Estimate of Growth Parameters of Penaeus kerathurus (Forskäl, 1775) (Crustacea, Penaeidae) in the Northern Adriatic Sea
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
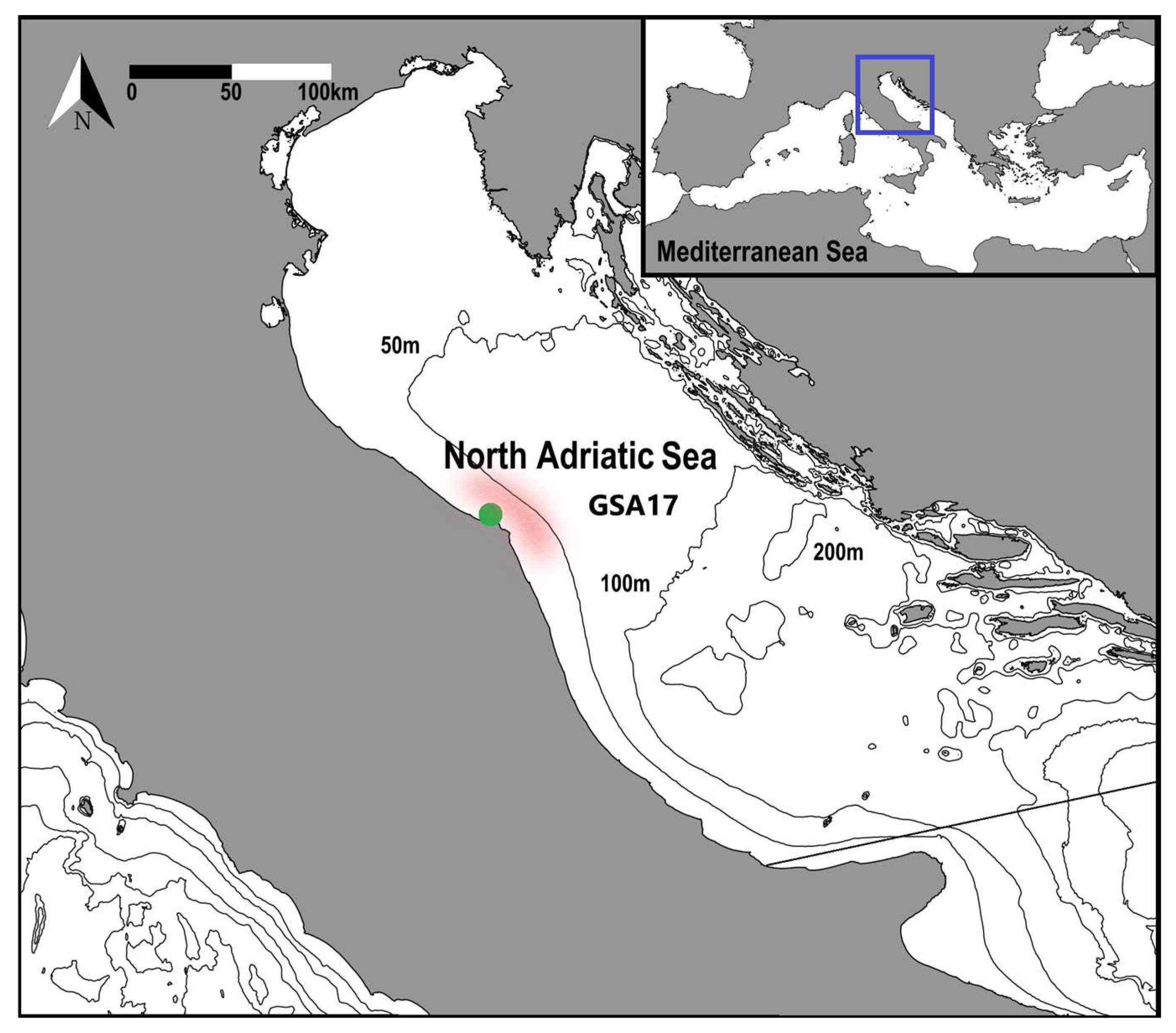
2.1. Study Area
2.2. Monthly LFDs Collection
2.3. VBGF Parameters Estimation
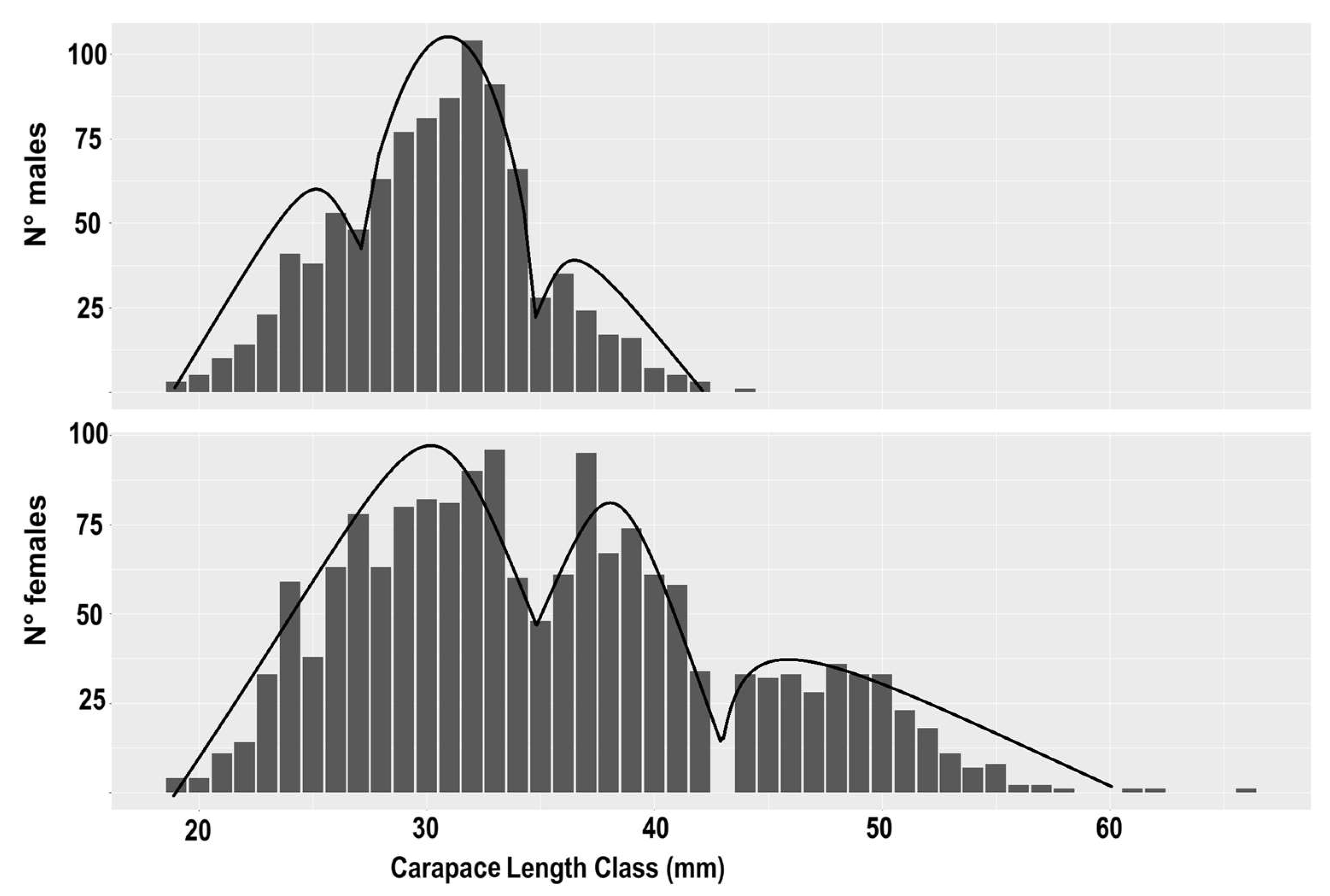
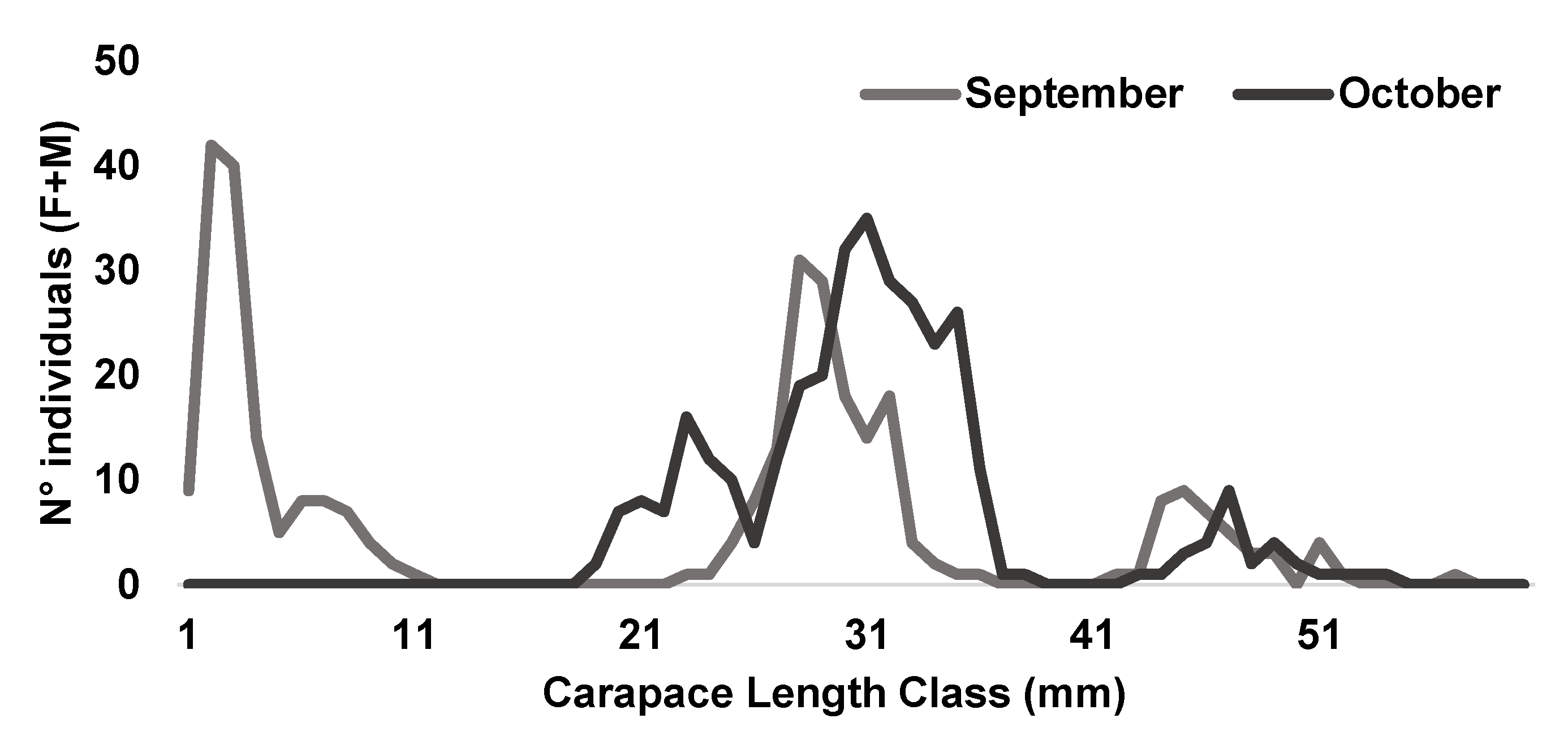
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boenish, R.; Kritzer, J.P.; Kleisner, K.; Steneck, R.S.; Werner, K.M.; Zhu, W.; Schram, F.; Rader, D.; Cheung, W.; Ingles, J.; et al. The Global Rise of Crustacean Fisheries. Front. Ecol. Environ. 2022, 20, 102–110. [Google Scholar] [CrossRef]
- FAO. The State of Mediterranean and Black Sea Fisheries 2023; FAO: Rome, Italy, 2023; ISBN 978-92-5-138411-4. [Google Scholar]
- Grati, F.; Fabi, G.; Lucchetti, A.; Consoli, P. Analisi Delle Catture Si Solea Vulgaris Quensel, 1806 Effettuate Con Reti Ad Imbrocco in Adriatico Settentrionale. Biol. Mar. Mediterr. 2002, 90, 154–160. [Google Scholar]
- Dayal, J.S.; Ponniah, A.G.; Khan, H.I.; Babu, E.P.M.; Ambasankar, K.; Kumarguru, K.P. Shrimps—A Nutritional Perspective. Curr. Sci. 2013, 104, 1487–1491. [Google Scholar]
- Champion, C.; Broadhurst, M.K.; Ewere, E.E.; Benkendorff, K.; Butcherine, P.; Wolfe, K.; Coleman, M.A. Resilience to the Interactive Effects of Climate Change and Discard Stress in the Commercially Important Blue Swimmer Crab (Portunus armatus). Mar. Environ. Res. 2020, 159, 105009. [Google Scholar] [CrossRef] [PubMed]
- Angelini, S.; Martinelli, M.; Santojanni, A.; Colella, S. Biological Evidence of the Presence of Different Subpopulations of Norway Lobster (Nephrops norvegicus) in the Adriatic Sea (Central Mediterranean Sea). Fish. Res. 2020, 221, 105365. [Google Scholar] [CrossRef]
