The Role of Progesterone in Elf5 Activation and Milk Component Synthesis for Cell-Cultured Milk Production in MAC-T Cells
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Cell Culture and Treatments
2.3. Protein Extraction and Western Blot
2.4. RNA Extraction and Real-Time Polymerase Chain Reaction (RT-PCR)
2.5. Immunofluorescence
2.6. Oil Red O Staining
2.7. Enzyme-Linked Immunosorbent Assay (ELISA), Triglyceride Assay, and Freeze Drying
2.8. Statistical Analysis
3. Results
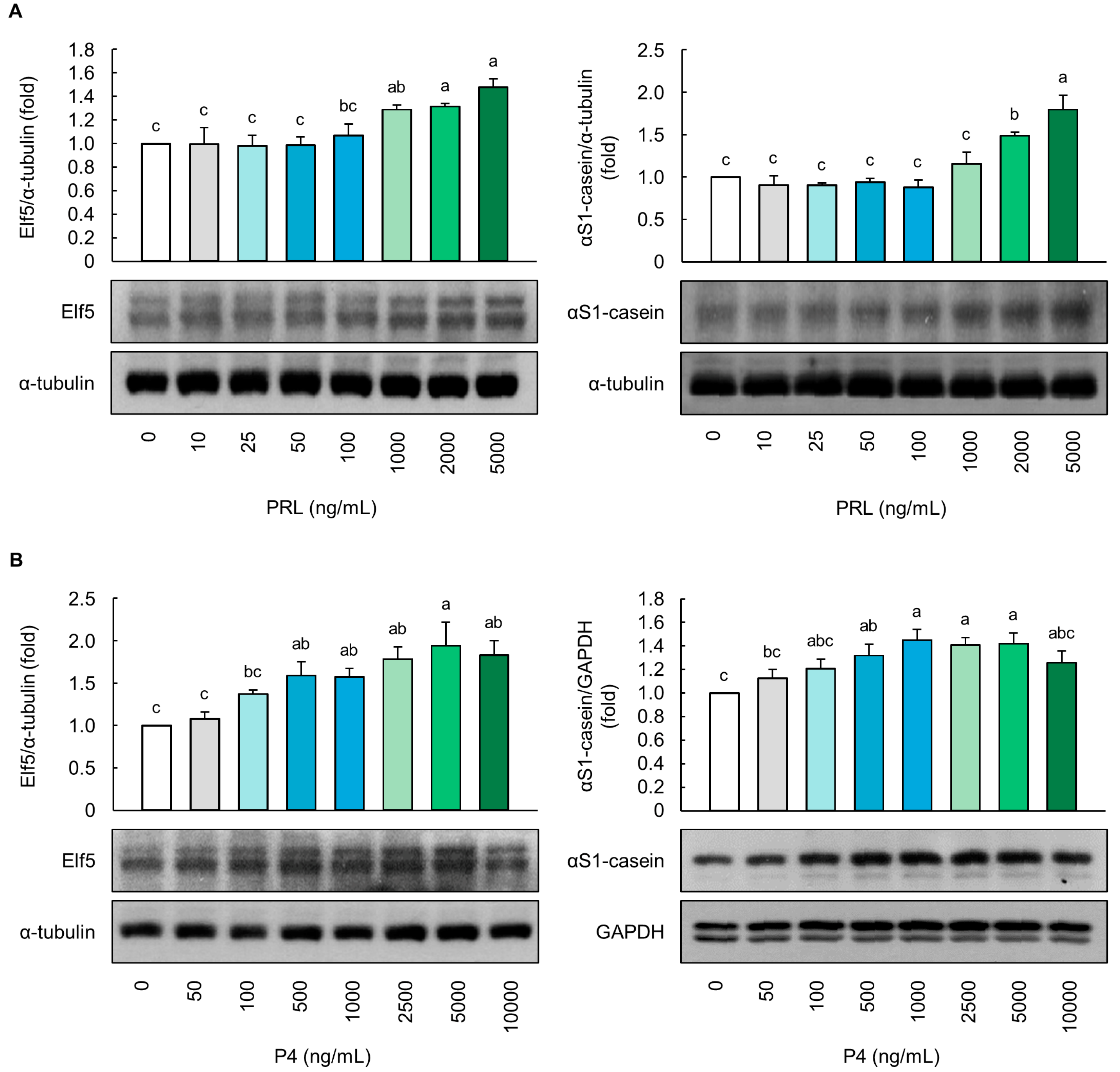
3.1. Selection of PRL and P4 Concentrations Based on Cell Differentiation Markers
3.2. Optimization of P4 Concentration Based on Cell Differentiation Markers
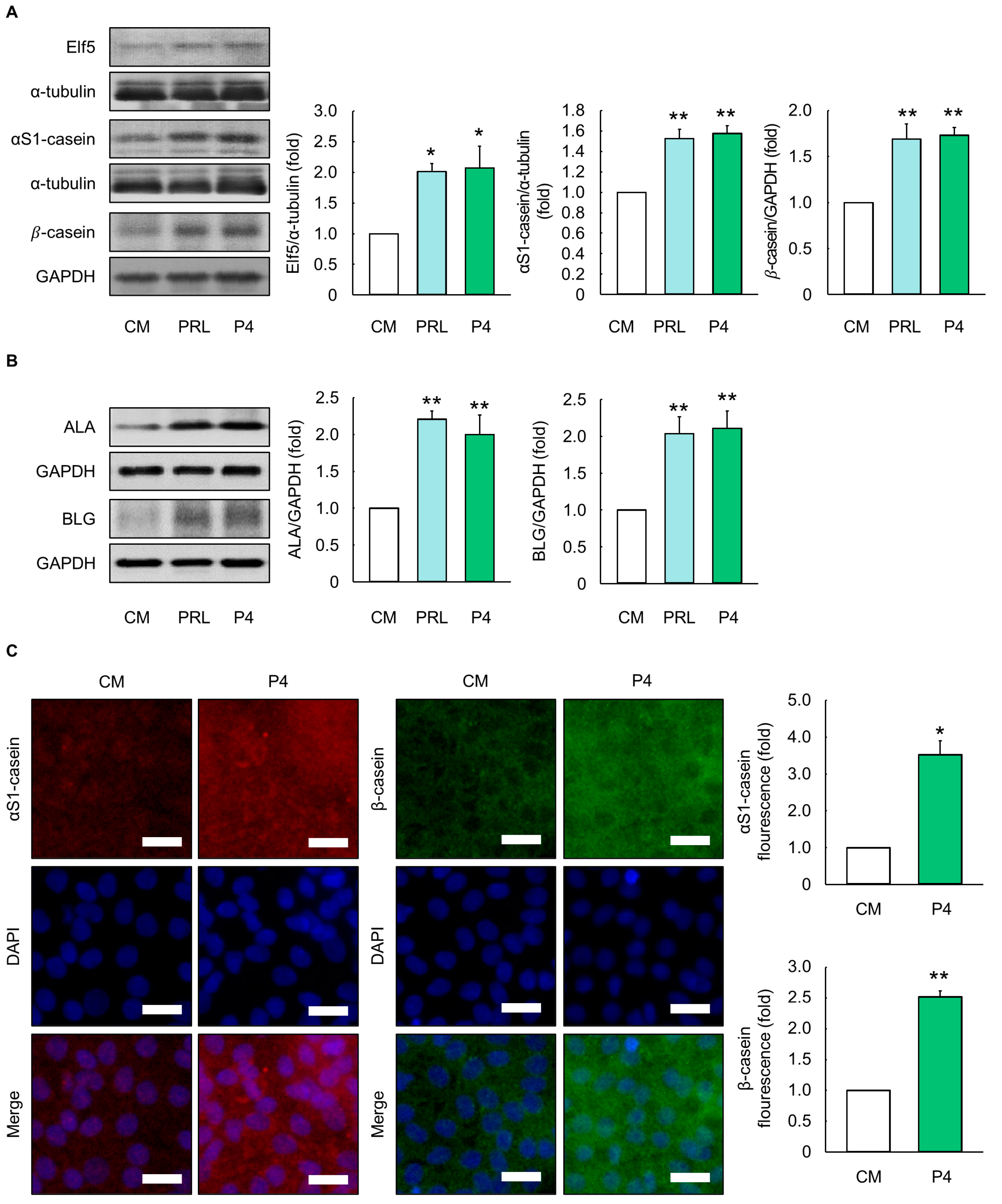
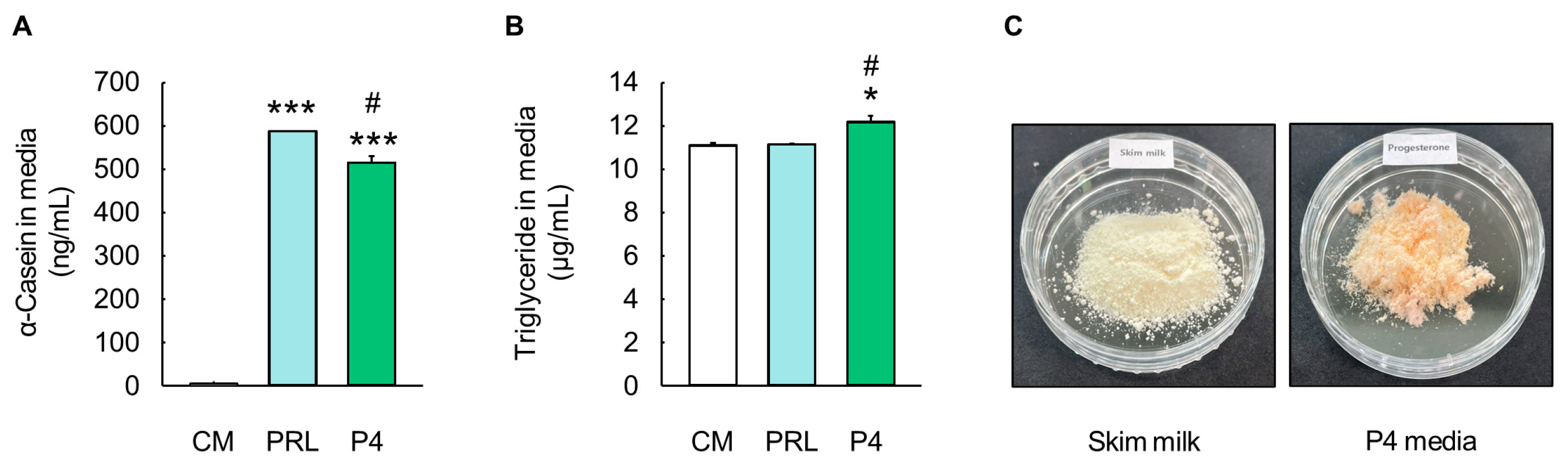
3.3. Effects of Optimal P4 Differentiation Media on Milk Protein Production
3.4. Effects of Optimal P4 Differentiation Media on Milk Fat Production
3.5. Effects of Optimal P4 Differentiation Media on Milk Component Production
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weaver, S.; Hernandez, L. Autocrine-paracrine regulation of the mammary gland. J. Dairy Sci. 2016, 99, 842–853. [Google Scholar] [CrossRef]
- Jaswal, S.; Jena, M.K.; Anand, V.; Jaswal, A.; Kancharla, S.; Kolli, P.; Mandadapu, G.; Kumar, S.; Mohanty, A.K. Critical Review on Physiological and Molecular Features during Bovine Mammary Gland Development: Recent Advances. Cells 2022, 11, 3325. [Google Scholar] [CrossRef]
- Hannan, F.M.; Elajnaf, T.; Vandenberg, L.N.; Kennedy, S.H.; Thakker, R.V. Hormonal regulation of mammary gland development and lactation. Nat. Rev. Endocrinol. 2023, 19, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Lacasse, P.; Ollier, S.; Lollivier, V.; Boutinaud, M. New insights into the importance of prolactin in dairy ruminants. J. Dairy Sci. 2016, 99, 864–874. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Liu, H.Y.; Zhou, M.M.; Liu, J.X. Establishment and characterization of a lactating bovine mammary epithelial cell model for the study of milk synthesis. Cell Biol. Int. 2010, 34, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, F.; Wei, C.; Liang, M.; Zhang, N.; Wang, C.; Li, Q.