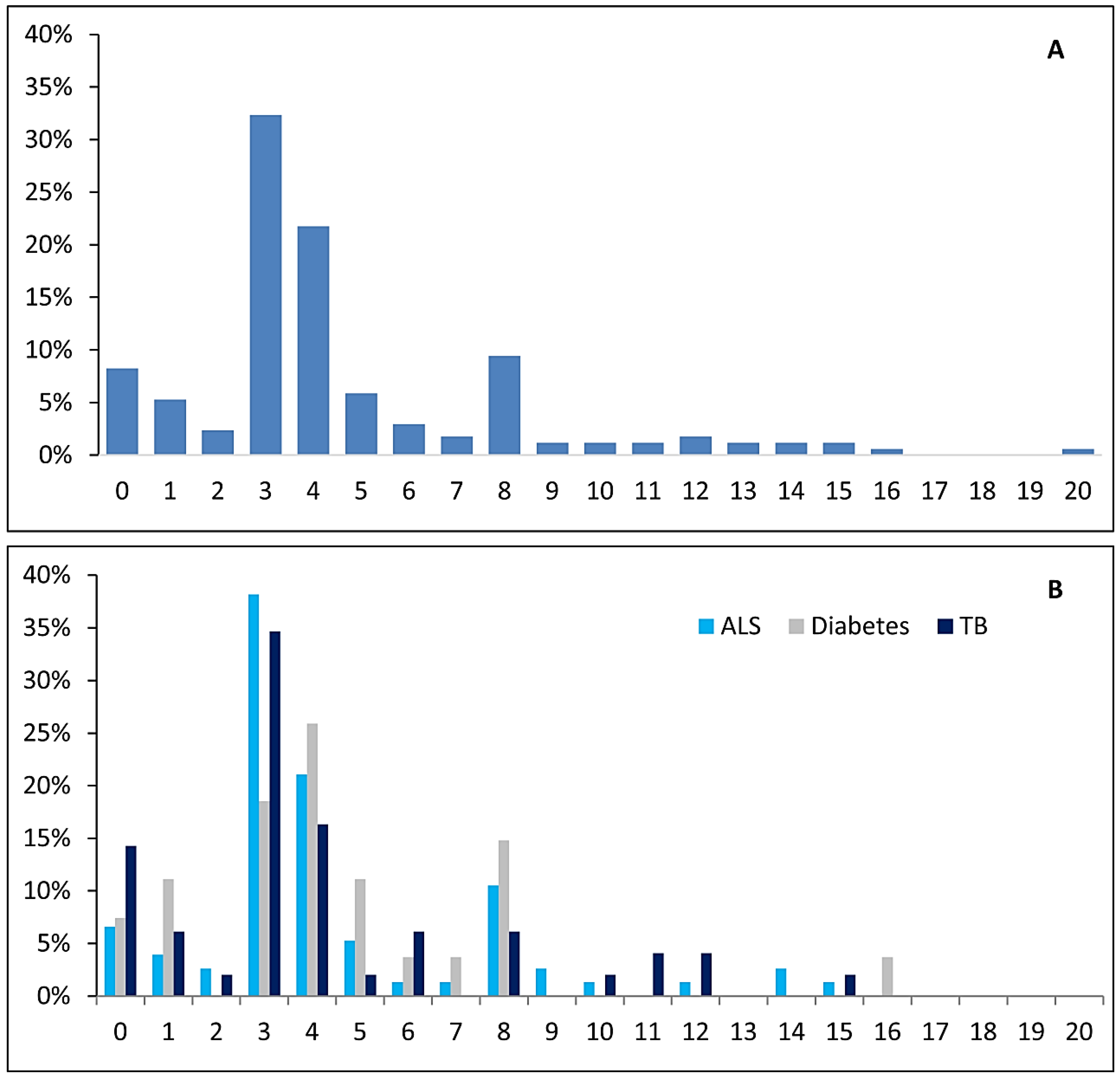
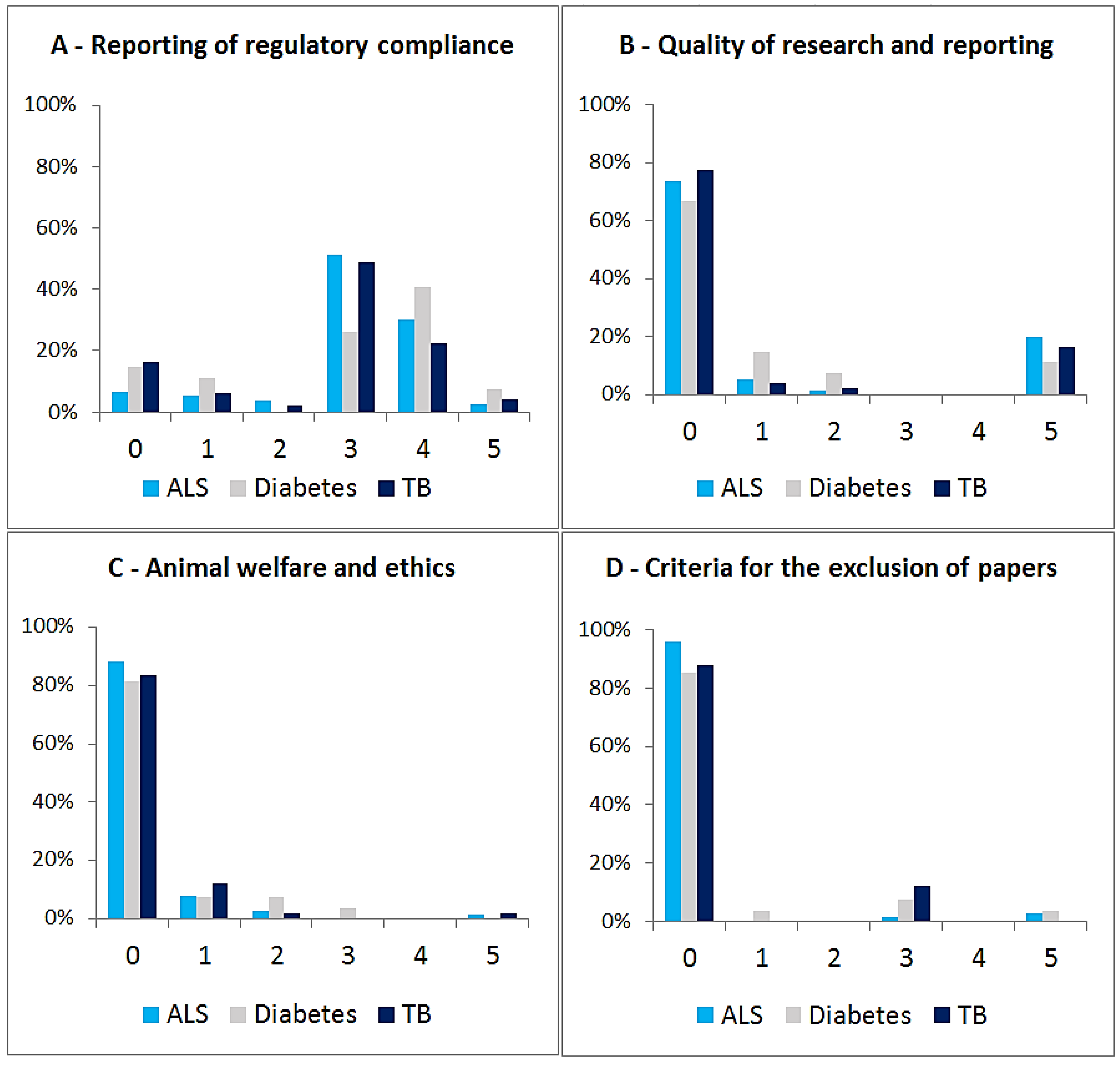
3.3. Relationship between EXEMPLAR Score and Other Parameters
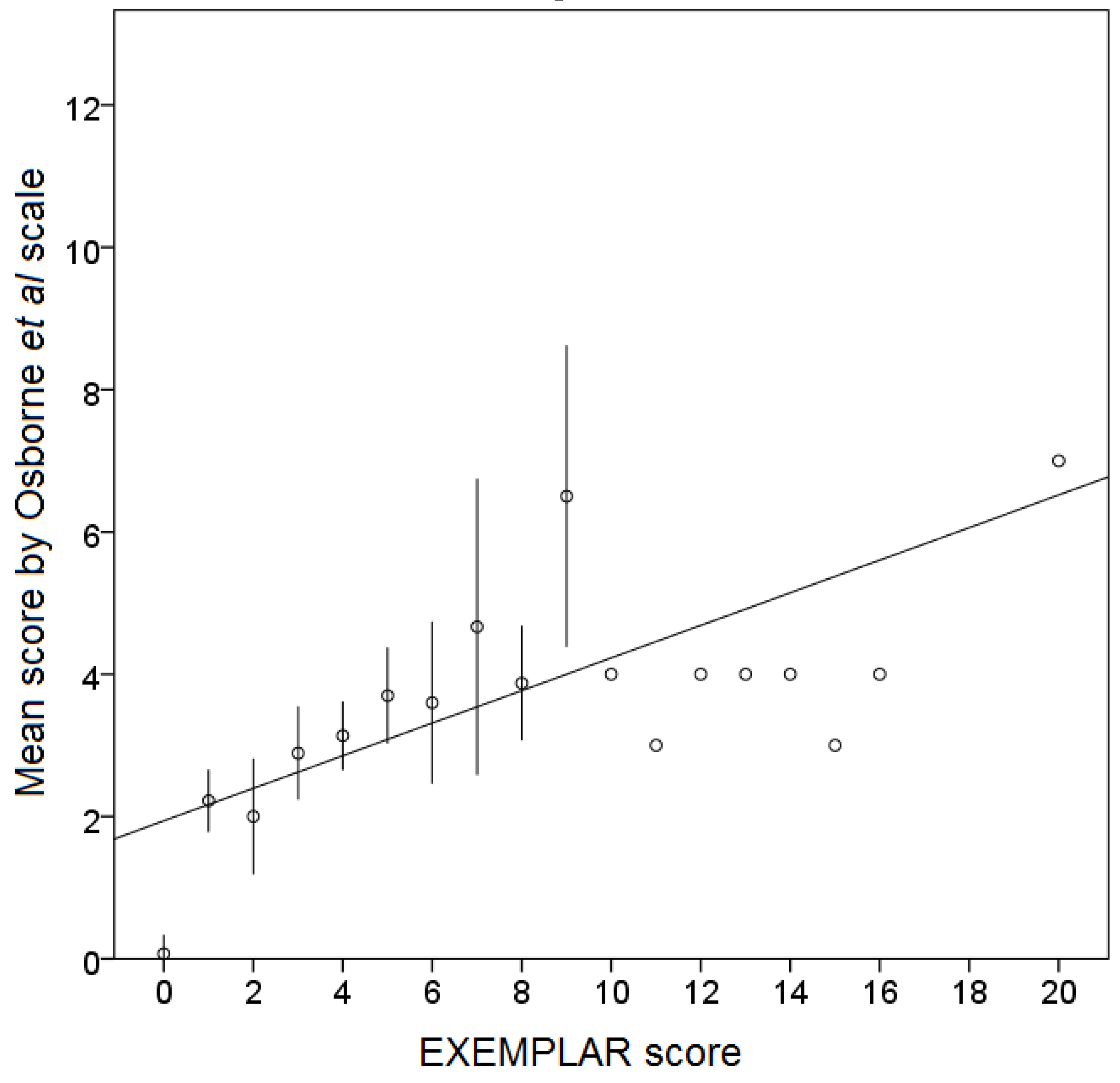
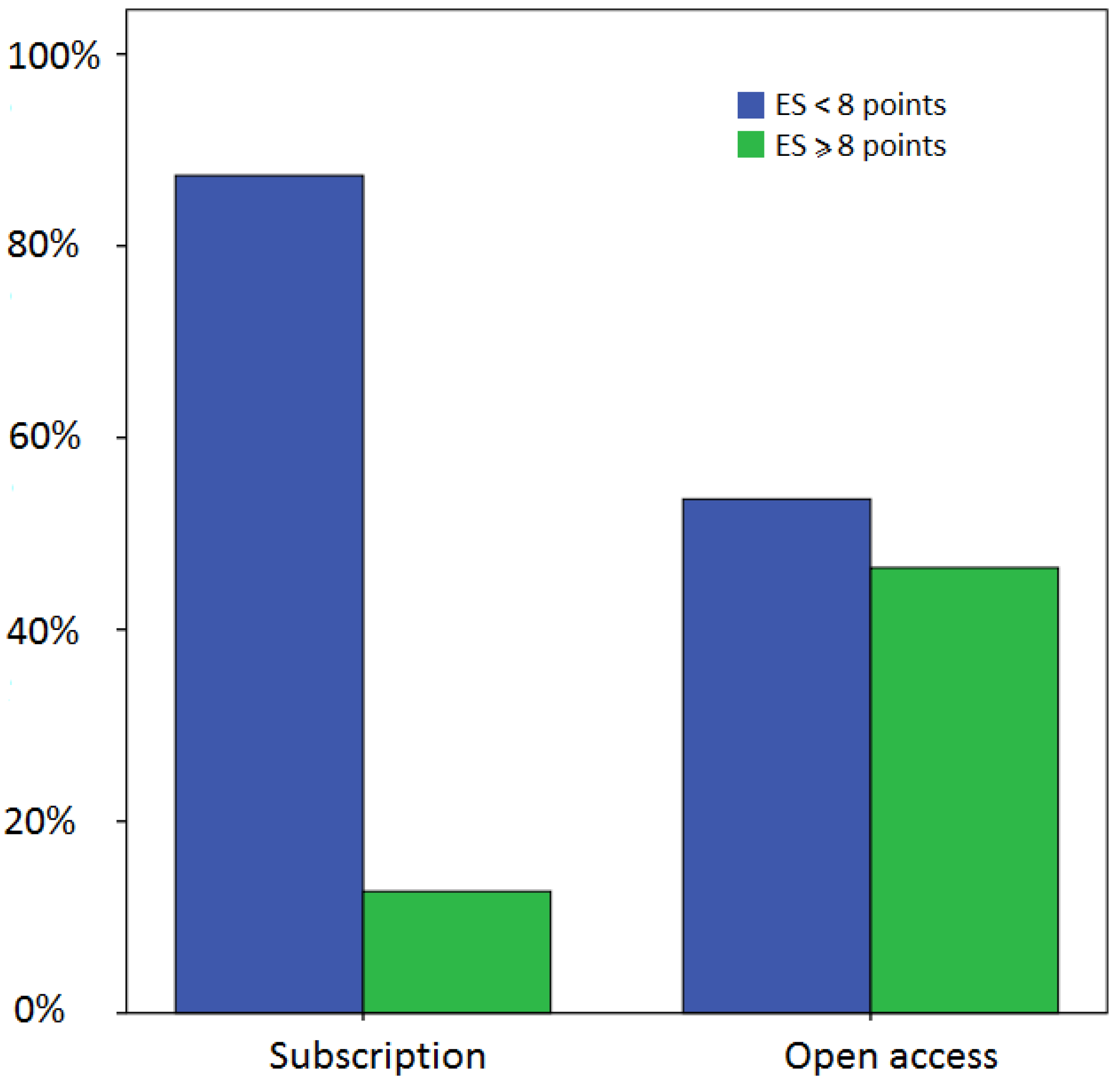
4. Discussion and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Franco, N.H. Animal experiments in biomedical research: A historical perspective. Animals 2013, 3, 238–273. [Google Scholar] [CrossRef]
- Hagelin, J.; Carlsson, H.-E.; Hau, J. An overview of surveys on how people view animal experimentation: Some factors that may influence the outcome. Public Underst. Sci. 2003, 12, 67–81. [Google Scholar] [CrossRef]
- Ormandy, E.H.; Schuppli, C.A.; Weary, D.M. Public attitudes toward the use of animals in research: Effects of invasiveness, genetic modification and regulation. Anthrozoos: Multidiscip. J. Interact. People Anim. 2013, 26, 165–184. [Google Scholar] [CrossRef]
- European Commission. Results of Questionnaire for the General Public on the Revision of Directive 86/609/EEC on the Protection of Animals Used for Experimental and Other Scientific Purposes. Available online: http://ec.europa.eu/environment/chemicals/lab_animals/pdf/results_citizens.pdf (accessed on 12 November 2014).