- Grati, F.; Aladzuz, A.; Azzurro, E.; Bolognini, L.; Carbonara, P.; Ҫobani, M.; Domenichetti, F.; Dragicevic, B.; Dulcic, J.; Ðurovic, M.; et al. Seasonal Dynamics of Small-Scale Fisheries in the Adriatic Sea. Mediterr. Mar. Sci. 2018, 19, 21. [Google Scholar] [CrossRef]
- European Commission (EC). Regulation (EU) 2017/1004 on the Establishment of a Union Framework for the Collection, Management and Use of Data in the Fisheries Sector and Support for Scientific Advice Regarding the Common Fisheries Policy and Repealing Council Regulation (EC) No 199/2008; European Commission (EC): Brussels, Belgium, 2017. [Google Scholar]
- Zitari-Chatti, R.; Chatti, N.; Elouaer, A.; Said, K. Genetic Variation and Population Structure of the Caramote Prawn Penaeus kerathurus (Forskäl) from the Eastern and Western Mediterranean Coasts in Tunisia. Aquac. Res. 2008, 39, 70–76. [Google Scholar] [CrossRef]
- Froglia, C.; Scarcella, G.; Lucchetti, A. On the Recent Increase of Penaeus (Melicertus) Kerathurus Stock in Northern and Central Adriatic Sea: Possible Explanations. Rapp. Comm. Int. Mer Médit. 2013, 40, 780. [Google Scholar]
- Lucchetti, A. La Mazancolla-Melicertus kerathurus (Forskal, 1755). Il Pesce 2006, 117. [Google Scholar]
- Heller, C. Die Crustaceen Des Südlichen Europa: Crustacea Podophthalmia. Mit Einer Übersicht Über Die Horizontale Verbreitung Sämmtlicher Europäischer Arten, von Camil Heller; W. Braumüller: Wien, NY, USA, 1863. [Google Scholar]
