Spatial and Temporal Habitat Use of an Asian Elephant in Sumatra
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Area
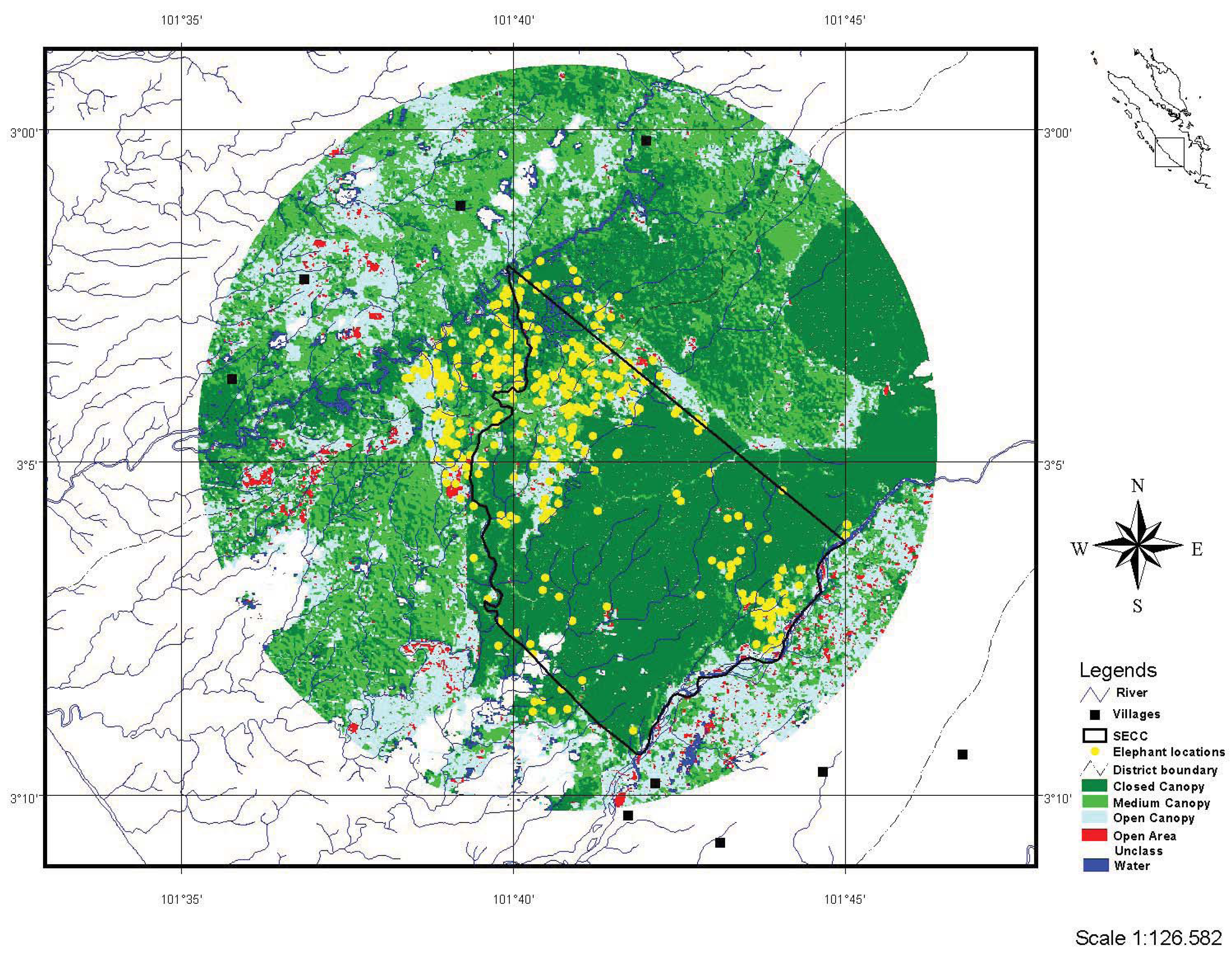
2.2. Telemetry
| Habitat category | Description | Proportion of study area (km2) |
| Closed canopy forest | Area with closed canopy forest and dense tree vegetation. Includes some mature (>10 years) palm plantation. | 0.455 (143) |
| Medium canopy | Area with broken canopy or rare standing tree vegetation. This area also mainly covered with secondary vegetation or tall shrub vegetation including bamboo vegetation. Includes some young palm plantation. | 0.283 (89) |
| Open canopy | Area with no tree vegetation and dominated with secondary vegetation, shrub or Alang-alang (Imperata cylindrica). Includes agricultural land. | 0.226 (71) |
| Open area | This area mostly bare ground or area with rare small vegetation, mostly grasses (e.g., Poaceae family) or small shrubs. Includes human settlement. | 0.017 (5) |
| Water | Water body including ponds, stream or river. | 0.019 (6) |
2.4. Habitat Use Analysis
2.5. Distance to Edge
3. Results
| Habitat | Population proportion (π) | Sample count (u) | Used sample proportion (o) | Expected count(π *ut) | Selection ratio (ŵ) | Manly standardize Index (B) | Bonferroni confidence limits | Selection level | Sig P < 0.05 | |
| Lower | Upper | |||||||||
| Closed canopy forest | 0.4549 | 126 | 0.353 | 162.413 | 0.7758 | 0.1687 | 0.75 | 2.46 | "-" | NS |
| Medium canopy | 0.2827 | 135 | 0.378 | 100.929 | 1.3376 | 0.2908 | 1.32 | 4.30 | "+" | S |
| Open canopy | 0.2264 | 87 | 0.244 | 80.837 | 1.0762 | 0.2340 | 1.06 | 3.46 | "+" | S |