-Z.; Gao, X.J. Function of SREBP1 in the milk fat synthesis of dairy cow mammary epithelial cells. Int. J. Mol. Sci. 2014, 15, 16998–17013. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.N.; Jeong, C.H.; Seo, H.G.; Han, S.G. Moringa extract attenuates inflammatory responses and increases gene expression of casein in bovine mammary epithelial cells. Animals 2019, 9, 391. [Google Scholar] [CrossRef]
- Barrington, G.; Besser, T.; Gay, C.; Davis, W.; Reeves, J.; McFadden, T.; Akers, R. Regulation of the immunoglobulin G1 receptor: Effect of prolactin on in vivo expression of the bovine mammary immunoglobulin G1 receptor. J. Endocrinol. 1999, 163, 25–32. [Google Scholar] [CrossRef]
- Davis, J.R. Prolactin and reproductive medicine. Curr. Opin. Obstet. Gynecol. 2004, 16, 331–337. [Google Scholar] [CrossRef]
- Choi, Y.S.; Chakrabarti, R.; Escamilla-Hernandez, R.; Sinha, S. Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: Failure of Stat5 activation and functional differentiation in the absence of Elf5. Dev. Biol. 2009, 329, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Ormandy, C.J. Elf5, hormones and cell fate. Trends Endocrinol. Metab. 2012, 23, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Fu, N.Y.; Nolan, E.; Lindeman, G.J.; Visvader, J.E. Stem cells and the differentiation hierarchy in mammary gland development. Physiol. Rev. 2020, 100, 489–523. [Google Scholar] [CrossRef]
- Lee, H.J.; Ormandy, C.J. Interplay between progesterone and prolactin in mammary development and implications for breast cancer. Mol. Cell. Endocrinol. 2012, 357, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Parlow, A.; Neville, M. Hormonal regulation of tight junction closure in the mouse mammary epithelium during the transition from pregnancy to lactation. J. Endocrinol. 2001, 170, 347–356. [Google Scholar] [CrossRef]
- Santos, S.J.; Haslam, S.Z.; Conrad, S.E. Estrogen and progesterone are critical regulators of Stat5a expression in the mouse mammary gland. Endocrinology 2008, 149, 329–338. [Google Scholar] [CrossRef]
