The “Super Chimpanzee”: The Ecological Dimensions of Rehabilitation of Orphan Chimpanzees in Guinea, West Africa
Simple Summary
Abstract
1. Introduction
2. Methods
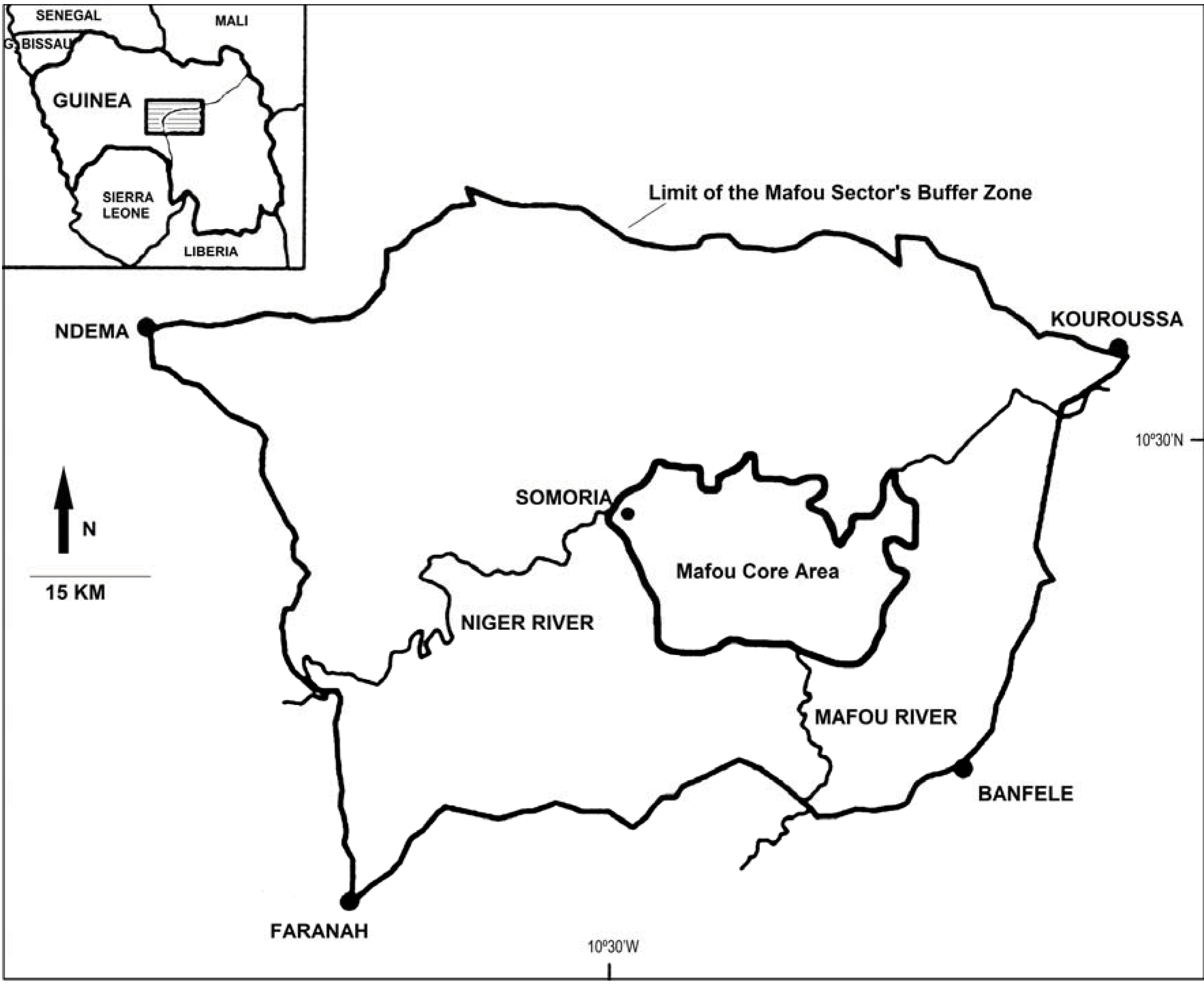
2.1. Study Site, Subjects
| Name | Sex | YOB | Group | Arrival Date (month/year) | Age of Individual (years) | Age at arrival (years) | Years at sanctuary | ABS ± 1SD |
|---|---|---|---|---|---|---|---|---|
| HAKIM | Male | 2004 | Nursery | 04/06 | 8 | 2 | 6 | 0 ± 0.0 |
| AMA | Female | 2006 | Nursery | 05/08 | 6 | 2 | 4 | 1.6 ± 0.5 |
| KIRIKOU | Male | 2006 | Nursery | 05/08 | 6 | 2 | 4 | 2.2 ± 0.4 |
| LILI | Female | 2005 | Nursery | 03/07 | 7 | 2 | 5 | 0 ± 0.0 |
| FLO | Female | 2005 | Nursery | 08/07 | 7 | 2 | 5 | 0.75 ± 0.5 |
| DOUMA | Male | 2006 | Nursery | 06/07 | 6 | 1 | 5 | 0.25 ± 0.5 |
| PANZA | Male | 2005 | Nursery | 09/06 | 8 | 1 | 7 | 0.25 ± 0.5 |
| TANGO | male | 2009 | TDT | 06/10 | 4 | 1 | 3 | 0.2 ± 0.4 |
| TYA | Female | 2010 | TDT | 01/11 | 2 | 1 | 1 | 0.25 ± 0.5 |
| DEMOU | Female | 2007 | TDT | 03/11 | 5 | 4 | 1 | 0 ± 0.0 |
| Likert Scale | Scale definition |
|---|---|
| 3 | Exhibits a range of abnormal behaviors, i.e., intense rocking, hair plucking and/or self-mutilation, these behaviors are seen throughout the day under both stressful and relaxed situations/environments. |
| 2 | Exhibits abnormal behaviors occasionally under stressful and less stressful conditions, e.g., rocking, on a daily basis. Abnormal behaviors seen several times a day. |
| 1 | Exhibits abnormal behaviors on occasion and only under stressful situations. Abnormal behaviors observed no more than once a day. |
| 0 | No abnormal behaviors |
2.2. Data Collection During Bush-Outings
| CATEGORIES | Definition |
|---|---|
| BEHAVIORS | |
| Social Behavior | An individual is engaged in social play or allo-grooming with humans or conspecifics. This category also includes rarely observed social sexual events. |
| Feeding | Individual eating a food item |
| Searching for food | An individual actively searching for food (may be collecting seed pods or fruit). |
| Subsistence tool-use | The use of an object, e.g., stick or stalk of vegetation, to probe or explore an opening, including termite mound holes, ground holes, or cracks. This behavioral category also included the use of a solid object to strike another to access potentially an embedded edible resource. |
| General Solitary Behaviors | Time spent self-grooming, playing alone or with an object, and solitary sexual events such as masturbation |
| Observing | Individual maintains gaze directed towards keeper or conspecific group member. Who is observed and their activity is also recorded. Gaze must be maintained for longer than 3 seconds and within 3 meters of the individual being observed. |