| Open area | 0.0174 | 5 | 0.014 | 6.220 | 0.8038 | 0.1748 | 0.80 | 2.60 | "-" | NS |
| Water | 0.0185 | 4 | 0.011 | 6.601 | 0.6059 | 0.1317 | 0.61 | 1.96 | "-" | NS |
| 1 | 357 | 4.5994 | 1 | |||||||
| (A) | Closed canopy forest | Open area | Medium canopy | Open canopy | Water | Rank |
| Closed canopy forest | +++ | --- | --- | +++ | 3 | |
| Open area | --- | --- | --- | - | 5 | |
| Medium canopy | +++ | +++ | - | +++ | 2 | |
| Open canopy | +++ | +++ | + | +++ | 1 | |
| Water | --- | + | --- | --- | 4 | |
| (B) | Closed canopy forest | Open area | Medium canopy | Open canopy | Water | Rank |
| Closed Canopy forest | +++ | --- | + | +++ | 2 | |
| Open area | --- | --- | --- | - | 5 | |
| Medium canopy | +++ | +++ | + | +++ | 1 | |
| Open canopy | - | +++ | - | +++ | 3 | |
| Water | --- | + | --- | --- | 4 |
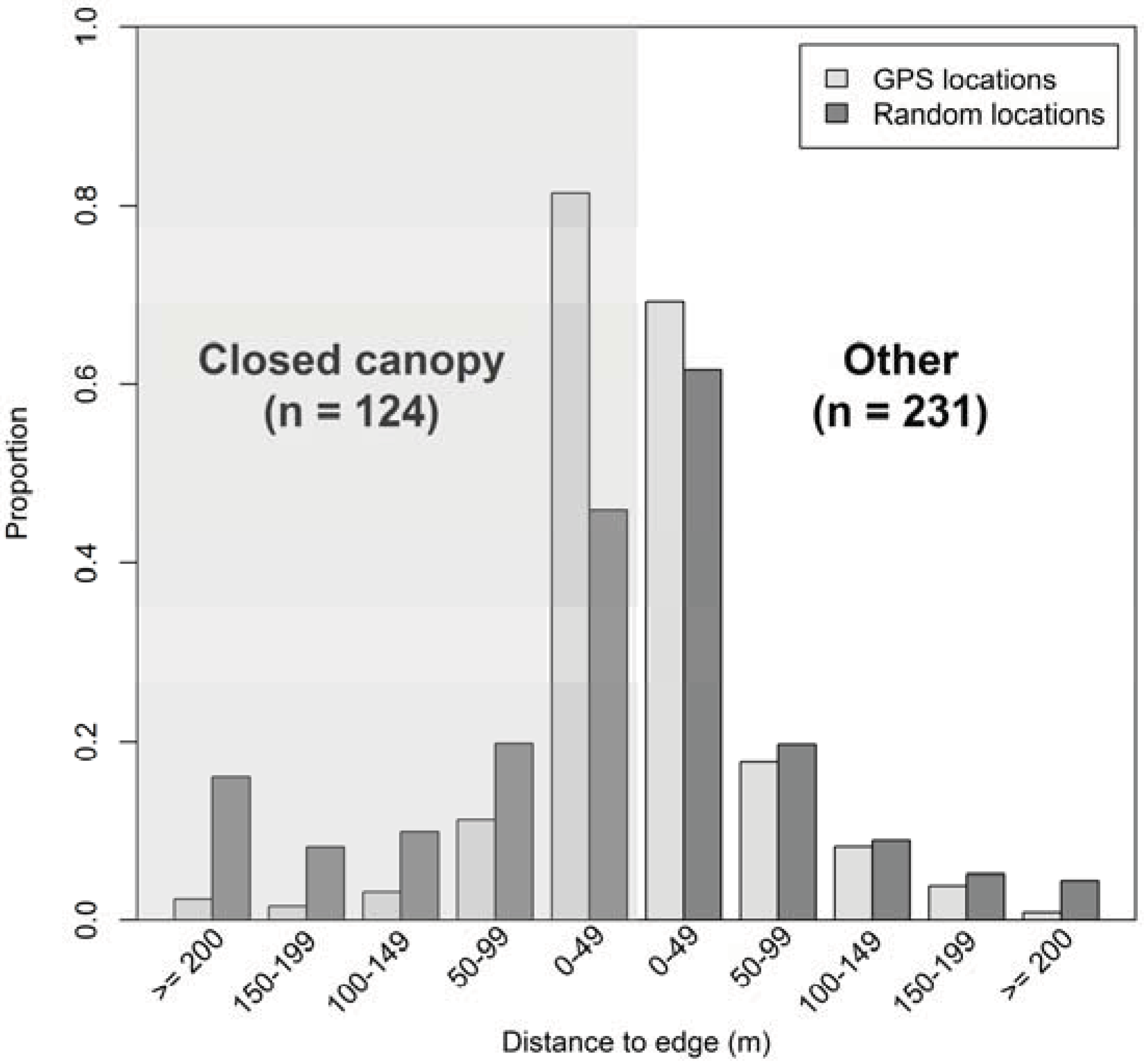
4. Discussion
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Soehartono, T.; Susilo, H.D.; Sitompul, A.F.; Gunaryadi, D.; Purastuti, E.M.; Azmi, W.; Fadhli, N.; Stremme, C. The Strategic and Action Plan for Sumatran and Kalimantan Elephant; Departemen Kehutanan (Ministry of Forestry): Jakarta, Indonesia, 2007. [Google Scholar]
- Leimgruber, P.; Gagnon, J.B.; Wemmer, C.M.; Kelly, D.S.; Songer, M.A.; Sellig, E.R. Fragmentation of Asia’s remaining wild lands: Implications for Asian elephant conservation. Anim. Conserv. 2003, 6, 347–359. [Google Scholar] [CrossRef]
- Hedges, S.; Tyson, M.; Sitompul, A.F.; Kinnaird, M.F.; Gunaryadi, D.; Aslan. Distribution, status and conservation needs of Asian elephant (Elephas maximus) in Lampung Province, Sumatra, Indonesia. Biol. Conserv. 2005, 124, 35–48. [Google Scholar] [CrossRef]