- Fernandez-Valdivia, R.; Mukherjee, A.; Creighton, C.J.; Buser, A.C.; DeMayo, F.J.; Edwards, D.P.; Lydon, J.P. Transcriptional response of the murine mammary gland to acute progesterone exposure. Endocrinology 2008, 149, 6236–6250. [Google Scholar] [CrossRef] [PubMed]
- Hilton, H.N.; Kalyuga, M.; Cowley, M.; Alles, M.C.; Lee, H.J.; Caldon, C.E.; Blazek, K.; Kaplan, W.; Musgrove, E.A.; Daly, R.J. The antiproliferative effects of progestins in T47D breast cancer cells are tempered by progestin induction of the ETS transcription factor Elf5. Mol. Endocrinol. 2010, 24, 1380–1392. [Google Scholar] [CrossRef]
- Lee, H.J.; Gallego-Ortega, D.; Ledger, A.; Schramek, D.; Joshi, P.; Szwarc, M.M.; Cho, C.; Lydon, J.P.; Khokha, R.; Penninger, J.M. Progesterone drives mammary secretory differentiation via RankL-mediated induction of Elf5 in luminal progenitor cells. Development 2013, 140, 1397–1401. [Google Scholar] [CrossRef]
- Kwon, H.C.; Jung, H.S.; Kim, D.H.; Han, J.H.; Han, S.G.; Keum, D.H.; Hong, S.J.; Han, S.G. Optimizing hormonal and amino acid combinations for enhanced cell proliferation and cell cycle progression in bovine mammary epithelial cells. Anim. Biosci. 2023, 36, 1757–1768. [Google Scholar] [CrossRef]
- Macias, H.; Hinck, L. Mammary gland development. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 533–557. [Google Scholar] [CrossRef]
- Slepicka, P.F.; Somasundara, A.V.H.; Dos Santos, C.O. The molecular basis of mammary gland development and epithelial differentiation. Semin. Cell Dev. Biol. 2021, 114, 93–112. [Google Scholar] [CrossRef]
- Ormandy, C.J.; Camus, A.; Barra, J.; Damotte, D.; Lucas, B.; Buteau, H.; Edery, M.; Brousse, N.; Babinet, C.; Binart, N. Null mutation of the prolactin receptor gene produces multiple reproductive defects in the mouse. Genes Dev. 1997, 11, 167–178. [Google Scholar] [CrossRef]
- Vomachka, A.J.; Pratt, S.L.; Lockefeer, J.A.; Horseman, N.D. Prolactin gene-disruption arrests mammary gland development and retards T-antigen-induced tumor growth. Oncogene 2000, 19, 1077–1084. [Google Scholar] [CrossRef]
- Atıcı, Ö.K.; Govindrajan, N.; Lopetegui-González, I.; Shemanko, C.S. Prolactin: A hormone with diverse functions from mammary gland development to cancer metastasis. Semin. Cell Dev. Biol. 2021, 114, 159–170. [Google Scholar] [CrossRef]