| Nest Making | Manipulation of branches and/or terrestrial herbaceous vegetation (THV) with the purpose to construct or modify a nesting structure either for resting or play. |
| Travel | Locomotion on the ground or in an arboreal setting (excludes individual displacement when actively searching for food—see above). |
| Resting | The individual is sleeping, standing or sitting and is not actively playing, grooming or partaking in any social behavior stated above, including not actively feeding, foraging, tool using or observing. |
| LOCATION | |
| Arboreal | Location of chimpanzee is in a tree or a vine, i.e., off the ground. |
| Terrestrial | Location of chimpanzee is at ground level. |
| HUMAN CONTACT | |
| Yes | Individual is in direct physical contact with a human caretaker. |
| No | Individual is not in direct physical contact with a human caretaker. |
| No. | Species | Family | Type of Plant | Part eaten |
|---|---|---|---|---|
| 1 | Adansonia digitata | Bombacaceae | Tree | fruit |
| 2 | Afzelia Africana | Caesalpinioideae | Tree | honey on leaves |
| 3 | Allophyllus africanus | Sapindaceae | Tree | fruit |
| 4 | Andropogon gayanus | Poaceae | Grass | leaf |
| 5 | Bombax costatum | Bombacaceae | Tree | flower, leaf |
| 6 | Cassia sieberiana | Caesalpinioideae | Tree | seed |
| 7 | Carapa procera | Meliaceae | Tree | fruit |
| 8 | Cola cordifolia | Sterculiaceae | Tree | flower, leaf stem, fruit, bark |
| 9 | Cordia myxa | Boraginaceae | Tree | fruit |
| 10 | Costus afer | Zingiberaceae | THV | stalk |
| 11 | Daniella oliveri | Caesalpinioideae | Tree | flower, seeds, new leaf |
| 12 | Detarium microcarpum | Caesalpinioideae | Tree | fruit, bark |
| 13 | Dialium guineensis | Caesalpinioideae | Tree | fruit |
| 14 | Diospyros mespiliformis | Ebenaceae | Tree | fruit, new leaf |
| 15 | Dioscorea sp. | Dioscoreaceae | Vine | leaf |
| 16 | Ficus sp. | Moraceae | Tree | fruit |
| 17 | Ficus sur | Moraceae | Tree | fruit |
| 18 | Ficus thonninguii | Moraceae | Tree | fruit |
| 19 | Gardenia erubescens | Rubiaceae | Tree | fruit |
| 20 | Hanna undulate | Simaroubaceae | Tree | fruit |
| 21 | Khaya senegalensis | Meliaceae | Tree | leaf, leaf stem |
| 22 | Kigelia africana | Bignoniaceae | Tree | fruit |
| 23 | Landolphia heudelotti | Apocynaceae | Vine | bark, fruit, leaf |
| 24 | Lannea acida | Anacardiaceae | Tree | seed |
| 25 | Lannea microcarpa | Anacardiaceae | Tree | leaf, bark |
| 26 | Manguifera indica | Anacardiaceae | Tree | leaf, fruit |
| 27 | Marantochloa cuspidate | Marantaceae | THV | stalk |
| 28 | Opilia celtidifolia | Opilianaceae | Vine | bark, leaf |
| 29 | Oxytenanthera abyssinica | Poaceae | Grass | stalk |
| 30 | Parkia biglobosa | Mimosaceae | Tree | flower, fruit |
| 31 | Piliostigma thoningui | Caesalpinioideae | Tree | seed |
| 32 | Pterocarpus erinaceus | Fabaceae | Tree | flower, new leaf |
| 33 | Pterocarpus sp. santalinoides | Fabaceae | Tree | flower |