- Jaziri, H.; Khoufi, W.; Meriem, S.B. Assessment Approach of Melicertus kerathurus Stock along the North-Eastern Tunisian Coast Using a Surplus Production Model Incorporating Temperature Parameter. Am. J. Clim. Chang. 2015, 4, 417–430. [Google Scholar] [CrossRef]
- Pikitch, E.K.; Santora, C.; Babcock, E.A.; Bakun, A.; Bonfil, R.; Conover, D.O.; Dayton, P.; Doukakis, P.; Fluharty, D.; Heneman, B.; et al. Ecosystem-Based Fishery Management. Science 2004, 305, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Ligas, A.; Sartor, P.; Colloca, F. Trends in Population Dynamics and Fishery of Parapenaeus longirostris and Nephrops norvegicus in the Tyrrhenian Sea (NW Mediterranean): The Relative Importance of Fishery and Environmental Variables. Mar. Ecol. 2011, 32, 25–35. [Google Scholar] [CrossRef]
- Galimany, E.; Baeta, M.; Durfort, M.; Lleonart, J.; Ramón, M. Reproduction and Size at First Maturity in a Mediterranean Exploited Callista chione Bivalve Bed. Sci. Mar. 2015, 79, 233–242. [Google Scholar] [CrossRef]
- Punt, A.E. Those Who Fail to Learn from History Are Condemned to Repeat It: A Perspective on Current Stock Assessment Good Practices and the Consequences of Not Following Them. Fish. Res. 2023, 261, 106642. [Google Scholar] [CrossRef]
- Conides, A.; Glamuzina, B.; Jug-dujakovic, J.; Papaconstantinou, C.; Kapiris, K. Age, Growth, and Mortality of the Karamote Shrimp, Melicertus kerathurus (Forskål, 1775), in the East Ionian Sea (Western Greece). Crustaceana 2006, 79, 33–52. [Google Scholar] [CrossRef]
- Kevrekidis, K.; Thessalou-Legaki, M. Population Dynamics of Melicertus kerathurus (Decapoda: Penaeidae) in Thermaikos Gulf (N. Aegean Sea). Fish. Res. 2011, 107, 46–58. [Google Scholar] [CrossRef]