- Tsugami, Y.; Suzuki, N.; Kawahara, M.; Suzuki, T.; Nishimura, T.; Kobayashi, K. Establishment of an in vitro culture model to study milk production and the blood–milk barrier with bovine mammary epithelial cells. Anim. Sci. J. 2020, 91, e13355. [Google Scholar] [CrossRef]
- Wang, B.; Shi, L.; Men, J.; Li, Q.; Hou, X.; Wang, C.; Zhao, F. Controlled synchronization of prolactin/STAT5 and AKT1/mTOR in bovine mammary epithelial cells. Vitr. Cell. Dev. Biol. Anim. 2020, 56, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; van Baal, J.; Ma, L.; Gao, X.; Dijkstra, J.; Bu, D. MRCKα is a novel regulator of prolactin-induced lactogenesis in bovine mammary epithelial cells. Anim. Nutr. 2022, 10, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K.; Banerjee, S.; Baker, G.W.; Kuo, C.-Y.; Chowdhury, I. The mammary gland: Basic structure and molecular signaling during development. Int. J. Mol. Sci. 2022, 23, 3883. [Google Scholar] [CrossRef] [PubMed]
- Lydon, J.P.; DeMayo, F.J.; Funk, C.R.; Mani, S.K.; Hughes, A.R.; Montgomery, C.; Shyamala, G.; Conneely, O.M.; O’Malley, B.W. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995, 9, 2266–2278. [Google Scholar] [CrossRef]
- Brisken, C.; Park, S.; Vass, T.; Lydon, J.P.; O’Malley, B.W.; Weinberg, R.A. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl. Acad. Sci. USA 1998, 95, 5076–5081. [Google Scholar] [CrossRef]
- Pang, W.W.; Hartmann, P.E. Initiation of human lactation: Secretory differentiation and secretory activation. J. Mammary Gland Biol. Neoplasia 2007, 12, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Hardy, N.; Enger, K.; Hanson, J.; Eastridge, M.; Moraes, L.; Enger, B. Organization of mammary blood vessels as affected by mammary parenchymal region and estradiol administration in Holstein heifer calves. J. Dairy Sci. 2021, 104, 6200–6211. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 99–117. [Google Scholar] [CrossRef]