| 34 | Pterocarpus senegalensis | Fabaceae | Tree | flower |
| 35 | Raphia sp. | Arecaceae | Tree | stalk |
| 36 | Saba comorensis | Apocynaceae | Vine | fruit |
| 37 | Saba senegalensis | Apocynaceae | Vine | fruit |
| 38 | Siphonochilus sp. | Zingiberaceae | THV | root, flower |
| 39 | Smilax anceps | Smilaceae | Vine | new leaf |
| 40 | Tamarindus indica | Caesalpinioideae | Tree | bark |
| 41 | Uapaca somon | Euphorbiaceae | Tree | fruit |
| 42 | Ximenia Americana | Olacaceae | Shrub | fruit |
| 43 | Xylopia aethiopica | Annonaceae | Tree | seed |
| 44 | Oecophylla longinoda | Formicidae | Insect | ant |
| 45 | Procubitermes | Termitidae | Insect | termite |
| 46 | Trigona sp. | Apidae | Honey | honey |
2.3. Analysis
3. Results
3.1. Activity Budget, Location and Human Contact

3.2. Dietary Knowledge

3.3. Observing Conspecifics Foraging
4. Discussion
4.1. Activity Budget
4.2. Dietary Knowledge
4.3. The Role of Observation
4.4. The “Super Chimpanzee” Theory
5. Conclusion
Acknowledgments
Conflict of Interest
References and Notes
- Noon, C. Chimpanzees and Retirement. J. Appl. Anim. Welf. Sci. 1999, 2, 141–146. [Google Scholar]
- Teleki, G. Sanctuaries for Ape Refugees. In Great Apes and Humans, the Ethics of Coexistence; Beck, B., Stoinski, T., Hutchins, M., Maple, T., Norton, B., Rowan, A., Stevens, E., Arluke, A., Eds.; Smithsonian Press: Washington, DC, USA, 2001; pp. 133–149. [Google Scholar]
- Farmer, K. Pan-African Sanctuary Alliance: Status and Range of Activities for Great Ape Conservation. Am. J. Primatol. 2002, 58, 117–132. [Google Scholar]
- Campbell, G.; Kuehl, H.; N’Goran Kouamé, P.; Boesch, C. Alarming Decline of West African Chimpanzees in Côte d’Ivoire. Curr. Biol. 2008, 18, R903–R904. [Google Scholar]
- Schipper, J.; Chanson, J.S.; Chiozza, F.; Cox, N.A.; Hoffmann, M.; Katariya, V.; Lamoreux, J.; Rodrigues, A.S.; Stuart, S.N.; Temple, H.J.; et al. The Status of the World’s Land and Marine Mammals: Diversity, Threat, and Knowledge. Science 2008, 322, 225–230. [Google Scholar]
- Beck, B. Chimpanzee Orphans: Sanctuaries, Reintroduction and Cognition. In The Mind of the Chimpanzee: Ecological and Experimental Perspectives; Lonsdorf, E., Ross, S., Matsuzawa, T., Eds.; University of Chicago Press: Chicago, IL, USA, 2010; pp. 332–346. [Google Scholar]
- Morimura, N.; Idani, G.; Matsuzawa, T. The First Chimpanzee Sanctuary in Japan: An Attempt to Care for the “Surplus” of Biomedical Research. Am. J. Primatol. 2011, 73, 226–232. [Google Scholar]
- Faust, L.; Cress, D.; Farmer, K.; Ross, S.; Beck, B. Predicting Capacity Demand on Sanctuaries for African Chimpanzees (Pan troglodytes). Int. J. Primatol. 2011, 32, 849–864. [Google Scholar] [CrossRef]
- Beck, B.; Walkup, K.; Rodrigues, M.; Unwin, S.; et Tara Stoinski, D.T. Best Practice Guidelines for the Reintroduction of Great Apes; IUCN: Abu Dhabi, UAE, 2007. [Google Scholar]