- Uryu, Y.; Mott, C.; Foead, N.; Yulianto, K.; Budiman, A.; Setiabudi; Takakai, F.; Nursamsu; Sunarto; Purastuti, E.; Fadhli, N.; Hutajulu, C.; Jaenicke, J.; Hatano, R.; Siegert, F.; Stüwe, M. Deforestation, Forest Degradation, Biodiversity Loss and CO2 Emissions in Riau Sumatra, Indonesia; WWF Indonesia Technical Report; WWF: Jakarta, Indonesia, 2008. [Google Scholar]
- Nyhus, P.J.; Tilson, R.; Sumianto. Crop-raiding elephants and conservation implications at Way Kambas National Park, Sumatra, Indonesia. Oryx 2000, 34, 262–274. [Google Scholar]
- Linkie, M.; Dinata, Y.; Nofrianto, A.; Leader-Williams, N. Patterns and perceptions of wildlife crop raiding in and around Kerinci Seblat National Park, Sumatra. Anim. Conserv. 2007, 10, 127–135. [Google Scholar] [CrossRef]
- Santiapilai, C.; Jackson, P. The Asian Elephant: An Action Plan for its Conservation; IUCN/SSC Asian Elephant Specialist Group: Gland, Switzerland, 1990. [Google Scholar]
- Hedges, S.; Gunaryadi, D. Reducing human-elephant conflict: Do chillies help deter elephants from entering crop fields? Oryx 2010, 44, 139–146. [Google Scholar] [CrossRef]
- Kinnaird, M.F.; Sanderson, E.W.; O’Brien, T.G.; Wibisono, H.T.; Woolmer, G. Deforestation trends in a tropical landscape and implications for endangered large mammals. Conserv. Biol. 2003, 17, 245–257. [Google Scholar] [CrossRef]
- Rood, E.; Ganie, A.A.; Nijman, V. Using presence-only modelling to predict Asian elephant habitat use in a tropical forest landscape: Implications for conservation. Diver. Distrib. 2010, 16, 975–984. [Google Scholar] [CrossRef]
- Sitompul, A.F. Ecology and conservation of Sumatran elephants (Elephas maximus sumatranus) in Sumatra, Indonesia. Ph.D. Thesis, University of Massachusetts, Amherst, MA, USA, 2011. [Google Scholar]