- Lund, T.B.; Sørensen, T.I.A.; Olsson, I.A.S.; Hansen, A.K.; Sandøe, P. Is it acceptable to use animals to model obese humans? A critical discussion of two arguments against the use of animals in obesity research. J. Med. Ethics 2014, 40, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Aldhous, P.; Coghlan, A.; Copley, J. Animal experiments—Where do you draw the line?: Let the people speak. New Sci. 1999, 162, 26–31. [Google Scholar] [PubMed]
- Worcester, R.M. Science and society: What scientists and the public can learn from each other. Proc. R. Inst. 2001, 71, 97–160. [Google Scholar]
- Lund, T.B.; Mørkbak, M.R.; Lassen, J.; Sandøe, P. Painful dilemmas: A study of the way the public’s assessment of animal research balances costs to animals against human benefits. Public Underst. Sci. 2014, 23, 428–444. [Google Scholar] [CrossRef] [PubMed]
- TNS Opinion & Social. Special Eurobarometer 340/Wave 73.1: Science and Technology; Brussels; Directorate General Research, European Commission, 2010. [Google Scholar]
- Pifer, L.; Shimizu, K.; Pifer, R. Public attitudes toward animal research: Some international comparisons. Soc. Anim. 1994, 2, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Serpell, J.A. Factors influencing human attitudes to animals and their welfare. Anim. Welf. 2004, 13, S145–S152. [Google Scholar]
- Holmberg, T.; Ideland, M. Secrets and lies: “Selective openness” in the apparatus of animal experimentation. Public Underst. Sci. 2012, 21, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Von Roten, F.C. Public perceptions of animal experimentation across Europe. Public Underst. Sci. 2012. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen & Co. Ltd: London, UK, 1959. [Google Scholar]
- Festing, S.; Wilkinson, R. The ethics of animal research. EMBO Rep. 2007, 8, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Louhimies, S. Eu directive 2010/63/eu: “Implementing the three rs through policy”. ALTEX Proc. 2012, 1, 27–33. [Google Scholar]
- Dixon-Woods, M.; Ashcroft, R. Regulation and the social licence for medical research. Med. Health Care Philos. 2008, 11, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Varga, O.; Hansen, A.K.; Sandoe, P.; Olsson, I.A.S. Improving transparency and ethical accountability in animal studies. Embo Rep. 2010, 11, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.J. Animal welfare and the 3rs in european biomedical research. Ann. N.Y. Acad. Sci. 2011, 1245, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.; Smith, J.A. Ethical review of animal experiments: Current practice and future directions. ALTEX Proc. 2012, 1, 275–279. [Google Scholar]
- Forni, M. Laboratory animal science: A resource to improve the quality of science. Vet. Res. Commun. 2007, 31, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Smaje, L.H.; Smith, J.A.; Combes, R.D.; Ewbank, R.; Gregory, J.A.; Jennings, M.; Moore, G.J.; Morton, D.B. Advancing refinement of laboratory animal use. Lab. Anim. 1998, 32, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Leenaars, M.; Ritskes-Hoitinga, M. A gold standard publication checklist to improve the quality of animal studies, to fully integrate the three rs, and to make systematic reviews more feasible. ATLA-Altern. Lab. Anim. 2010, 38, 167–182. [Google Scholar]
- Basel Declaration Society. Basel declaration: A call for more trust, transparency and communication on animal research. In Research at a Crossroads; Basel Declaration Society: Basel, Switzerland, 2010. [Google Scholar]
- Rollin, B.E. Animal research, animal welfare, and the three Rs. Available online: http://jpsl.org/archives/animal-research-animal-welfare-and-three-rs/ (accessed on 3 November 2014).
- Osborne, N.J.; Phillips, B.J.; Westwood, K. Journal editorial policies as a driver for change—Animal welfare and the 3Rs. In New Paradigms In Laboratory Animal Science, Proceedings of the Eleventh FELASA Symposium and the 40th Scand-LAS Symposium, Helsinki, Finland, 14–17 June 2010; FELASA: Helsinki, Finland, 2011; pp. 18–23. [Google Scholar]
- Osborne, N.J.; Payne, D.; Newman, M.L. Journal editorial policies, animal welfare, and the 3Rs. Am. J. Bioeth. 2009, 9, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Rands, S. Ethical policies on animal experiments are not compromised by whether a journal is freely accessible or charges for publication. Animal 2009, 3, 1591–1595. [Google Scholar] [CrossRef] [PubMed]
- Franco, N.H.; Olsson, I. “How sick must your mouse be?”—An analysis of the use of animal models in huntington’s disease research. ATLA 2012, 40, 271–283. [Google Scholar] [PubMed]
- Franco, N.H.; Correia-Neves, M.; Olsson, I.A.S. Animal welfare in studies on murine tuberculosis: Assessing progress over a 12-year period and the need for further improvement. PLOS ONE 2012, 7, e47723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plous, S.; Herzog, H. Animal research. Reliability of protocol reviews for animal research. Science 2001, 293, 608–609. [Google Scholar] [CrossRef] [PubMed]
- Varga, O.; Olsson, I.A.S. 6.2. Animal ethics committees: Different regions—Imilar tasks and difficulties?—European union. In Ethical Aspects of Animal Research—Life in the Lab; Gjerris, M., Olsson, I.A.S., Röcklinsberg, H., Eds.; Springer; in press.