- Kevrekidis, K.; Thessalou-Legaki, M. Catch Rates, Size Structure and Sex Ratio of Melicertus kerathurus (Decapoda: Penaeidae) from an Aegean Sea Trawl Fishery. Fish. Res. 2006, 80, 270–279. [Google Scholar] [CrossRef]
- Akyol, O.; Ceyhan, T. Catch per Unit Effort of Coastal Prawn Trammel Net Fishery in Izmir Bay, Aegean Sea. Medit. Mar. Sci. 2009, 10, 19. [Google Scholar] [CrossRef]
- Ihsanoglu, M.A.; Daban, I.B.; Işmen, A.; Cabbar, K.; Yığin, C.Ç. Reproductive Biology of Penaeus kerathurus (Forskål, 1775) (Decapoda: Penaeidae) in the Sea of Marmara, Turkey. Oceanol. Hydrobiol. Stud. 2021, 50, 33–37. [Google Scholar] [CrossRef]
- Schwamborn, R.; Mildenberger, T.K.; Taylor, M.H. Assessing Sources of Uncertainty in Length-Based Estimates of Body Growth in Populations of Fishes and Macroinvertebrates with Bootstrapped ELEFAN. Ecol. Model. 2019, 393, 37–51. [Google Scholar] [CrossRef]
- Morgan, M.J. Integrating Reproductive Biology into Scientific Advice for Fisheries Management. J. Northwest Atl. Fish. Sci. 2008, 41, 37–51. [Google Scholar] [CrossRef]
- Petersen, C.G.J. Eine Methode zur Bestimmung des Alters und Wuchses der Fische. Mitteilungen. Dtsch. Seefischerei-Ver. 1891, 11, 226–235. [Google Scholar]
- Pauly, D.; David, N. ELEFAN I, a BASIC Program for the Objective Extraction Datal of Growth Parameters from Length-Frequency. In Berichte der Deutshcen Wissenchaftlichen Kommission für Meeresforschung; P. Parey: Berlin, Germany, 1981; Volume 28, pp. 205–211. [Google Scholar]
- Mildenberger, T.K.; Taylor, M.H.; Wolff, M. TropFishR: An R Package for Fisheries Analysis with Length-Frequency Data. Methods Ecol. Evol. 2017, 8, 1520–1527. [Google Scholar] [CrossRef]