- Laumonier, Y.; Uryu, Y.; Stüwe, M.; Budiman, A.; Setiabudi, B.; Hadian, O. Eco-floristic sectors and deforestation threats in Sumatra: Identifying new conservation area network priorities for ecosystem-based land use planning. Biodiver. Conserv. 2010, 19, 1153–1174. [Google Scholar] [CrossRef]
- Sitompul, A.F. Sumatran elephant’s dietary ecology, movement and habitat use: Using ecological approach to save endangered Asian elephant and their habitat in Indonesia. Unpublished Final Report to the US Fish and Wildlife ServiceUnpublished Final Report to the US Fish and Wildlife Service. 2009. [Google Scholar]
- Sitompul, A.F.; Griffin, C.R.; Fuller, T.K.; Wardana, W.; Nazarudin. Use of tame elephants to find, immobilize, and mark wild elephants in Sumatran rainforest. Gajah 2012, 37, 11–15. [Google Scholar]
- Garshelis, D.L. Delusions in habitat evaluation: Measuring use, selection, and importance. In Research Techniques in Animal Ecology: Controversies and Consequences; Boitani, L., Fuller, T.K., Eds.; Columbia University Press: New York, NY, USA, 2000; pp. 111–164. [Google Scholar]
- Sitompul, A.F.; Griffin, C.R.; Fuller, T.K. Sumatran elephant ranging behavior in a fragmented rainforest landscape. Int. J. Biodiv. Conserv. 2013, 5, 66–72. [Google Scholar]
- Worton, B.J. Kernel methods for estimating the utilization distribution in home-range studies. Ecology 1989, 70, 164–168. [Google Scholar] [CrossRef]
- Beyer, H.L. Hawth’s analysis tools for ArcGIS. Version 3.27. 2006. Available online: http://www.spatialecology.com/htools (accessed on 23 April 2010).