- Accorsi, P.; Govoni, N.; Gaiani, R.; Pezzi, C.; Seren, E.; Tamanini, C. Leptin, GH, PRL, insulin and metabolic parameters throughout the dry period and lactation in dairy cows. Reprod. Domest. Anim. 2005, 40, 217–223. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine mammary protein synthesis during the lactation cycle. Bioinform. Biol. Insights 2011, 25, S7003. [Google Scholar] [CrossRef] [PubMed]
- Erb, R.; Chew, B.; Keller, H. Relative Concentration of Extrogen and Progestrone in Milk and Blood, and Excretion of Estrogene in Urine. J. Anim. Sci. 1977, 45, 617–626. [Google Scholar] [CrossRef]
- Mukherjee, J.; Mallick, S.; Chaudhury, M.; Prakash, B.; Dang, A. Infradian rhythmicity in milk leukocyte activity together with plasma cortisol and prolactin levels throughout the lactation period in high-yielding crossbred cows. Biol. Rhythm Res. 2015, 46, 909–917. [Google Scholar] [CrossRef]
- Fustini, M.; Galeati, G.; Gabai, G.; Mammi, L.; Bucci, D.; Baratta, M.; Accorsi, P.; Formigoni, A. Overstocking dairy cows during the dry period affects dehydroepiandrosterone and cortisol secretion. J. Dairy Sci. 2017, 100, 620–628. [Google Scholar] [CrossRef]
- Kamada, H. Effects of selenium-rich yeast supplementation on the plasma progesterone levels of postpartum dairy cows. Asian-Australas. J. Anim. Sci. 2017, 30, 347–354. [Google Scholar] [CrossRef]
- Hiew, M.; Megahed, A.; Horstman, L.; Constable, P. Clinical utility of plasma progesterone and blood and plasma glucose concentrations in predicting parturition in Holstein cows. J. Dairy Sci. 2020, 103, 5575–5590. [Google Scholar] [CrossRef]
- Fu, M.; Chen, Y.; Xiong, X.; Lan, D.; Li, J. Establishment of mammary gland model in vitro: Culture and evaluation of a yak mammary epithelial cell line. PLoS ONE 2014, 9, e113669. [Google Scholar] [CrossRef]
- Menzies, K.K.; Lefèvre, C.; Macmillan, K.L.; Nicholas, K.R. Insulin regulates milk protein synthesis at multiple levels in the bovine mammary gland. Funct. Integr. Genom. 2009, 9, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.L.; Hartig, S.M.; Edwards, D.P. Progesterone receptor signaling mechanisms. J. Mol. Biol. 2016, 428, 3831–3849. [Google Scholar] [CrossRef] [PubMed]