- García-Berthou, E.; Carmona-Catot, G.; Merciai, R.; Ogle, D.H. A Technical Note on Seasonal Growth Models. Rev. Fish Biol. Fish. 2012, 22, 635–640. [Google Scholar] [CrossRef]
- Campanelli, A.; Grilli, F.; Paschini, E.; Marini, M. The Influence of an Exceptional Po River Flood on the Physical and Chemical Oceanographic Properties of the Adriatic Sea. Dyn. Atmos. Ocean. 2011, 52, 284–297. [Google Scholar] [CrossRef]
- Marini, M.; Jones, B.H.; Campanelli, A.; Grilli, F.; Lee, C.M. Seasonal Variability and Po River Plume Influence on Biochemical Properties along Western Adriatic Coast. J. Geophys. Res. Ocean. 2008, 113, 1–18. [Google Scholar] [CrossRef]
- Bastari, A.; Micheli, F.; Ferretti, F.; Pusceddu, A.; Cerrano, C. Large Marine Protected Areas (LMPAs) in the Mediterranean Sea: The Opportunity of the Adriatic Sea. Mar. Policy 2016, 68, 165–177. [Google Scholar] [CrossRef]
- Pellini, G.; Gomiero, A.; Fortibuoni, T.; Ferrà, C.; Grati, F.; Tassetti, A.N.; Polidori, P.; Fabi, G.; Scarcella, G. Characterization of Microplastic Litter in the Gastrointestinal Tract of Solea solea from the Adriatic Sea. Environ. Pollut. 2018, 234, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Grati, F.; Scarcella, G.; Polidori, P.; Domenichetti, F.; Bolognini, L.; Gramolini, R.; Vasapollo, C.; Giovanardi, O.; Raicevich, S.; Celić, I.; et al. Multi-Annual Investigation of the Spatial Distributions of Juvenile and Adult Sole (Solea solea L.) in the Adriatic Sea (Northern Mediterranean). J. Sea Res. 2013, 84, 122–132. [Google Scholar] [CrossRef]
- Zhou, S.; Hutton, T.; Lei, Y.; Miller, M.; Van Der Velde, T.; Deng, R. Modelling Growth of Red Endeavour Prawns (Metapenaeus ensis) Using New ELEFAN and Bayesian Growth Models. 2021.