- Franco, N.H.; Olsson, I. Is the ethical appraisal of protocols enough to ensure best practice in animal research? ATLA 2013, 41, P5–P7. [Google Scholar] [PubMed]
- Grindlay, D.J.; Dean, R.S.; Christopher, M.M.; Brennan, M.L. A survey of the awareness, knowledge, policies and views of veterinary journal editors-in-chief on reporting guidelines for publication of research. BMC Vet. Res. 2014, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Festing, M.F.; Altman, D.G. Guidelines for the design and statistical analysis of experiments using laboratory animals. ILAR J. 2002, 43, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; de Vries, R.; Leenaars, M.; Curfs, J.; Ritskes-Hoitinga, M. Improving planning, design, reporting and scientific quality of animal experiments by using the gold standard publication checklist, in addition to the arrive guidelines. Br. J. Pharmacol. 2011, 162, 1259–1260. [Google Scholar] [CrossRef] [PubMed]
- Van der Worp, H.B.; Howells, D.W.; Sena, E.S.; Porritt, M.J.; Rewell, S.; O’Collins, V.; Macleod, M.R. Can animal models of disease reliably inform human studies? PLOS Med. 2010, 7, e1000245. [Google Scholar] [CrossRef] [PubMed]
- Landis, S.C.; Amara, S.G.; Asadullah, K.; Austin, C.P.; Blumenstein, R.; Bradley, E.W.; Crystal, R.G.; Darnell, R.B.; Ferrante, R.J.; Fillit, H.; et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 2012, 490, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Parsons, N.; Kadyszewski, E.; Festing, M.F.; Cuthill, I.C.; Fry, D.; Hutton, J.; Altman, D.G. Survey of the quality of experimental design, statistical analysis and reporting of research using animals. PLOS ONE 2009, 4, e7824. [Google Scholar] [CrossRef] [PubMed]
- Eisen, J.A.; Ganley, E.; MacCallum, C.J. Open science and reporting animal studies: Who’s accountable? PLOS Biol. 2014, 12, e1001757. [Google Scholar] [CrossRef] [PubMed]
- Olsson, I.A.S.; Robinson, P.; Sandøe, P. Ethics of animal research. Handb. Lab. Anim. Sci. 2011, 1, 21–35. [Google Scholar]
- Hirst, A.; Altman, D.G. Are peer reviewers encouraged to use reporting guidelines? A survey of 116 health research journals. PLOS ONE 2012, 7, e35621. [Google Scholar] [CrossRef] [PubMed]
- Galley, H.F. Mice, men, and medicine. Br. J. Anaesth. 2010, 105, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Lidster, K.; Sottomayor, A.; Amor, S. Two years later: Journals are not yet enforcing the arrive guidelines on reporting standards for pre-clinical animal studies. PLOS Biol. 2014, 12, e1001756. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, A.R.; Franco, N.H. A Critical Look at Biomedical Journals’ Policies on Animal Research by Use of a Novel Tool: The EXEMPLAR Scale. Animals 2015, 5, 315-331. https://doi.org/10.3390/ani5020315
Martins AR, Franco NH. A Critical Look at Biomedical Journals’ Policies on Animal Research by Use of a Novel Tool: The EXEMPLAR Scale. Animals. 2015; 5(2):315-331. https://doi.org/10.3390/ani5020315
Chicago/Turabian StyleMartins, Ana Raquel, and Nuno Henrique Franco. 2015. "A Critical Look at Biomedical Journals’ Policies on Animal Research by Use of a Novel Tool: The EXEMPLAR Scale" Animals 5, no. 2: 315-331. https://doi.org/10.3390/ani5020315