- Hannah, A.; McGrew, W. Rehabilitation of Captive Chimpanzees. In Primate Responses to Environmental Change; Chapman and Hall: London, UK, 2005; pp. 167–186. [Google Scholar]
- Yeager, C. Orangutan Rehabilitation in Tanjung Puting National Park, Indonesia. Conserv. Biol. 1997, 11, 802–805. [Google Scholar]
- Farmer, K.; Honig, N.; Goossens, B.; Jamart, A. Habitat Ecologique et Liberte des Primates: Re-Introduction of Central Chimpanzees to the Conkouati-Douli National Park, Republic of Congo. In IUCN/SSC: Global Re-Introduction Perspectives: Additional Case-Studies from Around the Globe; Soorae, P., Ed.; Re-Introduction Specialist Group: Abu Dhabi, UAE, 2010; pp. 231–237. [Google Scholar]
- Humle, T.; Colin, C.; Laurans, M.; Raballand, E. Group Release of Sanctuary Chimpanzees (Pan troglodytes) in the Haut Niger National Park, Guinea, West Africa: Ranging Patterns and Lessons So Far. Int. J. Primatol. 2010, 32, 456–473. [Google Scholar]
- Reimers, M.; Schwarzenberger, F.; Preuschoft, S. Rehabilitation of Research Chimpanzees: Stress and Coping after Long-Term Isolation. Hormones Behav. 2007, 51, 428–435. [Google Scholar] [CrossRef]
- Fritz, J.; Maki, S.; Nash, L.; Martin, T.; Matevia, M. The Relationship Between Forage Material and Levels of Coprophagy in Captive Chimpanzees (Pan troglodytes). Zoo Biol. 1992, 11, 313–318. [Google Scholar] [CrossRef]
- Wobber, V.; Hare, B. Psychological Health of Orphan Bonobos and Chimpanzees in African Sanctuaries. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Doran, D. Influence of Seasonality on Activity Patterns, Feeding Behavior, Ranging, and Grouping Patterns in Taï Chimpanzees. Int. J. Primatol. 1997, 18, 183–206. [Google Scholar] [CrossRef]
- Preutz, J. Feeding Ecology of Savanna Chimpanzees (Pan troglodytes verus) in Fongoli, Senegal. In Feeding Ecology in Apes and Other Primates: Ecological, Physical, and Behavioral Aspects; Hohmann, G., Robbins, M., Boesch, C., Eds.; Cambridge University Press: Cambridge, UK, 2006; pp. 161–182. [Google Scholar]
- Goodall, J. The Chimpanzees of Gombe: Patterns of Behavior; Harvard University Press: Cambridge, MA, USA, 1986. [Google Scholar]
- Boesch, C.; Boesch, H. Hunting Behavior of Wild Chimpanzees in the Taï National Park. Am. J. Phys. Anthropol. 1989, 78, 547–573. [Google Scholar] [CrossRef]
- Yamakoshi, G. Dietary Responses to Fruit Scarcity of Wild Chimpanzees at Bossou, Guinea: Possible Implications for Ecological Importance of Tool Use. Am. J. Phys. Anthropol. 1998, 106, 283–295. [Google Scholar] [CrossRef]
- Marshall, A.; Wrangham, R. Evolutionary Consequences of Fallback Foods. Int. J. Primatol. 2007, 28, 1219–1235. [Google Scholar] [CrossRef]
- van-Lawick-Goodall, J. Cultural Elements in a Chimpanzee Community. In Precultural Primate Behavior; Menzel, E.W., Ed.; Karger: Basel, Switzerland, 1973. [Google Scholar]