- Kilada, R.; Driscoll, J.G. Age Determination in Crustaceans: A Review. Hydrobiologia 2017, 799, 21–36. [Google Scholar] [CrossRef]
- Lucchetti, A.; Petetta, A.; Bdioui, M.; Gökçe, G.; Saber, M.; Sacchi, J.; Ozbilgin, H.; Carlson, A.; Carpentieri, P. Catalogue of Fishing Gear in the Mediterranean and Black Sea Region; FAO Fisher: Rome, Italy, 2023; ISBN 9789251380550. [Google Scholar]
- Pauly, D.; Murphy, G.I. Theory and Management of Tropical Fisheries; ICLARM: Manila, Philippines, 1982; p. 360. [Google Scholar]
- Taylor, M.H.; Mildenberger, T.K. Extending Electronic Length Frequency Analysis in R. Fish. Manag. Ecol. 2017, 24, 330–338. [Google Scholar] [CrossRef]
- Mildenberger, T.; Taylor, M.; Wolff, M.; TropFishR: Tropical Fisheries Analysis with R. R Package Version 1.6.0. Available online: https://cran.r-project.org/package=TropFishR (accessed on 15 December 2023).
- Xiang, Y.; Gubian, S.; Suomela, B.; Hoeng, J. Generalized Simulated Annealing for Global Optimization: The GenSA Package. R J. 2013, 5, 13–28. [Google Scholar] [CrossRef]
- Scrucca, L. GA: A Package for Genetic Algorithms in R. J. Stat. Softw. 2013, 53, 1–37. [Google Scholar] [CrossRef]
- Von Bertalanffy, L. A Quantitative Theory of Organic Growth (Inquiries on Growth Laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Ogle, D.H. An Algorithm for the von Bertalanffy Seasonal Cessation in Growth Function of Pauly et al. (1992). Fish. Res. 2017, 185, 1–5. [Google Scholar] [CrossRef]
- Cai, B.; Pellegrini, F.; Pang, M.; de Moor, C.; Shen, C.; Charu, V.; Tian, L. Bootstrapping the Cross-Validation Estimate. arXiv 2023, arXiv:2307.00260. [Google Scholar] [CrossRef]
- Stine, R. An Introduction to Bootstrap Methods. Sociol. Methods Res. 1989, 18, 243–291. [Google Scholar] [CrossRef]
- Rodríguez, A. Biología Del Langostino Penaeus kerathurus (Forskål, 1755) Del Golfo de Cádiz. II: Distribución y Estructura de La Población. Investig. Pesq. 1986, 49, 581–595. [Google Scholar]
- Sardà, F. Bio-Ecological Aspects of the Decapod Crustacean Fisheries in the Western Mediterranean. Aquat. Living Resour. 1993, 6, 299–305. [Google Scholar] [CrossRef]
- Pauly, D.; Munro, J.L. Once More on the Comparison of Growth in Fish and Invertebrates. Fishbyte 1984, 2, 1–21. [Google Scholar]
- Kevrekidis, K. Population Structure, Sex Ratio, CPUE and Reproductive Aspects of Melicertus kerathurus and Penaeus aztecus in the Thermaikos Gulf, Aegean Sea. Cah. Biol. Mar. 2021, 62, 268–283. [Google Scholar]
- Morghad, H.; Derbal, F.; Rachedi, M. Reproduction, Growth, Mortality and Exploitation of Penaeus kerathurus (Forskål, 1775) from the Eastern Coasts of Algeria. Thalassas 2023, 1–12. [Google Scholar] [CrossRef]
- Klaoudatos, S.; Tsevis, N.; Conides, A. Studies on Migratory Movements of the Prawn Penaeus kerathurus (Forskal, 1775) at Amvrakikos Gulf, Western Greece. Mar. Ecol. 1992, 13, 133–147. [Google Scholar] [CrossRef]
- Kevrekidis, K.; Thessalou-Legaki, M. Reproductive Biology of the Prawn Melicertus kerathurus (Decapoda: Penaeidae) in Thermaikos Gulf (N. Aegean Sea). Helgol. Mar. Res. 2013, 67, 17–31. [Google Scholar] [CrossRef]
- Meriem, S.B. Premire Approche de La Croissance de Penaeus kerathurus (Decapoda, Penaeidae) Dans le Golfe de Gabès, Tunisie [First Approach to the Growth of Penaeus kerathurus (Decapoda, Penaeidae) in the Gulf of Gabes, Tunisia]. Crustaceana 2004, 77, 277–297. [Google Scholar] [CrossRef]