- Obr, A.E.; Grimm, S.L.; Bishop, K.A.; Pike, J.W.; Lydon, J.P.; Edwards, D.P. Progesterone receptor and Stat5 signaling cross talk through RANKL in mammary epithelial cells. Mol. Endocrinol. 2013, 27, 1808–1824. [Google Scholar] [CrossRef] [PubMed]
- Schams, D.; Kohlenberg, S.; Amselgruber, W.; Berisha, B.; Pfaffl, M.W.; Sinowatz, F. Expression and localisation of oestrogen and progesterone receptors in the bovine mammary gland during development, function and involution. J. Endocrinol. 2003, 177, 305–318. [Google Scholar] [CrossRef]
- Yart, L.; Finot, L.; Lollivier, V.; Dessauge, F. Oestradiol enhances apoptosis in bovine mammary epithelial cells in vitro. J. Dairy Res. 2013, 80, 113–121. [Google Scholar] [CrossRef]


| Gene 1 | Primer Sequence 5′–3′ |
|---|---|
| CSN1S1 (Bos Taurus) | (F) ACT GAG GAT CAA GCC ATG GAA G (R) GAA TGT GCT TCT GCT CAA CAC T |
| CSN1S2 (Bos Taurus) | (F) AAT CCA TGC CCA ACA GAA AG (R) TCA GAG CCA ATG GGA TTA GG |
| CSN2 (Bos Taurus) | (F) CTG GAA TTA ACT GCT TCT ACC T (R) TAC TCT GCG ATT TGT CTT ATT GA |
| CSN3 (Bos Taurus) | (F) GGC GAG CCT ACA AGT ACA CCT A (R) GGA CTG TGT TGA TCT CAG GTG G |
| ALA (Bos Taurus) | (F) CCT GAA TGG GTC TGT ACC ACG TTT (R) ATG TTG CTT GAG TGA GGG TTC TGG |
| BLG (Bos Taurus) | (F) AGG CCT CCT ATT GTC CTC GT (R) GCA AAG GAC ACA GGG AGA AG |
| GAPDH (Bos Taurus) | (F) ATG ATT CCA CCC ACG GCA AGT T (R) ATC ACC CCA CTT GAT GTT GGC A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.C.; Jung, H.S.; Kim, D.H.; Han, J.H.; Han, S.G. The Role of Progesterone in Elf5 Activation and Milk Component Synthesis for Cell-Cultured Milk Production in MAC-T Cells. Animals 2024, 14, 642. https://doi.org/10.3390/ani14040642
Kwon HC, Jung HS, Kim DH, Han JH, Han SG. The Role of Progesterone in Elf5 Activation and Milk Component Synthesis for Cell-Cultured Milk Production in MAC-T Cells. Animals. 2024; 14(4):642. https://doi.org/10.3390/ani14040642
Chicago/Turabian StyleKwon, Hyuk Cheol, Hyun Su Jung, Do Hyun Kim, Jong Hyeon Han, and Sung Gu Han. 2024. "The Role of Progesterone in Elf5 Activation and Milk Component Synthesis for Cell-Cultured Milk Production in MAC-T Cells" Animals 14, no. 4: 642. https://doi.org/10.3390/ani14040642