- Pusey, A. Mother-Offspring Relationships in Chimpanzees after Weaning. Anim. Behav. 1983, 31, 363–377. [Google Scholar] [CrossRef]
- Hiraiwa-Hasegawa, M. Sex Differences in the Behavioral Development of Chimpanzees at Mahale. In Understanding Chimpanzees; Heltne, P., Marquardt, L., Eds.; Harvard University Press: Cambridge, MA, USA, 1989; pp. 104–115. [Google Scholar]
- Van Schaik, C. Orangutan Cultures and the Evolution of Material Culture. Science 2003, 299, 102–105. [Google Scholar] [CrossRef]
- Galef, B., Jr.; Giraldeau, L. Social Influences on Foraging in Vertebrates: Causal Mechanisms and Adaptive Functions. Anim. Behav. 2001, 61, 3–15. [Google Scholar] [CrossRef]
- Biro, D.; Inoue-Nakamura, N.; Tonooka, R.; Yamakoshi, G.; Sousa, C.; Matsuzawa, T. Cultural Innovation and Transmission of Tool Use in Wild Chimpanzees: Evidence from Field Experiments. Anim. Cognit. 2003, 6, 213–223. [Google Scholar] [CrossRef]
- Lonsdorf, E. Sex Differences in the Development of Termite-Fishing Skills in the Wild Chimpanzees, (Pan troglodytes schweinfurthii), of Gombe National Park, Tanzania. Anim. Behav. 2005, 70, 673–683. [Google Scholar] [CrossRef]
- Rapaport, L.; Brown, G. Social Influences on Foraging Behavior in Young Nonhuman Primates: Learning What, Where, and How to Eat. Evol. Anthropol. Issues News Rev. 2008, 17, 189–201. [Google Scholar] [CrossRef]
- Jaeggi, A.; Dunkel, L.; Van Noordwijk, M.; Wich, S.; Sura, A.; Van Schaik, C. Social Learning of Diet and Foraging Skills by Wild Immature Bornean Orangutans: Implications for Culture. Am. J. Primatol. 2010, 72, 62–71. [Google Scholar] [CrossRef]
- Riedler, B.; Millesi, E.; Pratje, P. Adaptation to Forest Life During the Reintroduction Process of Immature Pongo abelii. Int. J. Primatol. 2010, 31, 647–663. [Google Scholar] [CrossRef]
- Brugiere, D.; Dia, M.; Diakite, S.; Gbansara, M.; Mamy, M.; Saliou, B.; Magassouba, B. Large- and Medium-Sized Ungulates in the Haut Niger National Park, Republic of Guinea: Population Changes 1997–2002. Oryx 2005, 39, 50–55. [Google Scholar]
- Fleury-Brugiere, M.; Brugiere, D. High Population Density of Pan troglodytes verus in the Haut Niger National Park, Republic of Guinea: Implications for Local and Regional Conservation. Int. J. Primatol. 2010, 31, 383–392. [Google Scholar] [CrossRef]
- Likert, R. A Technique for the Measurement of Attitudes. Arch. Psychol. 1932, 140, 55–59. [Google Scholar]
- Altmann, J. Observational Study of Behavior: Sampling Methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Martin, P.; Bateson, P. Measuring Behaviour: An Introductory Guide, 3rd ed.; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Humle, T. School of Anthropology and Conservation, University of Kent: Canterbury, UK, Unpublished data. 2008.