- Manly, B.F.J.; Mcdonald, L.L.; Thomas, D.L.; McDonald, T.L.; Erickson, W.P. Resource Selection by Animals: Statistical Design and Analysis for Field Studies; Springer: New York, NY, USA, 2002. [Google Scholar]
- Aebischer, N.J.; Robertson, P.A.; Kenward, R.E. Compositional analysis of habitat use from animal radio-tracking data. Ecology 1993, 74, 1313–1325. [Google Scholar] [CrossRef]
- Johnson, D.H. The comparison of usage and availability measurement for evaluating resource preference. Ecology 1980, 61, 65–71. [Google Scholar] [CrossRef]
- Neu, C.W.; Byers, C.R.; Peek, J.M. A technique for the analysis of utilization-availability data. J. Wildl. Manage. 1974, 38, 541–545. [Google Scholar] [CrossRef]
- Chen, J.; Deng, X.B.; Zhang, L.; Bai, Z.L. Diet composition and foraging ecology of Asian elephants in Shangyong, Xishuaangbanna, China. Acta Ecol. Sinica 2006, 26, 309–316. [Google Scholar] [CrossRef]
- Olivier, R. On the ecology of the Asian elephant. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 1978. [Google Scholar]
- Sukumar, R. The Asian Elephant: Ecology and Management; Cambridge University Press: Cambridge, UK, 1989. [Google Scholar]
- Demarchi, M.W.; Bunnell, F.L. Estimating forest canopy effects on summer thermal cover for Cervidae (deer family). Can. J. For. Resourc. 1993, 23, 2419–2426. [Google Scholar] [CrossRef]
- Valeix, M.; Fritz, H.; Matsika, R.; Matsvimbo, F.; Madzikanda, H. The role of water abundance, thermoregulation, perceived predation risk and interference competition in water access by African herbivore. Afr. J. Ecol. 2007, 46, 402–410. [Google Scholar]
- Harper, K.A.; MacDonal, S.E.; Burton, P.J.; Chen, J.; Bosofske, K.D.; Saunders, S.C.; Euskirchen, E.S.; Roberts, D.; Jaiteh, M.S.; Esseen, P. Edge influence on forest structure and composition in fragmented landscapes. Conserv. Biol. 2005, 19, 768–782. [Google Scholar] [CrossRef]
- Olupot, W. A variable edge effect on trees of Bwindi Impenetrable National Park, Uganda, and its bearing on measurement parameters. Biol. Conserv. 2009, 142, 789–797. [Google Scholar] [CrossRef]
- Masse, A.; Cote, S.D. Habitat selection of a large herbivore at high density and without predation: Trade-off between forage and cover? J. Mammal. 2009, 90, 961–970. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sitompul, A.F.; Griffin, C.R.; Rayl, N.D.; Fuller, T.K. Spatial and Temporal Habitat Use of an Asian Elephant in Sumatra. Animals 2013, 3, 670-679. https://doi.org/10.3390/ani3030670
Sitompul AF, Griffin CR, Rayl ND, Fuller TK. Spatial and Temporal Habitat Use of an Asian Elephant in Sumatra. Animals. 2013; 3(3):670-679. https://doi.org/10.3390/ani3030670
Chicago/Turabian StyleSitompul, Arnold F., Curtice R. Griffin, Nathaniel D. Rayl, and Todd K. Fuller. 2013. "Spatial and Temporal Habitat Use of an Asian Elephant in Sumatra" Animals 3, no. 3: 670-679. https://doi.org/10.3390/ani3030670