- Bolognini, L.; Froglia, C.; Guicciardi, S.; Scanu, M.; Grati, F. Effects of Breakwater Deployment on the Life-History Traits of the Caramote Prawn Penaeus kerathurus (Forskål, 1775) in the Adriatic Sea. In Proceedings of the ECSA 58—EMECS 13. Estuaries and Coastal Seas in the Anthropocene-Structure, Functions, Services and Management, Hull, UK, 7–11 September 2020. [Google Scholar]
- Ferrà, C.; Tassetti, A.N.; Grati, F.; Pellini, G.; Polidori, P.; Scarcella, G.; Fabi, G. Mapping Change in Bottom Trawling Activity in the Mediterranean Sea through AIS Data. Mar. Policy 2018, 94, 275–281. [Google Scholar] [CrossRef]
- Amoroso, R.O.; Pitcher, C.R.; Rijnsdorp, A.D.; McConnaughey, R.A.; Parma, A.M.; Suuronen, P.; Eigaard, O.R.; Bastardie, F.; Hintzen, N.T.; Althaus, F.; et al. Bottom Trawl Fishing Footprints on the World’s Continental Shelves. Proc. Natl. Acad. Sci. USA 2018, 115, E10275–E10282. [Google Scholar] [CrossRef] [PubMed]
- Pauly, D. Growth Performance in Fishes: Rigorous Description of Patterns as a Basis for Understanding Causal Mechanisms. Aquabyte 1991, 4, 3–5. [Google Scholar]
- Conides, A.; Klaoudatos, S.; Tsevis, N. Study on the Growth Rates of the Prawn Penaeus kerathurus in Amvrakikos Gulf. In Proceedings of the 3rd Panhellenic Congress of Oceanography and Fisheries, Hersonissos, Greece, 6–9 May 1990; pp. 610–619. [Google Scholar]
- Righini, P.; Baino, R.; Cecchi, A. Note Sulla Biologia e Sui Parametri di Crescita di Penaeus kerathurus (Crustacea: Decapoda) Lungo la Costa Toscana. Biol. Mar. Mediterr. 1998, 5, 836–838. [Google Scholar]
- Jaziri, H.; Khoufi, W.; Ben Meriem, S. Characterization of Two Substocks of Penaeus kerathurus (Forskål, 1775) Using the Life-History Parameters. Russ. J. Mar. Biol. 2020, 46, 421–430. [Google Scholar] [CrossRef]
- Vitale, S.; Cannizzaro, L.; Lumare, L.; Mazzola, S. Population Parameters of Melicertus kerathurus (Decapoda, Penaeidae) in Southwest Sicilian Shallow Waters (Mediterranean Sea) Using Length-Frequency Analysis. Crustaceana 2010, 83, 997–1007. [Google Scholar] [CrossRef]
- İhsanoğlu, M. Less Known Aspects of Penaeus kerathurus (Forskål, 1775) (Decapoda, Penaeidae) Obtained from the Fishermen in the Sea of Marmara: Age, Growth, and Mortality Rates. Crustaceana 2020, 93, 1185–1195. [Google Scholar] [CrossRef]
- Rodríguez, A. Biología Del Langostino Penaeus kerathurus (Forskål, 1775) Del Golfo de Cádiz. III: Biometría, Edad y Cresimiento. Investig. Pesq. 1987, 51, 23–37. [Google Scholar]
- Kuparinen, A.; Mäntyniemi, S.; Hutchings, J.A.; Kuikka, S. Increasing Biological Realism of Fisheries Stock Assessment: Towards Hierarchical Bayesian Methods. Environ. Rev. 2012, 20, 135–151. [Google Scholar] [CrossRef]
- Spinelli, A.; Sendín Baquero, P.; Tiralongo, F. Westward Expansion of the Brown Shrimp Penaeus aztecus Ives 1891 (Decapoda: Penaeidae) in the Mediterranean Sea: A Review on the Mediterranean Distribution and First Record from Spain. Nat. Hist. Sci. 2023, 1891. [Google Scholar] [CrossRef]
- Froglia, C.; Scanu, M. Notes on the Spreading of Penaeus aztecus Ives 1891 (Decapoda, Penaeidae) in the Mediterranean Sea and on Its Repeated Misidentifications in the Region. Biology 2023, 12, 793. [Google Scholar] [CrossRef]