- Humle, T. Location and Ecology. In The Chimpanzees of Bossou and Nimba; Matsuzawa, T., Humle, T., Sugiyama, Y., Matsuzawa, T., Yamagiwa, J., Eds.; Springer: Tokyo, Japan, 2011; pp. 13–21. [Google Scholar]
- Boesch, C.; Boesch-Achermann, H. The Chimpanzees of the Taï Forest: Behavioural Ecology and Evolution; Oxford University Press: New York, NY, USA, 2000. [Google Scholar]
- Humle, T.; Snowdon, C.; Matsuzawa, T. Social Influences on Ant-Dipping Acquisition in the Wild Chimpanzees (Pan troglodytes verus) of Bossou, Guinea, West Africa. Anim. Cognit. 2009, 12, 37–48. [Google Scholar] [CrossRef]
- Baker, K. Guidelines for Nonhuman Primate Re-Introductions. Re-Introduction News 2002, 21, 29–57. [Google Scholar]
- Humle, T.; Ongman, L. Personal observation. School of Anthropology and Conservation, University of Kent: Canterbury, UK, 2012. [Google Scholar]
- Humle, T. Primate Material Culture. In The Oxford Handbook of Material Culture Studies; Hicks, D., Beaudry, M., Eds.; Oxford University Press: Oxford, UK, 2010; pp. 406–424. [Google Scholar]
- Whiten, A.; Spiteri, A.; Horner, V.; Bonnie, K.; Lambeth, S.; Schapiro, S.; de Waal, F. Transmission of Multiple Traditions within and between Chimpanzee Groups. Curr. Biol. 2007, 17, 1038–1043. [Google Scholar]
- Humle, T.; Snowdon, C. Socially Biased Learning in the Acquisition of a Complex Foraging Task in Juvenile Cottontop Tamarins, (Saguinus oedipus). Anim. Behav. 2008, 75, 267–277. [Google Scholar] [CrossRef]
- Chimpanzee Conservation Center, Somoria, Faranah, Republic of Guinea. Unpublished dataset. 2012.
- Lonsdorf, E. What is the Role of Mothers in the Acquisition of Termite-Fishing Behaviors in Wild Chimpanzees (Pan troglodytes schweinfurthii)? Anim. Cognit. 2006, 9, 36–46. [Google Scholar] [CrossRef]
- Nishida, T.; Turner, L. Food Transfer Between Mother and Infant Chimpanzees of the Mahale Mountains National Park, Tanzania. Int. J. Primatol. 1996, 17, 947–968. [Google Scholar] [CrossRef]
- Hawthorne, W.; Jongkind, C. Woody Plants of Western African Forests; Royal Botanic Gardens: Kew, UK, 2006. [Google Scholar]
- Humle, T.; Matsuzawa, T. Oil Palm Use by Adjacent Communities of Chimpanzees at Bossou and Nimba Mountains, West Africa. Int. J. Primatol. 2004, 25, 551–581. [Google Scholar] [CrossRef]
- Ongman, L. Personal observation. School of Anthropology and Conservation, University of Kent: Canterbury, UK, 2012. [Google Scholar]
- Ongman, L.; Colin, C.; Raballand, E.; Humle, T. Individual History and the Social Dimensions of Rehabilitation in Chimpanzees. 2013; in preparation. [Google Scholar]
- Raballand, E.; Colin, C. Personal observation. Chimpanzee Conservation Center, Somoria, Faranah, Republic of Guinea. 2012. [Google Scholar]
- Farmer, K.; Buchanan-Smith, H.M.; Jamart, A. Behavioral Adaptation of Pan troglodytes troglodytes. Int. J. Primatol. 2006, 27, 747–765. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ongman, L.; Colin, C.; Raballand, E.; Humle, T. The “Super Chimpanzee”: The Ecological Dimensions of Rehabilitation of Orphan Chimpanzees in Guinea, West Africa. Animals 2013, 3, 109-126. https://doi.org/10.3390/ani3010109
Ongman L, Colin C, Raballand E, Humle T. The “Super Chimpanzee”: The Ecological Dimensions of Rehabilitation of Orphan Chimpanzees in Guinea, West Africa. Animals. 2013; 3(1):109-126. https://doi.org/10.3390/ani3010109
Chicago/Turabian StyleOngman, Lissa, Christelle Colin, Estelle Raballand, and Tatyana Humle. 2013. "The “Super Chimpanzee”: The Ecological Dimensions of Rehabilitation of Orphan Chimpanzees in Guinea, West Africa" Animals 3, no. 1: 109-126. https://doi.org/10.3390/ani3010109
APA StyleOngman, L., Colin, C., Raballand, E., & Humle, T. (2013). The “Super Chimpanzee”: The Ecological Dimensions of Rehabilitation of Orphan Chimpanzees in Guinea, West Africa. Animals, 3(1), 109-126. https://doi.org/10.3390/ani3010109