| Sex | VBGF | CLinf | K | t_anchor 1 | φ’ 2 | C | ts |
|---|---|---|---|---|---|---|---|
| Females | Classic | 64.18 (58.47–67.18) | 0.42 (0.40–0.57) | 0.63 (0.58–0.88) | 3.23 (3.13–3.41) | ||
| Seasonal | 63.77 (58.22–65.72) | 0.43 (0.40–0.71) | 0.63 (0.10–0.90) | 3.24 (3.11–3.50) | 0.41 (0.03–0.96) | 0.48 (0.15–0.87) | |
| Males | Classic | 39.30 (37.90–47.30) | 0.73 (0.43–0.89) | 0.47 (0.20–0.75) | 3.05 (2.80–3.34) | ||
| Seasonal | 45.74 (38.16–47.27 | 0.85 (0.42–0.99) | 0.62 (0.16–0.72) | 3.25 (2.79–3.34) | 0.81 (0.23–0.99) | 0.60 (0.03–0.9) |
| Sex | VBGF | CLinf | K | t_anchor 1 | φ’ 2 | C | ts |
|---|---|---|---|---|---|---|---|
| Females | Classic | 61.76 (57.71–65.63) | 0.47 (0.41–0.78) | 0.64 (0.18–0.90) | 3.25 (3.13–3.57) | ||
| Seasonal | 61.80 (58.22–65.72) | 0.49 (0.41–0.74) | 0.63 (0.14–0.89) | 3.27 (3.15–3.50) | 0.46 (0.09–0.88) | 0.47 (0.22–0.81) | |
| Males | Classic | 41.69 (38.12–46.90) | 0.75 (0.50–0.90) | 0.44 (0.20–0.85) | 3.11 (2.86–3.30) | ||
| Seasonal | 43.57 (38.27–46.63) | 0.77 (0.49–0.95) | 0.46 (0.15–0.84) | 3.16 (2.85–3.31) | 0.55 (0.19–0.87) | 0.61 (0.10–0.87) |
| Sex | CLinf * | K | φ’ | Area | Reference |
|---|---|---|---|---|---|
| Females | 54.25 * | 0.6 | 3.252 | Gulf of Gabès (Tunisia) | [53] |
| 64.14 | 0.8 | 3.52 | Gulf of Annaba (Algeria) | [53] | |
| 66.15 * | 0.57 | - | Amvrakikos Gulf (Greece) | [58] | |
| 69.04 | 1.06 | 3.71 | Amvrakikos Gulf (Greece) | [18] | |
| 62.48 | 1.15 | 3.65 | Thermaikos Gulf (Greece) | [19] | |
| 64.9 | 0.7 | - | Off N coast Tuscany (Italy) | [59] | |
| 77.5 | 0.55 | 3.54 | Gulf of Annaba (Algeria) | [50] | |
| 61.45 * | 0.7 | 2.58 2 | Gulf of Tunis (Tunisia) | [60] | |
| 60.23 * | 0.76 | 2.64 | Gulf of Gabès (Tunisia) | [60] | |
| 62.84 1 | 0.46 1 | 3.24 | Northern Adriatic Sea (Italy) | Current study | |
| Males | 37.46 * | 0.78 | 3.04 2 | Gulf of Gabès (Tunisia) | [53] |
| 45.5 | 1 | 3.52 | Gulf of Annaba (Algeria) | [53] | |
| 57.53 * | 0.47 | - | Amvrakikos Gulf (Greece) | [58] | |
| 62.66 | 1.25 | 3.69 | Amvrakikos Gulf (Greece) | [18] | |
| 47.78 | 1.28 | 3.47 | Thermaikos Gulf (Greece) | [19] | |
| 46 | 0.9 | - | Off N coast Tuscany (Italy) | [59] | |
| 64.92 | 0.59 | 3.40 | Gulf of Annaba (Algeria) | [50] | |
| 58.59 * | 0.73 | 2.55 2 | Gulf of Tunis (Tunisia) | [60] | |
| 59.61 * | 0.76 | 2.63 | Gulf of Gabès (Tunisia) | [60] | |
| 43.27 1 | 0.77 1 | 3.16 | Northern Adriatic Sea (Italy) | Current study | |
| Combined | 72 | 0.78 | 3.61 | South of Sicily (Italy) | [61] |
| 60.9 | 0.24 | - | Marmara Sea (Turkey) | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scanu, M.; Froglia, C.; Grati, F.; Bolognini, L. Estimate of Growth Parameters of Penaeus kerathurus (Forskäl, 1775) (Crustacea, Penaeidae) in the Northern Adriatic Sea. Animals 2024, 14, 1068. https://doi.org/10.3390/ani14071068
Scanu M, Froglia C, Grati F, Bolognini L. Estimate of Growth Parameters of Penaeus kerathurus (Forskäl, 1775) (Crustacea, Penaeidae) in the Northern Adriatic Sea. Animals. 2024; 14(7):1068. https://doi.org/10.3390/ani14071068
Chicago/Turabian StyleScanu, Martina, Carlo Froglia, Fabio Grati, and Luca Bolognini. 2024. "Estimate of Growth Parameters of Penaeus kerathurus (Forskäl, 1775) (Crustacea, Penaeidae) in the Northern Adriatic Sea" Animals 14, no. 7: 1068. https://doi.org/10.3390/ani14071068





